Abstract
Nitrate (NO3 −) is major pollutant in groundwater worldwide. Karst aquifers are particularly vulnerable to nitrate contamination from anthropogenic sources due to the rapid movement of water in their conduit networks. In this study, the isotopic compositions (δ15N–NO3 −, δ15N–NH4 +) and chemical compositions(e.g., NO3 −, NH4 +, NO2 −, K+) were measured in groundwater in the Zunyi area of Southwest China during summer and winter to identify the primary sources of contamination and characterize the processes affecting nitrate in the groundwater. It was found that nitrate was the dominant species of nitrogen in most of the water samples. In addition, the δ15N–NO3 − values of water samples collected in summer were lower than those collected in winter, suggesting that the groundwater received a significant contribution of NO3 − from agricultural fertilizer during the summer. Furthermore, the spatial variation in the concentration of nitrate and the δ15N–NO3 − value indicated that some of the urban groundwater was contaminated with pollution from point sources. In addition, the distribution of δ15N–NO3 − values and the relationship between ions in the groundwater indicated that synthetic and organic fertilizers (cattle manure) were the two primary sources of nitrate in the study area, except in a few cases where the water had been contaminated by urban anthropogenic inputs. Finally, the temporal and spatial variation of the water chemistry and isotopic data indicated that denitrification has no significant effect on the nitrogen isotopic values in Zunyi groundwater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Excessively high levels of nitrogen pose a serious threat to drinking water supplies, promote eutrophication in lakes and reservoirs, and increase the release of the greenhouse gas, nitrous oxide (Heaton 1986; Kendall 1998; Silva et al. 2002; Widory et al. 2004). High nitrate concentrations in drinking water are also believed to be a health hazard. Nitrate is possibly the most widespread groundwater contaminant in the world. This contamination is primarily caused by anthropogenic activities, including the use of inorganic fertilizers, cattle manure, and other organic residues in agricultural regions, sewage effluent in urban environments, and increased atmospheric deposition of nitrogen (Silva et al. 2002; Liu et al. 2006).
Many studies have shown that stable isotope techniques are useful for evaluating sources of nitrogen because certain sources of nitrate have characteristic or distinctive isotopic compositions (Heaton 1986; Mariotti et al. 1988; Kendall 1998; Silva et al. 2002). Nitrate, which can be derived from soil organic nitrogen, synthetic fertilizer, livestock waste, sewage effluent, and atmosphere precipitation, is generally the dominant species of nitrogen in groundwater. In many cases, these sources produce nitrate with distinguishable 15N/14N ratios. For example, the δ15N values range from −1 to +2‰ for synthetic fertilizer, but from +2 to +8‰ for soil organic nitrogen (Kendall 1998). The extent of isotopic fractionation between ammonium and nitrate during nitrification can be ignored when the reaction of nitrification is quick and unlimited. In addition, mineralization usually causes only a small fractionation between soil organic nitrogen and ammonium if there is not a strong volatilization process in the system (Heaton 1986; Wassenaar 1995; Kendall 1998). Liu et al. (2006) observed that the δ15N values of livestock waste were approximately 7.0 ± 3.2‰ in Guiyang. However, fractionation can occur during mineralization and nitrification of excreta and cattle manure; therefore, the isotopic compositions of nitrate derived from excreta and cattle manure generally range from 8 to 20‰ (Heaton 1986; Kendall 1998). In rain, the dominant species of nitrogen is NH4 +, which generally has δ15N values of approximately −12‰ (Xiao and Liu 2002).
Groundwater is the major drinking water source for Zunyi, which is a city on a karstic landform in southwestern China. Karstic groundwater is particularly vulnerable to anthropogenic pollution due to its conduit networks and sinkholes, which quickly respond to rain events and other anthropogenic inputs (Han and Jin 1996; Panno et al. 2001; Katz et al. 2004). Surface and groundwater move quickly within karstic flow systems, and the residence time of groundwater in the aquifers is relatively short when compared to those in non-karstic settings. Therefore, it is important to understand the sources and the variability of nitrate in the groundwater to improve the effects of best management practices on water quality. In this study, the nitrogen isotopes of nitrate and ammonia, as well as the chemical composition (e.g., NO3 −, NH4 +, NO2 −, K+) of the karstic groundwater were analyzed in Zunyi prefecture to identify the sources of nitrate in the groundwater.
