Abstract
Feasibility of using straw as sole substrate for in situ bioremediation of acidic mine drainage (AMD) was studied. The result showed that straw was more suitable than woodchips, which had been successfully used for bioremediating AMD at the source, for establishing bioremediation layer. The sulfate removal rate of rice straw treatment was almost two times higher than that of the woodchips treatment when the initial pH of the synthetic AMD was set to 3.0. Straw treatment may be more efficient at reducing sulfate than woodchips treatment under stressful conditions. The sulfate removal rate of the rice straw treatment increased from 8.67 to 21.77 mg L−1 day−1 when initial pH increased from 1 to 7 while the removal rate of woodchips treatment increased from 3.80 to 11.95 mg L−1 day−1. The sulfate removal rate of the rice straw treatment decreased from 13.93 to 9.91 mg L−1 day−1 when temperature decreased from 25 to 5°C while the removal rate of woodchips treatment decreased from 7.43 to 4.98 mg L−1 day−1. Differences in soluble organic carbon release between rice straw and woodchips led to the differences in bioremediation efficiency. Concentrations of Cu2+ maintained at low level in the column effluent during the whole bioremediation period. Cu2+ was removed by forming sulfide precipitates. Microbial community analysis showed that sulfate reducing bacteria in the bioremediation layer together with microorganisms capable of degrading rice straw caused the bioremediation of AMD. These findings have significant environmental implications in terms of in situ bioremediation of AMD using straw as sole substrate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mining and milling of sulfide ore generate large quantities of waste rock and finely crushed mill tailings (Jurjovec et al. 2002). Tailings, particularly metal sulfide mine tailings, are likely to cause serious pollution to the surface water, groundwater and soil. Exposure of the sulfide minerals to atmospheric oxygen may ultimately lead to the formation of acidic mine drainage (AMD). Acidic waters generated in the unsaturated zone of tailings impoundments contain elevated concentrations of sulfate and heavy metals. The oxidation of sulfide minerals within tailings may continue to release metals to the surrounding environment for decades to millennia (Hulshof et al. 2006).
Conventional chemical treatment of AMD is expensive, produces voluminous amounts of sludge, and produces high-sulfate water, containing high concentrations of dissolved heavy metals (Benner et al. 1999). The alternative and economically attractive process for the decontamination of AMD is metal precipitation by using anaerobicly generated sulfide. Considerable research has been conducted to investigate the remediation of AMD by using bacteria, dominantly sulfate reducing bacteria (SRB) (Chang et al. 2000; Jin et al. 2008). The added organic carbon can promote SRB to convert sulfate into sulfide, which forms metal sulfide precipitates (Hulshof et al. 2003). An alternative approach for remediating mine drainage is the direct addition of organic carbon to the saturated tailings as layers (Hulshof et al. 2003). The direct addition of an organic carbon source to the tailings has the potential to promote sulfate reduction and the subsequent removal of heavy metals from the tailings pore water, remediating AMD at the source. This remediation approach can be implemented while tailings deposition is underway (Hulshof et al. 2003). While there are many desirable aspects of in situ bioremediation for mine drainage, little guidance is available regarding the low cost organic carbons.
Careful selection of a suitable carbon source is of paramount importance to ensure performance and longevity in biological AMD treatment (Zagury et al. 2006). Various organic matters such as milk (Jin et al. 2008; Tsukamoto and Miller 1999; Hulshof et al. 2006) have been used as organic carbon for promoting bacterial sulfate reduction in mine drainage remediation process. However, only woodchips and pulp waste have been successfully used in in situ layer for treating mine drainage (Hulshof et al. 2006; Hulshof et al. 2003). Studies have shown that poor biodegradability of woodchips may lead to low sulfate reduction rate (Chang et al. 2000). Moreover, harmful contents in woodchips have inhibition effect on sulfate bioreduction (Chang et al. 2000). The successful usage of alfalfa for promoting bacterial sulfate reduction in previous studies (Bechard et al. 1994) suggested that other cellulosic material might be an alternative solid carbon for establishing in situ biolayer. Cereal straw is one of the cheapest and most abundant resources in the world. The total worldwide production of cereal straw was estimated to exceed 2.9 × 109 t a−1 (Sun et al. 2004). So far, little information is available on the successful in situ biotreatment of acidic drainage in tailings impoundment using straw as substrate. A mixed aerobic–anaerobic microbial treatment process for AMD was developed previously using straw as substrate (Bechard et al. 1994). However, this process was effective only if AMD was supplemented with sucrose.
