Abstract
This research has been performed to determine the differences in microbial communities according to physicochemical properties such as concentrations of volatile aromatic hydrocarbons such as benzene, toluene, ethylbenzene, and xylene (BTEX), dissolved oxygen (DO), electron acceptors, etc., in oil-contaminated groundwaters at Kyonggi-Do, South Korea. The properties of bacterial and microbial communities were analyzed by 16S polymerase chain reaction (PCR) denaturing gradient gel electrophoresis (DGGE) fingerprinting method and community-level physiological profiling (CLPP) using Eco-plate, respectively. Based on the DGGE fingerprints, the similarities of bacterial community structures were high with similar DO levels, and low with different DO levels. Whereas the dominant bacterial groups in GW13 (highest BTEX and lowest DO) were acidobacteria, α-proteobacteria, β-proteobacteria, γ-proteobacteria, δ-proteobacteria, and spirochetes, those in GW7 (highest BTEX and highest DO) were actinobacteria, α-proteobacteria, β-proteobacteria, γ-proteobacteria, δ-proteobacteria, and sphingobacteria. Based on the CLPP results, the groundwater samples were roughly divided into three groups: above 4 mg/L in DO (group 1: GW3 and GW7), below 4 mg/L in DO (group 2: GW8, W1, W2, W3, and BH10), and highly contaminated with BTEX (group 3: GW13). Shannon index showed that the microbial diversities and equitabilities were higher in shallower aquifer samples. Overall, this study verified that the greatest influencing factors on microbial/bacterial communities in groundwaters were DO and carbon sources, although BTEX concentration was one of the major factors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In groundwater environments, petroleum hydrocarbons are degraded by anoxic zone development near the contaminant source, with reduced concentrations of electron acceptors and accumulation of dissolved metabolites such as organic acids and carbon dioxide (Skubal et al. 2001). Change in groundwater geochemistry from aerobic to anaerobic is accompanied by changes in terminal electron acceptors of higher energy potential (oxygen or nitrate) to lower energy potential (sulfate or carbon dioxide), which may result in shifts in microbial communities (Song and Katayama 2005). The absence of dissolved oxygen may cause the decrease of aerobic bacterial populations and increase of anaerobic populations (Findlay et al. 1990; Rajendran et al. 1994). In addition, there are many factors influencing shift of microbial community such as kinds or concentrations of various contaminants and physicochemical properties.
The diversity of microbial community has been investigated to date using traditional methods, which have the disadvantage of being too selective to determine the whole picture. The reason is that about 0.1% of total microbial species and about 10% of total microbial number can be cultivated (Amann et al. 1995). Therefore, data from conventional cultural methods cannot represent the reality of microbial community structure. However, the relatively recent advent of microbial community analysis based on the amplification of the 16S rDNA gene, and techniques such as denaturing gradient gel electrophoresis (DGGE), have provided researchers with a direct, cultivation-independent method for examining microorganism populations (Cho et al. 2005; Baldwin et al. 2009).
Community-level physiological profiling (CLPP) using Eco-plates is often described as a method of determining the functional diversity or functional potential of microbial communities (Haack et al. 1995; Konopka et al. 1998; Preston-Mafham et al. 2002). CLPP has been used effectively to establish spatial and temporal changes in microbial communities (Garland 1997) as well as providing insight into functional ability of microbial community members (Preston-Mafham et al. 2002).
To determine what major factors influence microbial communities in oil-contaminated groundwater, this study examined relations between bacterial/microbial community structures and physicochemical properties such as benzene, toluene, ethylbenzene, and xylene (BTEX), dissolved oxygen (DO), and electron acceptors. The characteristics of bacterial communities were analyzed using 16S polymerase chain reaction (PCR)-DGGE fingerprinting method, and the features of microbial communities were estimated through CLPP method by using Eco-plate.