Materials and methods
Site description
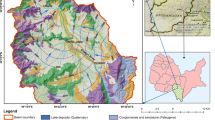
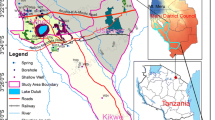
Zunyi prefecture is located in the northeastern portion of the Yunnan-Guizhou Plateau between 27º08′ to 29º12′ N latitude and 105º36′ to 108º13′ E longitude, in a karstic basin. The city has a wide range of karstic landforms and abundant karstic groundwater sources. The strata exposed in Zunyi consist primarily of Pre-Jurassic carbonate (limestone and dolomite) and a few Jurassic clastic rocks that only outcrop in the center of the urban area, with sulfate evaporite (gypsum) and coal seeming to be occurring locally. The main aquifers in the region are primarily composed of carbonate rocks (limestone and dolomite) and clastic rocks. The climate in Zunyi is subtropical, with an average temperature of 15°C and an annual precipitation of approximately 1100 mm. The monsoonal climate often results in a high level of precipitation during wet seasons (generally from April to October) and much less precipitation during dry seasons, although the humidity is often high year round (Mao 1995; Han and Jin 1996). Zunyi prefecture is an important agricultural center that provides food to Guizhou province. As a result, agricultural practices are developing rapidly and farming is becoming industrialized. This has resulted in a rapid influx of livestock waste and sewage effluent directly into the shallow karstic aquifer in the study area; therefore, the water quality in this region has become seriously deteriorated, and there has been a high nitrogen content in many domestic and municipal wells for several years.
Sampling and analytical methods
Water samples were collected in July and December 2002, corresponding to the high-flow (summer) and low-flow (winter) seasons, respectively. The sampling sites for groundwater and surface water are shown in Fig. 1. Samples G15, G17, and G39 were collected at the outlet of the underground river. Samples S34 and S38 were assigned contaminated waters that have a bad odor and unusual color. Field observations revealed that S34 with rural sewage flowed into the local groundwater system through a sinkhole, and that, part of the surface water from S38 with industrial effluents seeped into an underground river. Samples G1, G2, G13, and G14 were collected from boreholes with depths greater than 100 m. The remainder of the samples was collected from artesian wells or various types of springs. Xiangjiang, which is the major river flowing through the Zunyi prefecture, flows into the Wujiang River from SE Zunyi.
The temperature, electrical conductivity (EC), and pH were measured by portable meter (pIONeer 65) in the field. Alkalinity was measured by titrating samples with HCl within 12 h of sample collection. Major cations (Mg2+, Ca2+, K+, and Na+) were analyzed by atomic absorption spectrometry (AAS) and the anions (SO4 2−, Cl−, and NO3 −) were analyzed by high performance liquid chromatography (HPLC). Ammonium and nitrite concentrations were determined by spectrophotometry. The measured precisions for the major ions, NH4 + and NO2 −, were within ±5%.
In order to analyze the nitrogen isotopes in the water, a portion of each water sample (200–2000 ml) was filtered, through 0.45 μm cellulose–acetate filter paper into polyethylene bottles. These samples were then preserved with HCl and sealed with air-tight caps to prevent biological activity. The method described by Liu et al. (2006) was then used to analyze the nitrogen isotopes in the water samples. In brief, nitrate and ammonium were collected on ion-exchange columns that contained anion resin (Dowex 1-X8) and cation resin (Dowex 50-X8), respectively (Garten 1992; Silva et al. 2000; Xiao and Liu 2002). Next, excess 2 M KCl was used to elute the nitrate or ammonium from the resins. N from the eluant was then trapped with an acid trap (H2SO4) using the diffusion method. The combustion tube method was then used for nitrogen isotopic analysis (Kendall and Grim 1990).
Nitrogen isotope ratios were determined using a Finnigan MAT 252 mass spectrometer and was reported in the δ notation relative to N2 (air) in permil. The isotope results are represented in δ units defined as follows:
The δ15N values have a precision of 0.2‰ on average. The δ15N standards, N-1(IAEA), N-3(IAEA), and KNO3, which were provided by Shoko Co., Ltd., Tokyo, Japan (+1.9‰), and the working standards were converted to N2 and used to check the reproducibility of the results.