In the in situ bioremediation system, SRB play a crucial role in sulfate reduction, which is to consume protons and produce sulfide, thus increasing pH and decreasing heavy metal concentrations by forming metal–sulfide precipitates. However, SRB have been observed to represent a relatively low proportion of the microbial community in many sulfate-reducing mine drainage treatment systems (Jin et al. 2008; Pruden et al. 2007). The high sulfate reduction efficiency in the bioremediation system relies on the good cooperation between SRB and other microorganisms (Jin et al. 2008; Pruden et al. 2007). Therefore, efforts to improve microbiological design criteria for sulfate reduction bioremediation systems must consider the entire microbial community and not merely SRB. Although in situ bioremediation technique is biologically catalyzed treatment system, the microbial community in the biolayer has not been well characterized.
The objectives of this paper are to evaluate the feasibility of adding straw in biolayer for in situ biotreatment of mine drainage. The final goal is to obtain initial information on the performance of in situ biotreatment of acidic drainage in tailings impoundment using straw as substrate.
Materials and methods
Materials
Woodchips were obtained from local landscaping firm. They were dried at 105°C and crushed to an average size of 3 cm in diameter. The rice straw (Oryza sativa L.) was harvested from local field, and was dried at 105°C. The straw was cut into pieces approximately 3 cm in length.
The synthetic AMD water contained 450 mg L−1 SO4 2− and 20 mg L−1 heavy metal ion. Samples were obtained by dissolving weighted amounts of reagent grade chemicals. The pH was adjusted using 1 M HCl. Dissolved oxygen was removed by purging the medium with high pure N2 for at least 15 min. To avoid the interaction of heavy metal toxicity between different heavy metals and gain a more accurate result, only Cu2+, which was the most common heavy metal ion in AMD, was added as heavy metal. The initial pH value was 3.0 without special description.
Anaerobic sludge used as inoculum was obtained from the anaerobic bioreactor of Wang Xiaoying sewage treatment plant, Hefei, Anhui Province, China. Glass jars were filled to capacity with slurry, sealed, and transported to the laboratory. To avoid the disturbance of the organic contaminants in the anaerobic sludge, the sludge was collected by centrifugation (1,500g) in a tightly sealed centrifuge tube, washed twice with the oxygen-free medium and resuspended in 50 mL oxygen-free medium. After being vigorously mixed, this slurry was then settled for half an hour. The sludge-free aqueous phase was finally used as inoculum for subsequent experiments.
Batch experiments
Comparison studies between rice straw and woodchips were performed in batch experiments. Biodegradation tests were carried out in 150 mL serum bottles. Synthetic AMD (90 mL) and sediment-free culture (10 mL) were added to each bottle. Serum bottles were capped with rubber stoppers and crimped with aluminum seals. The head space of the bottles was high pure N2. The incubation was performed at 25°C in darkness. Woodchips (1 g) or rice straw (1 g) was added to all culture samples as solid carbon source. All treatments were performed in triplicate. Strict anaerobic microbial techniques were used throughout experiment manipulations. At each sampling point the cultures were rigorously shaken and sampled with sterile syringes flushed with high pure N2. All treatments were performed in triplicate.