Materials and methods
Study site description and groundwater samples
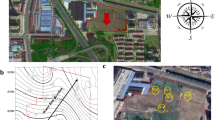
Groundwaters were sampled at site S, Kyonggi-Do, South Korea (Fig. 1) in February, 2004, where contaminated petroleum had been discovered. Based on sampling depth, samples were divided into two groups: the deep group (GW13, GW3, GW8, and GW7), with depth range of 15–30 m, and the shallow group (W2, W3, W1, and BH10), with depth range of 5–15 m. Samples from each group were arranged in order of BTEX concentration, which was the same as the order in distance away from the contamination source except for W1, which seemed to have a different streamline from the other three sites (Table 1). In the deep group, except for sample GW3, DO exhibited an inverse relation with BTEX concentration; in the shallow group, DO values were relatively low and did not exhibit dependence on BTEX concentration (Table 1). The nitrate concentration was also inversely proportional to BTEX concentration, with an exceptionally high value of nitrate in sample GW8 in the deep group, a negligibly low value for the two highest BTEX concentrations (W2 and W3), and a slightly higher value for the two lowest BTEX concentrations (W1 and BH10) (Table 1). As a result of iron reduction, the concentration of Fe2+ seems to be proportional to the BTEX concentration in both groups (Table 1). H2S concentration, which depends on the product of sulfate reduction, was low in all the deep samples, but sulfate and its reduced form were inversely related with BTEX concentration, except for sample W1 in the shallow group (Table 1).
Analysis of bacterial community structure using DGGE
The cells in each groundwater sample were harvested by centrifugation (7,600×g, 20 min) or filtration (0.2 μm pore size). Genomic DNA was extracted from ~0.5 g of each collected cells using the BIO101 FastDNA SPIN KIT for soil (Q-Biogene, USA). PCR was used to amplify a 177-bp portion of the 16S rDNA using bacterial primers 341fGC (5′-CGC CCG CCG CGC GCG GCG GGC GGG GCG GGG GCA CGG GGG GCC TAC GGG AGG CAG CAG-3′) and 518r (5′-ATT ACC GCG GCT GCT GG-3′). All reactions were carried out in 25 μL volumes containing 20 pmol of each primer, 10 mM of each deoxynuleoside triphosphate, 12.5 mg/mL bovine serum albumin, 2.5 μL 10× PCR buffer (20 mM Tris–HCl, 100 mM KCl, pH 8.0), 2 μL 25 mM MgCl2, 0.5 U Taq DNA polymerase (Promega, USA), and 1 μL DNA extract. The amplification conditions were as follows: 93°C for 2 min, followed by 35 cycles of 92°C for 1 min, 55°C for 1 min, and 68°C for 45 s, followed by a final extension at 72°C for 2 min (Kim et al. 2006).
DGGE was performed with a 16 × 16 cm 8% (w/v) polyacrylamide gel using the Dcode™ System (Bio-Rad, USA) maintained in 7 L 0.5× TAE buffer (20 mM Tris, 10 mM acetate, 0.5 mM Na2EDTA, pH 7.8). Gradient gels were prepared with 40% and 60% denaturant [100% denaturant contains 7 M urea and 40% (v/v) formamide]. Gels were run at 60°C with 50 V for 11.5 h, stained with ethidium bromide, and documented with a Mupid-21 gel imager (Cosmo Bio, Japan) (Kim et al. 2006). DGGE images were used for microbial community structure comparisons using GelCompar II software (version 3.5; Applied Maths, Belgium), which applied unweighted pair group method with arithmetic mean (UPGMA) clustering using the Jaccard coefficient based on band position.
DNA sequencing and phylogenic analysis
Bands were excised from the gel and mixed with 20 μl sterile deionized water, and DNA was extracted by freeze–thaw treatment (freezing at −20°C for 10 min and thawing at 65°C for 3 min, repeated three times). One microliter of the resulting DNA extract was used as the template for nested PCR amplification with the 341 forward primer (lacking the GC clamp) and the 518 reverse primer under the PCR conditions described above. The amplified products were purified with the QIAquick PCR purification kit (Qiagen, Germany), and 1 μL PCR product was cloned into the pGEM-T vector (Promega, USA), following the manufacturer’s protocol. Plasmid clones were amplified in E. coli according to standard procedures, extracted from broth cultures using the Wizard® Plus SV Miniprep DNA purification system (Promega, USA), and the DNA insert was excised with EcoRI. The cloned PCR fragments (100 μL) were sequenced using the T7 (5′-TAA TAC GAC TCA CTA CAG GG-3′) and SP6 (5′-ATT TAG GTG ACA CTA AGA AT-3′) primers and an ABI Prism model 373A automated DNA sequencer (Perkin Elmer, USA). The obtained sequences were compared with the GenBank database using the basic local alignment search tool (BLAST) algorithm feature of the National Center for Biotechnology Information (NCBI) website, which identified the subclass level of each sequence.