Results and discussion
Chemical composition of groundwater
The pH values of groundwater ranged from 6.8 to 8.4, with a mean value of 7.3. Ca2+ and Mg2+ were found to be the dominant cations in the groundwater, whereas HCO3 − and SO4 2− were the dominant anions. The chemical compositions of the water samples were primarily characterized as a HCO3–Ca type, HCO3·SO4–Ca type, and HCO3·SO4–Ca·Mg type according to mass balance. These findings indicate extensive dissolution of carbonate rocks and a high solubility of the dissolved solutes (Lang 2005). The variation in the water chemistry of the groundwater is in accordance with the lithology of the Zunyi area. The outcrops in the study area are primarily carbonate rocks (limestone and dolomite) of the Triassic and Permian age, although clastic rocks also show a minor presence. Coal-bearing formations that are enriched with sulfide minerals are also widely distributed; therefore, oxidation of sulfide minerals such as FeS2 results in the addition of sulfate to the groundwater.
Concentration and distribution of nitrogen species
Concentrations of NO3 − in the groundwater ranged from BDL (below detection limit) to 1.46 mmol/l in summer and from BDL to 1.74 mmol/l in winter, with the mean values being 0.32 and 0.31 mmol/l, respectively. In addition, the range of NO3 − in the surface water was from 0.04 to 0.53 mmol/l. The mean concentrations of NO3 − in the surface water were 0.10 and 0.17 mmol/l in summer and in winter, respectively, which are lower than those of the groundwater.
Nitrate was the dominant species of nitrogen in many water samples (Fig. 2); however, high concentrations of NH4 + above 0.2 mg/l were detected in sample G13, G18, G25, and G40 and contaminated waters (S34, S38). Nitrite was found to have the same spatial distribution as NH4 +, but the nitrite concentration was generally low due to its unstable nature. Concentrations of N–NO2 − in the groundwater ranged from BDL to 0.19 mg/l in summer and from BDL to 0.04 mg/l in winter. However, the nitrite concentration was higher in sewage effluent and surface water than in the groundwater. The distribution of NH4 + and NO2 − in several waters suggests that point pollution sources have deteriorated the water quality. Figure 3 provides a general indication of the extent of NO3 − contamination and shows the heterogeneous characteristic of karstic groundwaters. Also, the groundwaters with high nitrate concentrations are generally located in urban areas and the southern suburbs. In addition, the results of this study indicate that it is likely that the spatial distribution of nitrate in the study area changes over seasons. The World Health Organization has set a limit of 50 mg/l NO3 − for drinking water (World Health Organization 2008). However, nitrate contents higher than 50 mg/l were detected in two wells of this study (G24, G40), which indicated that point pollution might directly input the wells.
Co-variation of NO3 − with water chemistry in groundwater
Potential sources of NO3 − in the study area included organic fertilizer, synthetic fertilizer (urea, ammonium sulfate, and N/P/K fertilizer compounds) used in suburban agriculture, soil organic matter, effluent derived from septic systems, and wet deposition. According to analysis of the local fertilizer use from 1950 to 1996, the organic fertilizers used in the area generally consist of cattle manure (generally above 90%), green manure, and cake manure. In addition, the fertilizers used in the study area account for approximately 1/3 of all the fertilizer used in the Zunyi prefecture during the 1990s (Zeng and Sun 1999; Chen 2001).
The chemical behavior of chloride in natural water is conservative when compared with other major ions. Concentrations of Cl− in the groundwater were in the range of 0.06–4.77 mmol/l in summer and 0.01–4.51 mmol/l in winter (Table 1). The Potential sources of Cl− include natural sources (dissolution of minerals), agricultural chemicals (potash or KCl), animal waste, septic effluent, and road salt. Figure 4 shows the variation of the NO3 −/Cl− molar ratios with Cl− concentrations in the groundwater samples. In this study, there was no positive or negative relationship observed between Cl− and NO3 − (not shown), and there was a wide range of NO3 −/Cl− ratios in the groundwater (Fig. 4). The variable and temporal input of Cl− to the groundwater from animal waste or agricultural chemicals suggests that NO3 −/Cl− ratios would not be a good indicator of mixing or denitrification in this area.