Column experiments
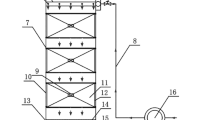
Column experiments were performed to gain further information on the performance of in situ biotreatment of acidic drainage in tailings impoundment using straw as substrate. Plexiglas cylinders used for the columns were made according to Hulshof et al. (2003). Each column was 80 cm in length and 10 cm in diameter. Layers of clean river sands with thickness of 30 cm were placed at the top and bottom of the column. River sands were washed twice by distilled water before being used for tailings. Straw (100 g) was packed between the sand layers to form an organic layer. This organic layer was replaced by the same volume of clean quartz sand in control. The packed column was flushed with high pure N2 before the experiment started up. The residence time of AMD in column is 8 days.
Oxygen-free AMD was pumped into infusion bag to store the synthetic AMD under anaerobic conditions (Wu et al. 2008). The stored AMD was fed into column by a pump. Each column was operated in upward flow mode at an average rate of 800 mL day−1. 800 mL sediment-free culture was fed on the first day.
Analytical methods
Compositions of woodchips and rice straw were determined by extraction methods (Harper and Lynch 1981).The pH and ORP were measured by using Ultrameter IITM 6P (Myron L Company, USA). Samples were subject to inductively coupled plasma atomic emission spectroscopy (Iris Advantage 1000, Thermo Jarrell Ash Corporation, USA) for analysis of heavy metals (copper nickel, and zinc). Sulfate was measured by a MIC ion chromatograph (Metohm, Switzerland). Volatile fatty acids (VFAs) were assayed using a GC-2010AF GC (Angilent Instruments Co., USA) equipped with a flame ionization detector.
The surface morphology of the black precipitant was observed by a Field Emission Scanning Electron Microscope. Micrograph was taken by a FEI SIRION 200 SEM (FEI Company, USA). An accelerating voltage of 5 kV was used during operation, with resolution 3 nm. The element analysis of the sample surface was determined by energy dispersive spectrometer (EDS). An OXFORD INCA EDS (OXFORD Instruments, UK) was employed during analysis.
The black precipitant sample was characterized by X-ray powder diffraction (XRD). X-ray analysis was performed by D8 ADVANCE X-ray polycrystaline diffractometer (Bruker axs GmbH, Karlsruhe, Germany). X-ray analysis employed Cu Kα radiation (λ = 1.5406 Ǻ), with the graphite filter and the position sensitive detector (PSD). XRD was operated at 40 kV and 40 mA. The scattered intensities were measured with a NaI dynamic scintillation counter. Scans were run from 10° to 90° (2θ), with a step of 0.02° and a step time of 0.3 s.
To be sure that SRB play a crucial role in our bioremediation system, Enumeration of viable SRB was performed as previously described (Benner et al. 2000). 1 g sample was added to each of five serum bottles. Inoculated samples were sequentially diluted and incubated under anaerobic conditions for 30 days. Positive growth of SRB was indicated by precipitation of Fe-sulfides. Values are reported as most probable number (MPN) determinations (Alexander 1965).
DNA extraction
At the end of the experiment, genomic DNAs of microorganisms in straw were extracted by the following method as described by Piao et al. (2008) with slight modification. Briefly, 2 g of mixture, collected from the batch experiment, was repeatedly homogenized by vortexing it in 20 mL phosphate-buffered saline and centrifuged at 200g for 2 min. Bacterial cells in the combined supernatant liquid were collected by centrifugation at 12,000g for 10 min, washed three times with TENP buffer, and lysed by bead beating. After the bead beating procedure, 110 μL of sodium dodecyl sulfate (10%) was added and gently mixed, and the sample was incubated on ice for 10 min. After this, 150 μL chloroform–isopropanol (25:1, vol/vol) was added, gently mixed, and then centrifuged at 15,000g for 10 min. The supernatant liquid was mixed with 1/10 volume of 3 M sodium acetate and 1 volume of phenol, followed by centrifugation at 15,000g for 10 min. The supernatant was then extracted twice with chloroform–isopropanol (24:1, vol/vol). Nucleic acids in the supernatant were precipitated with cold ethanol and resuspended in double-distilled water and stored at −20°C.
