Abstract
Background
Vitamin D plays a key role in gut immunity and maintenance of the mucosal barrier. Vitamin D deficiency (VDD) worsens ulcerative colitis (UC) and its supplementation ameliorates the disease in mouse models. The prevalence and predictors of VDD in UC are not known.
Methods
Consecutive patients with UC (n = 80) underwent clinical, endoscopic, and histological evaluation to assess the extent, severity using UC disease activity index (UCDAI) score, and duration of illness. An equal number of age and gender-matched healthy adults without any features of inflammatory bowel disease (IBD) living in the same latitude were identified as controls. The serum 25-hydroxy vitamin D3 level was estimated. The subjects were classified as deficient (< 20 ng/mL), insufficient (20–32 ng/mL), sufficient (32–80 ng/mL), and optimal (> 80 ng/mL) based on vitamin D levels. Chi-square test and Mann-Whitney U test were done to identify factors associated with vitamin D deficiency.
Results
The patients and controls were similar in age and gender (40 ± 11.4 years, 51% male vs. 40 ± 12 years, 51% male; p = 1.000). Median vitamin D levels among patients were lower than the controls (18.1 ng/mL [IQR 14] vs. 32.5 ng/mL [IQR 36]; p < 0.001). Patients were more often VDD (56% vs. 40%) or insufficient (34% vs. 9%) and less often sufficient (9% vs. 40%) or optimal (1% vs. 11%), in contrast to controls (p < 0.001). Median vitamin D levels were lower in those with UCDAI > 6 (15 vs. 21 ng/mL; p = 0.01), having pancolitis (13 vs. 21 ng/mL, p = 0.01), and longer duration of illness > 2 years (13.8 vs. 20.8; p = 0.025). Vitamin D levels showed a negative correlation with frequency of stools (rho = − 0.244, p = 0.05), disease duration (rho = − 0.244, p = 0.007) and UCDAI score (r = − 0.348, p = 0.002).
Conclusion
VDD is highly prevalent among patients with UC. Patients with longer disease duration, more severe symptoms, and pancolitis are likely to have lower vitamin D levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction

Vitamin D plays a primary role in calcium, phosphorus, and bone metabolism [1]. However, discovery of vitamin D receptors on lymphocytes, monocytes, and dendritic cells initiated studies, which have highlighted the role of vitamin D in regulating gut mucosal immunity and its barrier function [2,3,4,5]. Ulcerative colitis (UC) is characterized by inappropriate and exaggerated mucosal immune response to gastrointestinal (GI) antigens in genetically susceptible individuals [6]. In experimental IL-10 knockout mice models, vitamin D deficiency was found to result in severe colitis, progressive wasting, and high mortality. However, vitamin D supplementation not only prevented but also ameliorated symptoms of colitis in the mice model [7,8,9].
The key sources for vitamin D are adequate dietary intake and sunlight exposure. Patients with UC have diminished oral intake of vitamin D–rich food due to dietary restrictions, diminished outdoor activities resulting in lower sunlight exposure, and high GI losses of vitamin D and vitamin D binding protein due to chronic illness involving GI mucosa [10,11,12]. Vitamin D deficiency, if present, is likely to further perpetuate the illness as established in experimental animal models [7,8,9]. Objective assessment of vitamin D levels in patients with UC is likely to be beneficial to help physician identify and correct deficiency state. Vitamin D may have a therapeutic role in management of disease and preventing osteoporosis [9, 13]. We thus planned a prospective case control study to assess the serum vitamin D levels in consecutive patients with UC and determine its relationship to disease severity, extent, and duration of illness.