Analysis of microbial community using Eco-plate
Eco-plates (Biolog Eco-plate™, USA) were used to analyze the functional diversity of microbial communities of individual samples. An Eco-plate consists of 96 wells, which includes three sets of 31 substrates and control. The control wells do not contain any substrate. If microbial respiration occurs once microbes use a substrate in a well as a sole carbon source, tetrazolium dye contained in each well will turn violet. Therefore, substrate utilization of microbial communities can be comparatively evaluated using Eco-plates. Each groundwater sample (150 μL/well) was inoculated into each well in a plate and the plate was cultivated at 20°C. During cultivation, the degree of substrate color changes in each well was measured by optical density (OD) at 595 nm every 24 h using a microtiter plate reader (Multiskan Ascent, Thermo Labsystem, Finland), followed by comparative analysis of substrate utilization patterns according to sample and time. Cultivation was continued for 11 days to achieve steady state in growth curves.
Statistical analysis
The average well color developments (AWCDs) of all samples with time were calculated to compare control and samples using ODs of each well of Eco-plate (control has no substrate). AWCD measures overall color change due to microbial growth, according to the following equation (Garland and Mills 1991):
where C is the OD595 nm value of each well including a substrate, R is the OD595 nm value of control well, and n is the number of substrates (Garland and Mills 1991). Using these values, the potential utilization of various carbon sources by microbial communities can be understood.
To analyze statistically the similarity between carbon-source utilization by the microbial community of each sample, principal component analysis (PCA) (SPSS 12.0K for Windows) was conducted using the value obtained from Eq. 2.
where C id is the value of OD595 nm of each cultured well on day i (or the intensity of each DGGE band), R id is the value of OD595 nm of control on day i (or zero for DGGE), and AWCDid is the value of AWCD on day i (or the average intensity of total possible bands). The similarity in carbon-source utilization or DGGE band pattern can be obtained using PCA values representing each sample’s differences, shown on a graph with two axes (principal component 1, PC1; principal component 2, PC2). This means that samples shown on the same section of a PCA graph are similar in terms of carbon-source utilization or genetically (Smalla et al. 1998).
In addition, Shannon index (representing species diversity in the field of ecology) was calculated using the following Eq. 3 expressed with time and samples:
where H′ is the Shannon index, and P i is the value of OD595 nm in each substrate over the total value of OD595 nm in all the substrates (or intensity of individual DGGE band over the total intensity of all the bands), expressed as P i = C/∑(C − R). Furthermore, the distribution of individuals within species was referred to as equitability and measured using Shannon J′ given by the following equation (Moss and Bassall 2006):
where S is the total number of species in the sample.
Results
Properties of bacterial communities by DGGE fingerprinting
DGGE band patterns were compared, and a similarity tree is shown in Fig. 2. The comparison of DGGE band profiles resulted in the closest relationship between W1 and W3 with 76.0% similarity, which were sampled in the shallow aquifer and were similarly low in BTEX concentration. Also, the similarity between GW3 and W1 was very high (71.4%) because of similar DO levels (4.53 versus 3.33 mg/L), although they were sampled from different depths and had different BTEX concentrations. The most distant relationship occurred between GW7 and W2 (35.5% similarity), which were sampled from the deep and shallow aquifer, respectively (Table 2). Also, the similarity between GW7 and GW13 was very low (37.5%) because of different BTEX concentrations (not detectable versus 13,475 mg/L) and different DO levels (8.76 versus 1.96 mg/L), although they were sampled from similar depths.