16 S rDNA amplification and denaturing gradient gel electrophoresis (DGGE) analysis
Each DNA fragment encoding 16S rRNA (corresponding to the positions 50-341 to 927-30 in the Escherichia coli sequence) was amplified using the eubacterial primer GM5F and the universal primer 907R (Muyzer et al. 1995). A 40-base GC clamp was attached to the end-5′ of the GM5F primer for DGGE analysis. The primer sequences were 5′-CCTACGGGAGGCAGCAG-3′ for GM5F, 5′-CCGTCAATTCCTTTRAGTTT-3′ for 907R, and 5′-CGCCCGCCGCGCCCCGCGCCCGTCCCGCCGCCCCCGCCCGCCTACGGGAGGCAGCAG-3′ for GC-GM5F. PCR amplifications were performed with a Mastercycler gradient PCR system (Eppendorf China Ltd.) as described by Nakagawa et al. (2002a).
The PCR solution consisted of 76 μL of sterile water (Sangon, Shanghai, China), 10 μL of 10× Taq buffer with MgCl2 (Sangon, Shanghai, China), 25 pmol each of the primers, 10 μL of deoxynucleotide triphosphates mixture, and 1 μL of template DNA solution. To minimize nonspecific annealing of the primers to nontarget DNA, 2.5 U of Taq polymerase (Sangon, Shanghai, China) was added to the reaction mixture at 80°C after an initial denaturing step of 94°C for 5 min. The temperature was subsequently cooled to 65°C for 1 min. This temperature was decreased by 1°C every second cycle until a touchdown of 55°C, the temperature at which 10 cycles were carried out. Denaturation and primer extension were carried out at 94°C for 1 min and at 72°C for 3 min, respectively. Cycling was completed by a final extension at 72°C for 10 min. PCR products were verified by electrophoresis in a 0.8% agrose gel, and then analyzed by DGGE.
DGGE analysis was performed as described by Muyzer et al. (1995) by loading PCR-amplified DNA product onto an 6% (wt/vol) acrylamide gel containing a denaturant gradient of 20–60% [100% denaturant consisted of 7 M urea and 40% (vol/vol) formamide] parallel to the electrophoresis detection using the D-code system (Bio-Rad, Hercules, CA, USA). Electrophoresis was performed at 60°C and a constant voltage of 200 V for 4 h. After electrophoresis, the gels were incubated for 10 min in ethidium bromide (1.0 mg L−1), rinsed for 10 min in distilled water, and then been analyzed with UV transillumination (302 nm).
For further identification of predominant DGGE bands in individual samples, DGGE fragments were cut and eluted in 50 mL of TE buffer overnight. Recovered DNA was used as DNA template in a following PCR amplification with the same DGGE primer set as used previously. The PCR products were analyzed in a separate DGGE for purity, purified using UNIQ-10 DNA purification kit (Sangon, Shanghai, China), and then sequenced commercially (Sangon, Shanghai, China) with an ABI 3730 DNA analyzer (Applied Biosystems) using the GM5F primer.
Data calculation
The maximum bioreduction rate of sulfate was determined from the time course of sulfate disappearance, using points in the linear portion of graphs that released sulfate concentration to time following the previously described method (Lu et al. 2008).
Results and discussion
Chemical characterization of substrates
Chemical characterization of straw and woodchips were measured (Table 1) to know their potentials of organic carbon supply. Concentrations of cellulose (388 g kg−1 dry weight) and lignin (97 g kg−1 dry weight) in rice straw were much lower than those in woodchips while concentrations of solvent extractable carbon (69 g kg−1 dry weight), hot water extractable (113 g kg−1 dry weight), and hemi-cellulose (306 g kg−1 dry weight) in straw were much higher than those in woodchips. Chemical composition of an organic substrate controls the pattern of its degradability (Gibert et al. 2004). Since extractable carbon and hemi-cellulose are easily available carbon source in solid waste materials (Chang et al. 2000), their high concentrations in straw suggest that straw be more readily degraded than woodchips. Moreover, the high concentrations of poor digestable substances (cellulose and lignin) indicate that woodchips are more recalcitrant than rice straw. Relatively high concentration of typical heavy metals in rice straw might be caused by the high application frequency of fertilizer and pesticide in rice production.