Methods
Consecutive adult patients with UC attending the gastroenterology services of a tertiary care referral institution from July 2008 to July 2010 were prospectively studied. The study was conducted in full compliance with the guidelines of good clinical practice of the world medical assembly declaration of Helsinki and the Indian Council of Medical Research guidelines. The study was approved by the institute’s ethics committee. All the patients provided written informed consent before participating in the study. Cases were defined as patients with UC, the diagnosis of which was based on standard clinical, endoscopic, and histological criteria [14]. Patients older than 70 years, those with history of prior colectomy, evidence of malignancy, or toxic megacolon, presence of serious systemic comorbidities, and those who had received mega-doses of parenteral or oral vitamin D supplementation in the preceding 6 months were excluded from the study. Each study subjects underwent a detailed clinical evaluation and endoscopic assessment. Colonoscopy was done to assess the extent of the disease and endoscopic severity. The disease was classified as proctitis, proctosigmoiditis, left-sided colitis, and pancolitis depending on the extent of involvement. Endoscopic severity was classified according to mucosal appearance as grade 0–3 [15]. Biopsies were obtained from the involved mucosa and the rectum in all the patients. For each study subject, the ulcerative colitis disease activity index (UCDAI) was calculated based on the stool frequency, rectal bleeding, mucosal appearance, and physician’s global assessment [15]. The severity of UC was calculated on the basis of UCDAI score and classified as 0–2 as remission, 3–6 as mild disease, 7–10 as moderate disease, and 11–12 as severe disease [14, 15].
All the patients were evaluated for their risk of vitamin D deficiency in the form of adequacy of sun-exposure, rural/urban residence, socioeconomic status, body mass index (BMI), and quantity of milk/curd intake. Patients were also evaluated for clinical features of vitamin D deficiency in the form of proximal muscle weakness (assessed on asking the patient to stand up from a squatting position that was graded from 1 to 5), fatigue, divarication of recti (tested during leg raising), history of bone pain, and fractures. Routine hemogram and biochemistry was performed in all the patients. Serum samples were obtained at the time of enrolment in untreated vacutainers and refrigerated at − 20 °C until processing. 25-hydroxy vitamin D3 levels were estimated in the serum using enzyme linked immunosorbent assay (ELISA) using commercial kit (Immunodiagnostic Systems Ltd. UK). The ELISA was read at 450 nm against 650 nm as reference as recommended by the manufacturer. A dose-response curve of the absorbance unit vs. concentration was generated using values obtained from the standard provided by the manufacturer. 25-OH vitamin D3 level was determined from this curve. The inter-assay and intra-assay coefficients of variation were 4.6% and 5.3%, respectively. The test was known to have a < 0.01% cross reactivity with vitamin D3 and < 0.3% with vitamin D2. Values were expressed as ng/mL. Subjects were classified on the basis of vitamin D levels as deficient (<20 ng/mL), insufficient (20–32 ng/mL), sufficient (32–80 ng/mL), and optimal (>80 ng/mL) [10, 16, 17]. The healthy controls (n = 80) were recruited from the Department of Endocrinology in the same time period, and were similar in age, gender, socioeconomic class, and resided in the same latitude. The control group underwent estimation of 25-hydroxy vitamin D3 levels using a chemiluminescent radioimmunoassay (Biosource, Europe, SA). The inter-assay and intra-assay coefficients of variation was 5.2% and 3.3%, respectively.
Sample size calculation and statistical analysis
To detect a difference of 10 ng/mL between cases and controls with a power of 80% and alpha error of 0.05 given the SD of 20 ng/mL, we required 64 cases and 64 controls to be able to reject the null hypothesis. To account for dropouts and other technical issues, we recruited 80 cases and 80 controls. The data were analyzed using Statistical Package for the Social Sciences (SPSS) Version 17 (SPSS, Inc., Chicago, IL, USA). Vitamin D levels among the cases and controls were compared using the Mann-Whitney U test. The data were expressed as mean ± SD or median and interquartile range (IQR) if it had a non-parametric distribution. Continuous data were compared using unpaired t test or Mann-Whitney U test as was appropriate. ANOVA was used to compare across three groups. A p-value < 0.05 was considered significant. Vitamin D levels were compared to the disease severity, extent, and its duration. The independent risk factors for vitamin D deficiency were assessed using logistic regression analysis (conditional backward regression).