In the phylogenetic analysis (Table 3, Fig. 3), sample GW13, which was most highly contaminated with BTEX, included relatively diverse groups of bacteria (six groups). Overall, members of β-proteobacteria showed the highest level (27%), followed by members of α-proteobacteria, γ-proteobacteria, δ-proteobacteria, acidobacteria, and spirochetes (18%, 18%, 18%, 9%, and 9%, respectively). Typically, the W series (W1, W2, and W3) revealed relatively high levels of β-proteobacteria, whereas the GW series (GW3, GW7, GW8, and GW13) revealed relatively high levels of α-proteobacteria, with some exceptions. Strongly dominant bacterial groups were found in samples GW3 and W3: β-proteobacteria (50%) and δ-proteobacteria (63%), respectively. The most interesting feature is that bacteria from the GW sample series (from the deeper aquifer) were from a wider variety of groups (4–7 groups) than those from the W sample series (2–4 groups).
Neighbor-joining 16S rDNA phylogenetic trees showing the genetic distances among individual clones from eight libraries (BH10, GW3, GW7, GW8, GW13, W1, W2, and W3). Bootstraps based on 500 replicates are indicated by the numbers at the nodes. The scale bar indicates 10% nucleotide substitution per 16S rDNA position
Properties of microbial communities by CLPP and DGGE
Figure 4 illustrates changes of optical density with time as a microbial growth indicator. Each sample showed different pattern according to individual substrate. AWCD values, an expression of heterotrophic bacterial activity, were highest in W1 and W3, followed by W2, BH10, GW3, GW7, GW8, and GW13 (W series and BH10: shallow aquifer samples; GW series: deep aquifer samples) (Fig. 4). This might be the reason why the concentrations of HCO −3 were high in the W series (Table 1). All the shallow aquifer samples were higher than those of the deep aquifer, and the most highly contaminated GW13 was the lowest.
Figure 5a shows differentiation of DGGE band patterns according to band location and intensity. BH10, GW8, and GW13 were grouped together, whereas the others were scattered in distribution, meaning that these three samples were similar in terms of bacterial community structure while the others were different from one another. On the other hand, PCA representing differentiation by utilization properties of individual substrate showed three groups: GW3 and GW7; GW8, W1, W2, W3, and BH10; and GW13 (Fig. 5b). Unexpectedly, GW8 was not close to the GW series, but close to the W series. GW13 was located separately, maybe due to heavy contamination. However, we found the common differentiating property to be DO level: above 4 mg/L for group 1, below 4 mg/L for group 2, and lowest with highest BTEX concentration for group 3. These two PCA results were different from each other, indicating different patterns for bacterial community structure and substrate utilization.
Table 4 presents Shannon diversity (H′) and equitability (J′) results using DGGE band patterns and Eco-plates. H′ based on DGGE data was 2.37–2.73, showing a very narrow range, which indicates similar diversity in all samples in terms of bacterial community structure. In contrast, Shannon indices based on Eco-plates data indicated that W1, W2, W3, and BH10 were similar and higher than GW series (GW3, GW7, GW8, and GW13), which were themselves also similar (Table 3). Such similar diversities indicate that diversity was more influenced by depth of aquifer than by contamination. The value of zero for H′ and J′ for GW13 was due to AWCDs lower than threshold (0.25). Equitability values were in the range 0.82–0.94 and 0.82–0.98 for DGGE and Eco-plates data, respectively. The J′ values for W2 and W3 were typically relatively higher than the others in DGGE and Eco-plate analyses.
Discussion
Based on BTEX concentrations, it was possible to guess that the flow direction of contaminants might be from GW13 to GW3, to GW8, then to GW7 for the deep aquifer and from W2 to W3 then to W1 or BH10 for the shallow aquifer. Among GW series samples, GW13, the most highly contaminated groundwater, showed the lowest levels of dissolved oxygen and nitrate, and the highest levels of ferrous iron (Fe2+), a reduced form of Fe(III) oxyhydroxide (FeOOH), and of hydrogen sulfide (H2S), a reduced form of sulfate ions (SO 2−4 ). Based on comparison of concentration ratio between electron acceptors and their reduced forms (Table 1), it seems that the aerobic, hypoxic, and Fe(III)-reducing conditions had already passed and sulfate reduction was ongoing in GW13. Although the concentration of BTEX in GW8 was lower than in GW3, the level of DO was lower in GW8 than in GW3, indicating that carbon sources other than petroleum hydrocarbons might have flowed in and that aerobic degradation had quickly consumed dissolved oxygen. The highest concentration of nitrate in GW8 might indicate that some nitrogen sources came in and aerobic degradation was a major process before denitrification. Fe(III) reduction could not occur due to very low concentrations of total iron in GW8 and GW7, resulting in zero level of ferrous irons. Based on comparison between sulfate and hydrogen sulfide, one can infer that sulfate reduction did not occur rapidly in GW8. The most highly contaminated groundwater (W2) in the shallow aquifer was in the process of Fe(III) and sulfate reduction because of low levels of DO and nitrate and relatively high ratios of ferrous iron and hydrogen sulfide reduced from their former chemical forms. W1, W2, and BH10 were not yet in the process of Fe(III) and sulfate reduction due to low ratios of Fe(II)/Fe(III) and/or of H2S/SO4 (with the exception of W1).