The contents of potassium in the water were low due to the stability of the potassium-bearing aluminosilicate minerals and adsorption, as well as a result of assimilation by plants (Hem 1985). However, the K+ may partly originate from fertilizers in the agricultural regions in the study area because organic fertilizer and N/P/K fertilizer are widely used for crops due to the fast movement of waters in the karstic system. It has been reported that the N/K (molar ratio) values in organic fertilizer were approximately 10–12 between 1950 and 1996 and that those values in synthetic fertilizer was approximately 3–4 during the 1990s (Zeng and Sun 1999; Chen 2001). In this study, a higher K+ content was observed in contaminated waters with the N/K value lower than 1. Specifically, the following water samples had K+ concentrations that were greater than 0.30 mmol/l: G25, G27, G37, and G39. These samples were collected in/near urban area, which suggests that the waters were primarily contaminated by sewage effluent with a high K+ level that was generated by industrial activities or urban septic systems. The K+ and NO3 − content can be diluted by convective mixing, diffusion, and adsorption occurring along the hydraulic gradient (Datta et al. 1997). However, karstic groundwater is particularly vulnerable to anthropogenic pollution because its conduit networks and sinkholes quickly respond to rain events and other anthropogenic inputs. Therefore, a relationship between these two ions would be expected if both NO3 − and K+ originate primarily from fertilizers and industrial or domestic wastes. Figure 5 shows a scatter diagram of the NO3 − and K+ content of the groundwater. The samples located on the low N/K molar ratio trend might have been contaminated by urban sewage effluent. In addition, the general increase in NO3 − content with increasing K+ content suggests that the nitrate and potassium concentrations in groundwater in Zunyi are affected by organic fertilizer, synthetic fertilizer, and urban sewage effluents.
δ15N of nitrate in groundwater
The δ15N values of the NO3 −in groundwater were found to range from −1.8 to +20.7‰ (n = 27, mean +7.0‰) in summer and from –4.3 to +22.7‰ (n = 24, mean +10.4‰) in winter. There are, in general, higher δ15N values of nitrate in winter than those in summer. The higher δ15N values in the groundwater may have been caused by a shift in nitrate sources from summer to winter (Panno et al. 2001; Liu et al. 2006). The mean values of δ15N–NO3 −in the surface water were +3.2‰ (n = 11) in summer and +6.6‰ (n = 12) in winter. However, the δ15N–NH4 + values (range,–1.1 to +5.2‰, mean +1.9‰ (n = 18)) in the water samples were lower than the δ15N–NO3 − values. Finally, the δ15N–NO3 − values of the groundwater were higher than those of the surface water.
As shown in the histogram of δ15N values of NO3 − in Zunyi (Fig. 6), approximately 50% of the samples collected during winter, and 25% of those collected during summer had δ15N–NO3 − values greater than 10‰, and approximately 8% of samples collected during winter and 25% of those collected during summer had δ15N–NO3 − values lower than 2‰. Liu and Yuan (2000) reported that karstic groundwater chemistry in Southwest China changed quickly due to the high level of precipitation and the conduit networks and sinkholes in karstic flow system. Therefore, the difference in δ15N–NO3 − values between samples collected during different seasons suggests that there are greater inputs of nitrate from chemical fertilizers during high flow seasons, but that there is a steady input from sewage and livestock sources during low flow seasons. Thus, the nitrogen isotopic composition of domestic septic waste and sewage effluent would be greater in winter.
The spatial distribution of δ15N–NO3 −in groundwater is shown in Fig. 7. The large spatial variation of δ15N from NO3 −observed in the groundwater indicates that the heterogeneous karstic groundwaters were affected by many factors. Mayer et al. (2002) reported that a positive correlation exists between the percentage of land used for agricultural and urban purposes and the mean δ15N values of nitrate for 16 river systems in the northeastern USA. In this study, higher values (>+10‰) of δ15N–NO3 − were generally reported for groundwater samples collected from urban areas or near urban areas (Fig. 7). These findings indicate that most of the NO3 − in these waters was a result of urban activities. The abnormally heavy δ15N–NO3 − value (above 15‰) of groundwater with high concentrations of nitrate (G12, G24, G27) located in urban areas suggests that these wells had a significant contribution of NO3 − from anthropogenic sources that produce waste with a heavy δ15N value. The presence of a high δ15N–NO3 − value in sample G2, which had a low nitrate content, may have occurred as a result of minor denitrification.
Processes affecting nitrate in groundwater
A crossplot of δ15N–NO3 − versus 1/NO3 − and ln[NO3 −] can be used to evaluate the mixing processes and denitrification within hydrological systems (Kendall, 1998). In this study, no simple correlations were observed between δ15N and 1/[NO3 −], or between δ15N and ln[NO3 −] (Fig. 8), which indicates that there are a wide variety of factors controlling nitrate behavior in the groundwater. These findings are similar to those of several previous studies (Wassenaar 1995; Feast et al. 1998). Meanwhile, the results of this study suggest that denitrification had no significant effect on the shift in nitrogen isotopic values of nitrate in Zunyi groundwater (Fig. 8). Instead, the findings of this study indicate that mixing between multiple sources and nitrification were the controlling factors that affected nitrate in the groundwater of Zunyi.