Ethical considerations
The study was conducted in full compliance with the guidelines of good clinical practice of the world medical assembly declaration of Helsinki and the Indian Council of Medical Research guidelines. The study was approved by the Institute’s Ethics Committee. All the patients provided written informed consent before participating in the study.
Results
The mean age of 80 patients was 40 ± (11.4) years and males constituted 51% of the study population. The mean age of the control group was 40 ± 11.1 years, 51% were males (p = 1.000). The median duration of illness was 36 months (IQR 13 to 69 months). As per the UCDAI score, 22/80 (27.5%) patients had severe disease, 29/80 (36%) had moderate disease, 22/80 (27.5%) had mild disease, and 7/80 (9%) were in remission. The disease extent was as follows: pancolitis in 32%, left sided colitis in 28%, proctosigmoiditis in 37%, and proctitis in 3%. The disease course was remitting and relapsing in 63 (79%) patients, chronic continuous in 11 (14%) and single episode in 6 (7%) patients. All the patients were receiving mesalamine in the dose of 2.4 to 3.6 g per day. Of the 80 patients, 45 (56%) had history of prior steroid intake and 19% were on azathioprine (50–100 mg/day) at the time of enrollment. Extraintestinal manifestations were present in 21 (26%) patients that included arthritis in 13, uveitis in 5, and hypercoagulability in 5 patients. The mean BMI of patients was 21.3 ± 4.1 kg/m2 and there were 8 smokers (all men).
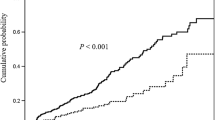
The median value of 25-hydroxy vitamin D3 was 18.1 (IQR 9.4−24 ng/mL). The control group showed a median 25-hydroxy vitamin D3 level of 32.5 ng/mL (IQR 13.3−49.8 ng/mL; p < 0.001) (Fig. 1). Cases were more often vitamin D deficient (56% vs. 40%) or insufficient (34% vs. 9%) and less often sufficient (9% vs. 40%) or optimal (1% vs. 11%) in contrast to the controls (p < 0.001).
The median serum vitamin D levels were significantly lower in patients with moderate or severe colitis (UCDAI score > 6) compared to those with mild disease or that in remission (UCDAI score ≤ 6) (14.7 [IQR 8.5–21] vs. 21.3 [IQR 14–29.8]; p = 0.01) (Fig. 2b). The median levels of vitamin D in patients with severe, moderate, mild disease, and those in remission were 13.9 (IQR of 9.4–20.8), 17.6 (8.3–23), 21.3 (13.7–27.3), and 18.5 (13–60), respectively; and the differences between them were significant (p = 0.013; Jonckheere–Terpstra test). The vitamin D levels also showed a negative correlation with the UCDAI score (r = − 0.348; p = 0.002).
a This box plot shows the comparison between vitamin D levels in patients with longer disease duration compared to those with shorter disease duration. Patients with longer disease duration had lower median value of vitamin D compared to those with shorter duration (13.8 ng/mL [IQR 8–22] vs. 20.8 ng/mL [IQR 13–24]; p < 0.025). b This box plot shows the comparison between vitamin D levels in patients with mild ulcerative colitis (UC) compared to those with moderate or severe disease. Patients with mild UC had higher median value of vitamin D compared to those with moderate or severe disease (21.3 ng/mL [IQR 14–29] vs. 14.7 ng/mL [IQR 9–21]; p < 0.01). c This box plot shows the comparison between vitamin D levels in patients with limited disease compared to those with pancolitis. Patients with limited disease had higher median value of vitamin D compared to those with pancolitis (13 ng/mL [IQR 8–20] vs. 21 ng/mL [IQR 14–28]; p < 0.01)
Patients with a higher frequency of stools (> 6/day) had lower levels of vitamin D compared to those with less frequent stools (< 6/day) (16 [IQR 9.6–21] vs. 21 [IQR 9.6–30.4]; p = 0.04). The vitamin D levels showed a significant negative correlation with frequency of stools (rho = − 0.244; p = 0.05). The median number of stools at presentation was higher in those with vitamin D levels ≤ 20 than those with vitamin D levels > 20 ng/mL (8 [4.75–12] vs. 5 [4–7.75]; p = 0.029).