Microbial community
To know the main functional bacteria in the bioremediation system, the microbial community in straw was studied. Six predominant DGGE bands were sequenced. The majority sequenced bands corresponded to bacteria associated with plant residue degradation, which belonged to Clostridiaceae, Eubacterium and Pseudobutyrivibrio. Two sequences showed 96–98% similarity to bacteria, which belonged to Clostridiaceae, associated with degradation of rice plant residue (Akasaka et al. 2003). Two sequences showed 98–99% similarity to the cellulose degraders Eubacterium cellulosolvens (Taguchi et al. 2008) and Pseudobutyrivibrio ruminis strain Ce1 (Vossenberg 2003), respectively. SRB were also detected. One sequence showed 95% similarity to the sulfate reducers Bacterium T-2 (Nakagawa et al. 2002b) and uncultured bacterium (Nakagawa et al. 2002a). As previously described, these SRB were capable of degrading complex organic pollutants under sulfate-reducing conditions. One sequence showed 98% similarity to sulfur compounds reducing bacterium Clostridium sp. U42 (Takahashi 2006) indicates that this bacterium may also correspond to SRB. To be sure that SRB play a crucial role in our bioremediation system, Enumeration of viable SRB was also performed. As expected, SRB (2.75 × 108 MPN g−1) were detected. These observations show that each kind of bacteria (sulfate reducing bacteria, cellulolytic bacteria, and fermentative bacteria) may have a significant and symbiotic role in the bioremediation process.
Batch experiment
Low pH can do harm to microorganisms which makes low pH be a common problem for AMD biotreatment. Treatment of AMD by SRB depended on the ability of SRB to reduce sulfate (Luptakova and Kusnierova 2005). For this reason, effect of initial pH on sulfate reduction in different substrate treatments was conducted. There is a linear relationship (r 2 = 0.907–0.995) between initial pH and the sulfate removal rate (Fig. 1). Increase in the initial pH resulted in increased removal rate. Low pH had inhibition effect on sulfate reduction in both treatments. The sulfate removal rate of straw treatment increases much more rapidly than that of woodchips treatment when the initial pH increased. The sulfate removal rate of the rice straw treatment increased from 13.93 to 21.77 mg L−1 day−1 when initial pH increased from 3 to 7 while the removal rate of woodchips treatment increased from 7.43 to 11.95 mg L−1 day−1. The result indicates that rice straw treatment may be more efficient in the sulfate reduction than woodchips treatment under acid stress conditions. When the initial pH of the synthetic AMD was set to 3.0, the sulfate removal rate of rice straw treatment was almost two times higher than that of the woodchips treatment. The result shows the feasibility of the in situ biotreatment of AMD using straw as the sole substrate. Though straw was often used for the biotreatment of AMD, successful reports on using straw as the sole substrate in in situ bioremediation treatment of AMD were quite rare. The sulfate removal rates are similar to the previous report of Gibert (Gibert et al. 2004). In their batch experiment, they used oak leaf or sheep manure as carbon sources.
In anaerobic treatment, the slow growth rate of microorganisms makes temperature more important for bioremediation design. To investigate the effect of low temperature on sulfate bioreduction, incubations were performed at 5, 10, and 15°C. Rice straw treatment had better bioremediation efficiencies than woodchips treatment at low temperatures (Fig. 2). The sulfate removal rate of the rice straw treatment decreased from 11.75 to 9.91 mg L−1 day−1 when temperature decreased from 15 to 5°C while the removal rate of woodchips treatment decreased from 6.08 to 4.98 mg L−1 day−1.