The levels of DO of the most highly contaminated sites (GW13 and W2) were less than 2 mg/L, considered as anaerobic conditions. Unexpectedly, BH10 also showed an anaerobic condition, which might be due to unknown carbon sources which came into the groundwater from the surface rice field. Therefore, GW13 seemed to be most closely related with BH10 instead of with the rest of the GW series based on comparison of DGGE profiles, and W2 appeared to be shifted from the rest of the W series, as expected based on significant changes from aerobic to anaerobic bacterial community structure in the anaerobic source zone (Table 2; Fig. 2), as also found in previous studies (Barcelona et al. 1995; Cozzarelli et al. 1990; Findlay et al. 1990; Preston-Mafham et al. 2002; Rajendran et al. 1994). As mentioned in the results, DO level most strongly influenced bacterial community structure, with strong relationships between samples with similar DO levels and weak relationships between those with different DO levels. However, there were some exceptions, e.g., low similarities between W2 and BH10 and between GW13 and W2, even though they had similar DO levels. It is considered that, for W2 and BH10, major reasons for this are different carbon sources, as mentioned above, and different concentrations of main electron acceptors, causing different bacterial growth; for GW13 and W2, different temperatures, different concentrations of ions and trace elements, and different aquifer environments such as depth, mineral texture, etc. may be major reasons (Table 1).
Whereas the W series samples (W1, W2, and W3) had high percentages of β-proteobacteria (31–37%), the GW series samples (GW3, GW7, GW8, and GW13) showed relatively high percentages of α-proteobacteria (18–34%). This appeared to be due to agricultural nutrients that were supplied to the shallow (W series) aquifer but not to the deep (GW series) aquifer. Such results are supported by those reported by MacNaughton et al. (1999), who found a member of α-proteobacteria in a natural attenuation plot. On the other hand, the most contaminated groundwater (GW13) among all the samples exhibited a relatively wide variety of phylogenetic groups, more than in the W series samples (Table 3). This leads one to infer that more diverse microbial communities occur in contaminated than in uncontaminated zones (Fang and Barcelona 1998). In addition, γ-proteobacteria could be found in uncontaminated and/or contaminated groundwaters from a subsurface soil contaminated with hydrocarbons (Song and Katayama 2005) and also in oil-contaminated seawater (Harayama et al. 2004). So γ-proteobacteria might be ubiquitous in any aquatic environment as well as soil environments, and effective on hydrocarbon degradation in various environments. Cavalca et al. (2004) reported that the BTEX-degrading strains belonged to α-, β-, and γ-proteobacteria, and high-G+C Gram-positive bacteria, which (which the exception of the latter) were also found in the most highly contaminated groundwater (GW13) in this study. Moreover, members of acidobacteria, fermicutes, and δ- and ε-proteobacteria were also found in that groundwater, and might be increased under anaerobic conditions, implying that they might be nitrate, iron, and/or sulfate reducers (Da Silva and Alvarez 2002; Hendrickx et al. 2005; Kim et al. 2006), although not sulfate reducers based on chemical analysis (Table 1). Holmes et al. (2007) suggested that Geobacteraceae (δ-proteobacteria) predominate in Fe(III)-reducing oil-contaminated groundwater, but this study found only two clones closely related to that family and not in the highly contaminated site (GW13), which might be due to the low concentration of iron ions (Table 1).