Patients with longer disease duration (≥ 2 years) had lower median levels of vitamin D than those with shorter duration of illness (< 2 years) (20.8 [13.3–24] vs. 13.8 [8.4–21.6]; 0.025) (Fig. 2a). The disease duration showed a significant negative correlation with vitamin D levels (rho = − 0.297; p = 0.007). The median duration of illness in patients with serum vitamin D ≤ 20 ng/mL was longer than in those with vitamin D > 20 ng/mL (48 months [16.5–102] vs. 24 months [11.5–36]; p = 0.007).
Patients with pancolitis had lower median levels of vitamin D than those without pancolitis (13 [8.4–20.4] vs. 21 [13.6–28]; p = 0.01) (Fig. 2c). Patients with extraintestinal manifestations had higher median vitamin D levels than those without these manifestations (21 [18–23.4] vs. 13.8 [9–22.5]; p = 0.04) (Table 1).
The risk factors associated with vitamin D deficiency were disease duration > 2 years, presence of pancolitis, and frequency of stools (cut-off of ≥ 6 stools/day; Table 2). Patients with vitamin D deficiency more often tended to have severe disease compared to those without deficiency, though not statistically significant. Serum vitamin D levels showed no relationship with age and gender. Though the median vitamin D levels among patients belonging to the upper socioeconomic strata tended to be higher than that of those belonging to lower socioeconomic strata, the difference was not statistically significant (19.3 ng/mL [13.6–25.4] vs. 14.1 ng/mL [8.25–21.9]; p = 0.096). Patients living in rural areas tended to have higher median vitamin D levels than those in urban areas (21 ng/mL [9.75–27.1] vs. 17.6 ng/ml [9.15–21]; p = 0.162). The mean BMI of patients with vitamin D ≤ 20 ng/mL was comparable to those with vitamin D > 20 ng/mL (20.7 ± 4 vs. 21.6 ± 4; p = 0.326). Risk factors for vitamin D deficiency were highly prevalent in the form of inadequate diary product intake (72.5%), inadequate sun exposure (94%), and indoor profile of daily activities (74%). The risk factors were among patients with UC only. The clinical features of vitamin D deficiency were highly prevalent among the patients with UC in the form of fatigue in 79%, proximal muscle weakness in 71%, divarication of recti in 35%, bone pain in 31%, and fractures in 5%.
On logistic regression analysis (conditional backward regression analysis), the independent predictors for vitamin D deficiency were found to be present of pancolitis (OR = 3.9, CI 1.1, 14.2; p = 0.035) and increased stool frequency (> six times) (OR. 4.37; CI 1.35, 14.1; p = 0.014). The other parameters entered into the model were age, gender, disease duration of > 2 years, adequate/inadequate sun exposure, milk intake > 200 mL, and rural/urban residence.
Discussion
This present study highlights a high prevalence of vitamin D deficiency among patients with UC living in a sun-rich environment. Optimal vitamin D levels were found in only 10% of patients with UC. Vitamin D levels were lower in those with longer disease duration, greater disease severity, and in those with pancolitis. Moreover, there has been no earlier study from sunshine-rich tropical countries like India on vitamin D levels in UC. In addition, the comparison between the vitamin D levels of UC patients with age- and sex-matched healthy controls shows that the difference between the levels in both the groups is due to disease-related factors rather than environmental causes as the control group had similar environmental and sociocultural background.