Based on Arrhenius equation, a simple equation (Rittmann and McCarty 2001) which was widely used in environmental engineering was developed to describe the relationship between kinetic parameter of anaerobic systems and temperature:
where Φ is the temperature coefficient, \( \hat{q}_{1} \) and \( \hat{q}_{2} \) are the maximum rate of substrate utilization at temperature T 1 and T 2, respectively. The maximum removal rates of sulfate at different temperatures (5, 10, 15 and 25°C) were used to calculate the temperature coefficient (Φ) according to Eq. 1. Straw treatment has lower temperature coefficient (0.017°C−1) than woodchips treatment (0.02°C−1) which indicates that rice straw treatment may be more efficient in the sulfate reduction than woodchips treatment at low temperatures. Relatively low temperature coefficient suggests that temperature changes have little effect on bioremediation efficiencies. Similar phenomenon had been observed by Tsukamoto et al. (2004).
Soluble organic carbon release in different substrate
The nature of organic matter is a determinant factor on the biotreatment efficiency. Solid material with low dissolved organic carbon (DOC) content is not a good solid carbon source for bacterial sulfate reduction (Zagury et al. 2006). For this reason, DOC and VFAs were measured as indicators of soluble organic contents in different substrate treatments to know if rice straw is more suitable for being used in in situ bioremediation treatment system than woodchips. Both DOC and VFAs reached their top within the first week (Fig. 3) owing to the intense prevailing activity of anaerobic celullolytic and fermentative bacteria over SRB. Therefore, DOC and VFAs accumulated in this period. Concentration of DOC in straw amendment treatment was much higher than that of woodchips amendment treatment during the whole operation period. The recalcitration of woodchips may lead to the low release rate of DOC. Changes in the concentrations of VFAs showed that acetate was the main content of the DOC. Moreover, concentration of acetate in straw amendment treatment was much higher than that in woodchips amendment treatment during the whole operation period. Relatively high concentration of DOC in the straw treatment resulted in much higher sulfate reduction rate. Differences in soluble organic carbon release between rice straw and woodchips led to the differences in sulfate reduction.
Although SRB catalyze the sulfate reducing process, which is the final reaction of the in situ bioremediation, they rely on the activity of anaerobic cellulolytic bacteria and fermentative bacteria to break down complex organic materials from rice straw or woodchips into easily available carbons such as acetate, to provide them with carbon and energy sources. For this reason, the recalcitrant woodchips, which have high concentrations of poorly digestable substances (cellulose and lignin), could not supply enough easily available carbons to maintain relatively high sulfate reduction rate.
Column experiment
To ensure that sulfate was reduced during the bioremediation period, changes in the concentration of sulfate were also monitored. Changes in the sulfate concentration (Fig. 4) show that heavy metal removal in straw treatment is mainly caused by sulfate reduction after day 5. The sulfate removal rate of straw treatment is almost 45.6 mg L−1 day−1 from day 5 to day 10, which are similar to the previous report of Gibert et al. (2004), confirming the feasibility of using straw as the sole substrate.
The initial pH of rice straw treatment or control was about 6.2, which was much higher than that of synthetic AMD (Fig. 5), as a result of the acidity neutralization by river sands. The decrease in acidity neutralization capacity of sands led to decrease in effluent pH in control during the operation period. After 120 days of operation, effluent pH in control slightly dropped to 4.4 while that in cover treatment slightly increased to 7.8. Increase in effluent pH was due to proton consumption by sulfate reduction during the bioremediation period. The biological reduction of sulfate can generate alkalinity, which contributes to neutralizing the acidity of the AMD (Luptakova and Kusnierova 2005), by consuming protons.
Treatment of AMD by SRB was dependent on the ability of SRB to reduce sulfate to hydrogen sulfide, which could form precipitates with heavy metals (Luptakova and Kusnierova 2005). Black precipitant, which was identified as metal sulfide by SEM with EDS (Fig. 6), and XRD (data not shown) was flushed out the column. Cu2+ was precipitated with S2−, forming the copper sulfide (CuS). Typical crystalloid of CuS was found in SEM micrograph. Concentration of Cu2+ in the effluent maintained at low level during the whole bioremediation period in straw treatment, ranging between 0.1 and 0.4 mg L−1 after a bioreactor startup period (Fig. 7). Low heavy metal concentration was also observed in control. However, this only occurred within the initial 5 days followed with a sharp increase within another 5 days. Concentration of Cu2+ had maintained at high level since day 10. As can be seen in Fig. 5, pH of effluent was around 6 at the initial days in control column. At this pH value it is reasonable to think that Cu2+ concentration decrease is due to adsorption processes. Typical break through curve for Cu2+ observed in control indicate that heavy metals removal during the preliminary period in all treatments is mainly caused by adsorption.