In AWCD analysis, the reason that W1 and W3 were higher than W2 and BH10 is that they were maintaining more aerobic conditions than the other two; i.e., more aerobic hetrotrophs perhaps showed more microbial activity with aerobic Eco-plates. GW3 appeared to exhibit the same effect: it showed the highest AWCD in the deep aquifer. However, GW8 (having the highest DO) had lower AWCD than did GW3, meaning that there might be a low concentration of carbon sources including hydrocarbons due to low HCO −3 , making microbes inactive and thus maintaining high oxygen concentration of 8.76 mg/L (Table 1). GW13 showed the lowest AWCD but the highest BTEX (carbon source). It might inhibit by the toxicity of high BTEX concentration and/or low DO concentration. Overall, samples in the shallow aquifer seemed to have higher AWCD than did samples in the deep aquifer, since some nutrients (including carbon sources) entered the shallow aquifer. PCA analysis of utilization properties of individual substrate showed slightly different patterns than did AWCD analysis because GW8 was grouped together with the shallow samples and GW13 was separated from the other samples. The characteristic of group 2 (GW8, W1, W2, W3, and BH10) was to have intermediate levels of DO (2–4.5 mg/L), as shown by common bacterial groups such as proteobacterias (Table 3). GW13, in group 3, included many unique members, different from those in other samples, presumably due to the highest BTEX concentration and lowest DO level.
Based on Shannon index, representing the microbial diversity in substrate utilization, there were two groups by depth of aquifers as well as equitability, showing that the shallower aquifer samples had higher values (H′ and J′) than the deeper ones (Table 4). This suggests that the shallower aquifer included more bacteria adjusted to a variety of carbon sources than did the deeper ones. However, the Shannon diversities and equitabilities based on DGGE revealed different patterns from the Eco-plate analyses and a much narrower range of values, especially for diversity. This result indicates that there were some differences between physical and genetic diversity, and Shannon diversity using DGGE data might not be a good way to differentiate diversities due to the narrow range of values (H′), although it was possible to obtain Shannon indices (Table 4). These differences were also reflected in PCA results (Fig. 5).
Conclusions
In this study, the relationship between microbial structure and water quality in petroleum-contaminated groundwater was investigated using genetic and metabolic profiles by DGGE and Eco-plates, respectively. DGGE fingerprints showed that DO (among various physicochemical properties of groundwater) was the most important factor to characterize eubacterial community structure. On the basis of community-level physiological activities using Eco-plates, the groundwater samples were grouped by BTEX concentration as well as DO. Therefore, this study demonstrated that important factors influencing the structure of microbial community in oil-contaminated groundwater were BTEX concentration and DO.
References
Amann RI, Ludwig W, Schleifer KH (1995) Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev 59:143–169
Baldwin BR, Nakatsu CH, Nebe J, Wickham GS, Parks C, Nies L (2009) Enumeration of aromatic oxygenase genes to evaluate biodegradation during multi-phase extraction at a gasoline-contaminated site. J Hazard Mater 163:524–530
Barcelona MJ, Lu J, Tomczak DM (1995) Organic acid derivatization techniques applied to petroleum hydrocarbon transformations in subsurface environments. Ground Water Monit Rem 15:114–124
Cavalca L, Della Amico E, Andreoni V (2004) Intrinsic bioremediability of an aromatic hydrocarbon-polluted groundwater: diversity of bacterial population and toluene monoxygenase genes. Appl Microbiol Biotechnol 64:576–587
Cho W, Lee EH, Shim EH, Kim J, Ryu HW, Cho KS (2005) Bacterial communities of biofilms sampled from seepage groundwater contaminated with petroleum oil. J Microbiol Biotechnol 15:952–964
Cozzarelli IM, Eganhouse RP, Baedecker MJ (1990) Transformation of monoaromatic hydrocarbons to organic acids in anoxic groundwater environment. Environ Geol Water Sci 16:135–141