In rain, NH4 + is the dominant nitrogen species and its δ15N value is negative. The nitrate content and δ15N values in rain in Guizhou province, Southwest China are often low (Xiao and Liu 2002). Therefore, rain contributes only a small portion to nitrate pollution of the groundwater. The wide range of δ15N–NO3 − values and inter-correlations between NO3 − and K+ observed in this study suggest that there are at least three major sources contributing to the nitrate pollution of the groundwater in Zunyi, which include organic manure and synthetic fertilizer, and urban sewage effluents.
Aquifers act as “bioreactors” that enable in situ denitrification. Therefore, denitrification should be taken into account when considering δ15N–NO3 − variation because it can remove nitrate from groundwater and lead to isotopic enrichment of the residual NO3 − (Böttcher et al. 1990; Aravena and Robertson 1998; Kendall 1998). Einsiedl and Mayer (2006) reported that denitrification may have occurred in the porous rock matrix of a karst aquifer in southern Germany. However, there is no direct evidence to show that denitrification occurred in the aquifer evaluated in this study. Therefore, further studies are needed to ascertain the extent to which nitrogen isotopes shift in response to denitrification in the Zunyi groundwater.
Conclusion
The results of this study demonstrate that nitrate is the dominant species of nitrogen in most groundwater samples in Zunyi. However, high concentrations of ammonium and nitrite were detected in several samples, which suggests that point-source pollution may play an important role in altering the water quality. Furthermore, the δ15N–NO3 − values in the groundwater were found to vary between summer and winter, which may have occurred due to the transition of nitrate sources. In addition, these findings reflect the rapid movement of waters in the karst aquifer in response to rain event. Furthermore, the lower δ15N–NO3 − observed in summer suggests that there are more synthetic fertilizer inputs during the wet seasons (generally from April to October). In addition, the large spatial variation in the δ15N–NO3 −value that was observed in this study indicates that the heterogeneous karst groundwaters were affected by many factors. When combined with the K+/NO3 − ratio, these variations in the isotope values of nitrate in the groundwater demonstrate that there are at least three major potential sources of nitrate in the groundwater in Zunyi.
The N-isotope analysis of groundwater nitrate indicated that synthetic fertilizers and organic fertilizers (cattle manure) were the two major contributors of nitrate in the study area, except for several waters that were primarily contaminated by urban anthropogenic inputs. However, the relationship between nitrogen isotopic values and water chemistry did not indicate that denitrification was responsible for the significant shift in δ15N–NO3 − values observed in this study. In summary, mixing processes and nitrification were the primary processes that controlled nitrate in groundwater in this area. In addition, the results of this study indicate that it is important to avoid point-source pollution in this area to protect the quality of the groundwater. However, additional systematic study is needed to assess the nitrogen transformation that occurs in this groundwater system as a result of mixing between point and non-point pollution and to evaluate the shift in isotopic composition that occurs as a result of microbial processes and the complicated conduit networks in the karst groundwater system.
References
Aravena R, Robertson WD (1998) Use of multiple isotope tracers to evaluate denitrification in ground water: study of nitrate from a large-flux septic system plume. Ground Water 36:975–982
Böttcher J, Strebel O, Voerkelius S, Schmidt HL (1990) Using isotope fractionation of nitrate-nitrogen and nitrate-oxygen for evaluation of microbial denitrification in a sandy aquifer. J Hydrol 114:413–424
Chen XH (2001) Variations of soil nutrient content and fertilization in Guizhou. Plant Nutrition and Fertilizer Science 7(2):121–128 (in Chinese)
Datta PS, Deb DL, Tyagi SK (1997) Assessment of groundwater contamination from fertilizers in the Delhi area based on 18O, NO3 − and K+ composition. J Contam Hydrol 27:249–262
Einsiedl F, Mayer B (2006) Hydrodynamic and microbial processes controlling nitrate in a fissured-porous karst aquifer of the Franconian Alb, Southern Germany. Environ Sci Technol 40(21):6697–6702