Conclusions
In the present study, we reported the in situ bioremediation of AMD using straw as the sole substrate. The result demonstrated that straw was more suitable than woodchips for in situ bioremediation of AMD. High bioremediation efficiency could be achieved when straw replaced woodchips as the sole substrate. Straw was more efficient in promoting sulfate reduction than woodchips under stressful conditions such as low pH and low temperature. Since straw is more easily available than woodchips, these findings have significant environmental implications in terms of AMD treatment for mine industry.
References
Akasaka H, Izawa T, Ueki A, Ueki K (2003) Phylogeny of numerically abundant culturable anaerobic bacteria associated with degradation of rice plant residue in Japanese paddy field soil. FEMS Microbiol Ecol 43:149–161. doi:10.1111/j.1574-6941.2003.tb01054.x
Alexander M (1965) Most-probable-number method for microbial populations. In: Black CA et al (eds) Methods of Soil Analysis, Part 2. Am. Soc. Agron. Wise, Madison, pp 1467–1472
Bechard G, Yamazaki H, Gould WD, Bedard P (1994) Use of cellulosic substrates for the microbial treatment of acid-mine drainage. J Environ Qual 23:111–116
Benner SG, Blowes DW, Gould WD, Herbert RB, Ptacek CJ (1999) Geochemistry of a permeable reactive barrier for metals and acid mine drainage. Environ Sci Technol 33:2793–2799. doi:10.1021/es981040u
Benner SG, Gould WD, Blowes DW (2000) Microbial populations associated with the generation and treatment of acid mine drainage. Chem Geol 169:435–448. doi:10.1016/S0009-2541(00)00219-9
Chang IS, Shin PK, Kim BH (2000) Biological treatment of acid mine drainage under sulphate-reducing conditions with solid waste materials as substrate. Water Res 34:1269–1277. doi:10.1016/S0043-1354(99)00268-7
Gibert O, Pablo J, Cortina JL, Ayora C (2004) Chemical characterisation of natural organic substrates for biological mitigation of acid mine drainage. Water Res 38:4186–4196. doi:10.1016/j.watres.2004.06.023
Harper SHT, Lynch JM (1981) The chemical components and decomposition of wheat straw leaves, internodes and nodes. J Sci Food Agric 32:1057–1062. doi:10.1002/jsfa.2740321103
Hulshof AHM, Blowes DW, Ptacek CJ, Gould WD (2003) Microbial and nutrient investigations into the use of in situ layers for treatment of tailings effluent. Environ Sci Technol 37:5027–5033. doi:10.1021/es020822r
Hulshof AHM, Blowes DW, Gould WD (2006) Evaluation of in situ layers for treatment of acid mine drainage: a field comparison. Water Res 40:1816–1826. doi:10.1016/j.watres.2006.03.003
Jin S, Fallgren PH, Morris JM (2008) Biological source treatment of acid mine drainage using microbial and substrate amendments: microcosm studies. Mine Water Environ 27:20–30. doi:10.1007/s10230-007-0026-0
Jurjovec J, Ptacek CJ, Blowes DW (2002) Acid neutralization mechanisms and metal release in mine tailings: a laboratory column experiment. Geochim Cosmochim Ac 66:1511–1523. doi:10.1016/S0016-7037(01)00874-2
Lu J, Jin Q, He YL, Wu J (2008) Biodegradation of nonylphenol polyethoxylates by denitrifying activated sludge. Water Res 42:1075–1082. doi:10.1016/j.watres.2007.09.031
Luptakova A, Kusnierova M (2005) Bioremediation of acid mine drainage contaminated by SRB. Hydrometallurgy 77:97–102. doi:10.1016/j.hydromet.2004.10.019