Da Silva MLB, Alvarez PJJ (2002) Effects of ethanol versus MTBE on benzene, toluene, ethylbenzene, and xylene natural attenuation in aquifer columns. J Environ Eng 128:862–867
Fang J, Barcelona MJ (1998) Biogeochemical evidence for microbial community change in a jet fuel hydrocarbons-contaminated aquifer. Org Geochem 29:899–907
Findlay RH, Trexler MB, Guckert JB, White DC (1990) Response of a benthic microbial community to biotic disturbance. Mar Ecol Prog Ser 62:135–148
Garland JL (1997) Analysis and interpretation of community-level physiological profiles in microbial ecology. FEMS Microbiol Ecol 24:289–300
Garland JL, Mills AL (1991) Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level, sole-carbon-source utilization. Appl Environ Microbiol 57:2351–2359
Haack SK, Garchow H, Klug MJ, Forney LJ (1995) Analysis of factors affecting the accuracy, reproducibility and interpretation of microbial community carbon source utilization patterns. Appl Environ Microbiol 61:1458–1468
Harayama S, Hara A, Baik S, Shutsubo K, Misawa N, Smits THM, van Beilen JB (2004) Cloning and functional analysis of AlkB genes in Alcanivorax borkumensis SK2. Environ Microbiol 6:191–197
Hendrickx B, Dejonghe W, Boenne W, Brennerova M, Cernik M, Lederer T, Bucheli-Witschel M, Bastiaens L, Verstraete W, Top EM, Diels L, Springael D (2005) Dynamics of an oligotrophic bacterial aquifer community during contact with a groundwater plume contaminated with benzene, toluene, ethylbenzene, and xylenes: an in situ mesocosm study. Appl Environ Microbiol 71:3815–3825
Holmes DE, O’Neil RA, Vrionis HA, N’Guessan LA, Ortiz-Bernad I, Larrahondo MJ, Adams LA, Ward JA, Nicoll JS, Nevin KP, Chavan MA, Johnson JP, Ling PE, Loveley DR (2007) Subsurface clade of Geobacteraceae that predominates in a diversity of Fe(III)-reducing subsurface environments. ISME J 1:663–677
Kim J, Kang SH, Min KA, Cho KS, Lee IS (2006) Rhizosphere microbial activity during phytoremediation of diesel-contaminated soil. J Environ Sci Health A Tox Hazard Subst Environ Eng 41:2503–2516
Konopka A, Oliver L, Turco RF (1998) The use of carbon substrate utilization patterns in environmental and ecological microbiology. Microb Ecol 35:103–115
MacNaughton SJ, Stephen JR, Venosa AD, Davis GA, Chang YJ, White DC (1999) Microbial population changes during bioremediation of an experimental oil spill. Appl Environ Microbiol 65:3566–3574
Moss A, Bassall M (2006) Effects of disturbance on the biodiversity and abundance of isopods in temperature grasslands. Eur J Soil Biol 42:S254–S268
Preston-Mafham J, Boddy L, Randerson PF (2002) Analysis of microbial community functional diversity using sole-carbon-source utilization profiles—a critique. FEMS Microbiol Ecol 42:1–14
Rajendran N, Matsuda O, Urushigawa Y, Simidu U (1994) Characterization of microbial community structure in the surface sediment of Osaka bay, Japan, by phospholipid fatty acid analysis. Appl Environ Microbiol 60:248–257
Skubal KL, Barcelona MJ, Adriaens P (2001) An assessment of natural biotransformation of petroleum hydrocarbons and chlorinated solvents at an aquifer plume transect. J Contam Hydrol 49:151–169
Smalla K, Wachtendorf U, Heuer H, Liu WT, Forney L (1998) Analysis of BIOLOG GN substrate utilization patterns by microbial communities. Appl Environ Microbiol 64:1220–1225
Song D, Katayama A (2005) Monitoring microbial community in a subsurface oil contaminated with hydrocarbons by quinone profile. Chemosphere 59:305–314
Acknowledgments
This research was supported by a grant by Sustainable Water Resources Research Center (1-0-3). Ms. Kim, J. Y. and Koo, S. Y. were financially supported by the Korea Science and Engineering Foundation through the Advanced Environmental Biotechnology Research Center at Pohang University of Science and Technology (R11-2003-006-06001-0). Ms. Lee, E. H. was also financially supported by the KOSEF NRL Program grant funded by the Korea government (MEST) (R0A-2008-000-20044-0).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, EH., Kim, J., Kim, JY. et al. Comparison of microbial communities in petroleum-contaminated groundwater using genetic and metabolic profiles at Kyonggi-Do, South Korea. Environ Earth Sci 60, 371–382 (2010). https://doi.org/10.1007/s12665-009-0181-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-009-0181-7