Feast NA, Hiscock KM, Dennis PF, Andrews JN (1998) Nitrogen isotope hydrochemistry and denitrification within the Chalk aquifer system of north Norfolk, UK. J Hydrol 211:233–252
Garten CT Jr (1992) Nitrogen isotope composition of ammonium and nitrate in bulk precipitation and forest throughfall. Int J Environ Anal Chem 47:33–45
Han ZJ, Jin ZS (1996) Hydrogeology of Guizhou Province, China. Seismic Publication, Beijing (in Chinese)
Heaton THE (1986) Isotopic studies of nitrogen pollution in the hydrosphere and atmosphere: a review. Chem Geol (Isotope Geochemistry Section) 59:87–102
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water, 3rd edn. U.S. Geological Survey Water Supply Paper, 2254, 263 pp
Katz BG, Chelette AR, Pratt TR (2004) Use of chemical and isotopic tracers to asses nitrate contamination and groundwater age, Woodville Karst Plain, USA. J Hydrol 289:36–61
Kendall C (1998) Tracing sources and cycling of nitrate in catchments. In: Kendall C, McDonnell JJ (eds) Isotope tracers in catchment hydrology. Elsevier, Amsterdam, pp 519–576
Kendall C, Grim E (1990) Combustion tube method for measurement of nitrogen isotope ratios using calcium oxide for total removal of carbon dioxide and water. Anal Chem 62:526–529
Lang YC (2005) Geochemical characteristics of cycling of substances in karstic ground water system: a case study from Guiyang and Zunyi Cities, China. PhD Thesis, Graduate School of the Chinese Academy of Sciences, Beijing (in Chinese)
Liu ZH, Yuan DX (2000) Geochemical dynamic features of the typical karst systems in China and their environmental implications. Geol Rev 46(3):324–327 (in Chinese)
Liu C-Q, Li S-L, Lang Y-C, Xiao H-Y (2006) Using δ15N- and δ18O-values to identify nitrate sources in karst ground water, Guiyang, Southwest China. Environ Sci Technol 40:6928–6933
Mao XY (1995) Analysis of factors on motion of groundwater and groundwater search in Zunyi, Guizhou. Guizhou Geol 12(1):78–83 (in Chinese)
Mariotti A, Landreau A, Simon B (1988) 15N isotope biogeochemistry and natural denitrification process in ground water: application to the chalk aquifer of northern France. Geochim Cosmochim Acta 52(7):1869–1878
Mayer B, Boyer EW, Goodale C et al (2002) Sources of nitrate in rivers draining sixteen watersheds in the northeastern US: isotopic constraints. Biogeochemistry 57(58):171–197
Panno SV, Hackley KC, Hwang HH, Kelly WR (2001) Determination of the sources of nitrate contamination in karst springs using isotopic and chemical indicators. Chem Geol 179:113–128
Silva SR, Kendall C, Wilkison DH, Zergler AC, Chang CCY, Avanzino R (2000) A new method for collection of nitrate from fresh water and the analysis of nitrogen and oxygen ratios. J Hydrol 228:22–36
Silva SR, Ging PB, Lee RW, Ebbert JC, Tesoriero AJ, Inkpen EL (2002) Forensic applications of nitrogen and oxygen isotopes in tracing nitrate sources in urban environments. Environ Forensic 3:125–130
Wassenaar LI (1995) Evaluation of the origin and fate of nitrate in the Abbotsford aquifer using the isotopes of 15N and 18O in NO3 −. Appl Geochem 10(4):391–405
Widory D, Kloppmann W, Chery L, Bonnin J, Rochdi H, Guinamant J-L (2004) Nitrate in groundwater: an isotopic multi-tracer approach. J Contam Hydrol 72:165–188
World Health Organization (2008) Guidelines for drinking-water quality, 3rd edn. Geneva
Xiao HY, Liu C-Q (2002) Sources of nitrogen and sulfur in wet deposition at Guiyang, southwest China. Atmos Environ 36:5121–5130
Zeng XZ, Sun J (1999) The actuality of organic fertilizer input into arable land and the measure to develop soil fertility in Zunyi Prefecture. Cult Plant 5:59–60 (in Chinese)
Acknowledgments
We are grateful to T.H.E Heaton who has kindly provided the δ15N working standard. We also thank reviewers who have given comments and suggestions. This study is financially supported by National Natural Science Foundation (Grant No. 40603005, 40721002), and the “973-program” (Grant No. 2006CB403205) of Ministry of Science and Technology of China.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, SL., Liu, CQ., Lang, YC. et al. Tracing the sources of nitrate in karstic groundwater in Zunyi, Southwest China: a combined nitrogen isotope and water chemistry approach. Environ Earth Sci 60, 1415–1423 (2010). https://doi.org/10.1007/s12665-009-0277-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-009-0277-0