Muyzer G, Teske A, Wirsen CO, Jannasch HW (1995) Phylognetic relationships of Thiomicrospira species and their identification in deep-sea hydrothermal vent samples by denaturing gradient gel electrophoresis of 16S rDNA fragments. Arch Microbiol 164:165–172. doi:10.1007/BF02529967
Nakagawa T, Sato S, Yamamoto Y, Fukui M (2002a) Successive changes in community structure of an ethylbenzene-degrading sulfate-reducing consortium. Water Res 36:2813–2823. doi:10.1016/S0043-1354(01)00502-4
Nakagawa T, Sato S, Fukui M (2002b) Microbial community enriched on crude oil and aromatic hydrocarbons (toluene, ethylbenzene, o-, p-xylene) from various coastal sediments under sulfate-reducing condition. Unpublished Genbank entry AB081555
Piao Z, Yang L, Zhao L, Yin S (2008) Actinobacterial community structure in soils receiving long-term organic and inorganic amendments. Appl Environ Microbiol 74:526–530. doi:10.1128/AEM.00843-07
Pruden A, Messner N, Pereyra L, Hanson RE, Hiibel SR, Reardon KF (2007) The effect of inoculum on the performance of sulfate-reducing columns treating heavy metal contaminated water. Water Res 41:904–914. doi:10.1016/j.watres.2006.11.025
Rittmann BE, McCarty PL (2001) Environmental biotechnology: principles and applications, 2nd edn. McGraw-Hill Companies, Inc., Boston, pp 604–606
Sun XF, Sun RC, Tomkinson J, Baird MS (2004) Degradation of wheat straw lignin and hemicellulosic polymers by a totally chlorine-free method. Polym Degrad Stabl 83:47–57. doi:10.1016/S0141-3910(03)00205-2
Taguchi H, Wasaki J, Watanabe J, Hamada S, Kobayashi Y (2008) Cloning and sequencing of the gene for cellobiose 2-epimerase from a ruminal strain of Eubacterium cellulosolvens. FEMS Microbiol Lett 287:34–40. doi:10.1111/j.1574-6968.2008.01281.x
Takahashi Y (2006) Isolation of sulfur compounds reducing bacteria. Unpublished Genbank entry AB277866
Tsukamoto TK, Miller GC (1999) Methanol as a carbon source for microbial treatment of acid mine drainage. Water Res 33:1365–1370. doi:10.1016/S0043-1354(98)00342-X
Tsukamoto TK, Killion HA, Miller GC (2004) Column experiments for microbial treatment of acid mine drainage: low-temperature, low-pH and matrix investigations. Water Res 38:1405–1418. doi:10.1016/j.watres.2003.12.012
Van de, Vossenberg, JLCM, Joblin KN (2003) Biohydrogenation of linoleic and linolenic acids by ruminal cellulolytic bacteria from a grazing cow. Unpublished Genbank entry AY178841
Wu J, Wu Y, Lu J (2008) Laboratory study of the clogging process and factors affecting clogging in a tailings dam. Environ Geol 54:1067–1074. doi:10.1007/s00254-007-0873-9
Zagury GJ, Kulnieks VI, Neculita CM (2006) Characterization and reactivity assessment of organic substrates for sulphate-reducing bacteria in acid mine drainage treatment. Chemosphere 64:944–954. doi:10.1016/j.chemosphere.2006.01.001
Acknowledgments
This work is financially supported by the National Key Basic Research Program of China (Grant No. 2007CB815603), National Natural Science Foundation of China (Grant No. 40801217), and Research Program of Hefei University of Technology (Grant No. 2008GDBJ002). The authors would like to thank the anonymous reviewers for their reading of the manuscript, and for their suggestions and critical comments.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wu, J., Lu, J., Chen, T. et al. In situ biotreatment of acidic mine drainage using straw as sole substrate. Environ Earth Sci 60, 421–429 (2010). https://doi.org/10.1007/s12665-009-0186-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-009-0186-2