Though a number of cut-off values of serum vitamin D levels have been suggested in the literature, a level < 20 ng/mL has been generally considered as deficient due to its association with increased risk of bone fracture [16,17,18]. Vitamin D levels of < 32 ng/mL is considered to be insufficient as below this level the various parameters of calcium metabolism are abnormal [17, 18]. As patients with UC, may have high degree of vitamin D loss and vitamin D deficiency may in itself accentuate the disease process, levels of > 32 ng/mL may be more optimal target. By these criteria, 90% of our patients of UC had suboptimal vitamin D levels.
In a study from Canada, a country with limited availability of sunlight, Leslie et al. showed that 43.5% of their patients with IBD (45 with UC) had vitamin D levels < 20 ng/mL [19]. Pappa et al. showed in children with UC (n = 36), 25% had vitamin D levels < 15 ng/mL [10]. We found a higher prevalence of deficiency than these two studies. This is possibly due to higher prevalence of vitamin D deficiency in the Indian subcontinent [20,21,22,23,24]. Vitamin D deficiency is highly prevalent in Indian subcontinent despite abundant sunshine. This paradox in India is explained by insufficient outdoor activities, darker skin color, modest clothing precluding adequate sun exposure, environmental pollution, and inadequate dietary calcium intake resulting in secondary vitamin D deficiency [20,21,22,23,24,25].
In a small study on 34 patients with Crohn’s disease from southern India, Joseph et al. found that 79% patients were deficient (< 20 ng/mL) in serum 25(OH) vitamin D, 12% were insufficient (20–32 ng/mL), and only 9% were normal (> 32 ng/mL) [20]. In our study, the prevalence of vitamin D deficiency in UC was lower than that found in patients with Crohn’s disease. Crohn’s disease is more often associated with small bowel involvement, which may result in malabsorption of vitamin D [10].
The mechanism of vitamin D deficiency in UC is multifactorial [3, 10]. UC is associated with insufficient dietary intake of vitamin D–rich food, inadequate sun exposure due to limited outdoor activities, and excessive GI losses due to loss of vitamin D binding protein as a part of protein losing enteropathy [3, 10,11,12]. Patients with UC often consume inadequate milk and its products due to fear of GI discomfort from lactose malabsorption, which results in diets inadequate in calcium, protein, and vitamin D especially in patients who are vegetarians. In our study, it was found that there was a high prevalence of clinical features of vitamin D deficiency in the form of fatigue, proximal muscle weakness, and bone pains, which though non-specific, would influence the physical activity. Poor physical activity would, in turn, result in diminished outdoor activities and thus reduce the duration of sunlight exposure [11]. These factors may act in combination or alone to result in vitamin D deficiency. In our study, we found longer disease duration was associated with lower vitamin D levels. This is possibly due to the cumulative effect of inadequate intake of vitamin D and calcium, chronic GI losses, and inadequate sunlight exposure. A study from Japan showed that vitamin D levels significantly correlated with disease duration (r = 0.46; p = 0.003) [3]. In our study, we also found that disease severity and extent were associated with lower vitamin D levels. An actively inflamed colon, particularly over a larger surface area is more likely to be associated with protein losing enteropathy resulting in loss of vitamin D binding protein [26, 27]. Inadequate vitamin D binding protein is likely to further perpetuate vitamin D deficiency. Though we did not find any relationship between vitamin D levels and steroid intake, our study was not designed primarily to assess this relationships. In a study by Leslie et al. also, no difference in serum 25(OH) vitamin D was found between subjects with prior corticosteroid exposure compared to those without [19]. We did not find any difference between both the genders. We found that patients of upper socioeconomic status had marginally higher levels of vitamin D, which might be related to greater access to vitamin D–rich diet. Patients in rural area had marginally higher vitamin D levels, which might be related to their clothing pattern, as well as greater exposure to sunlight [28]. Some recent studies have eluded to the role of vitamin D deficiency in the clinical manifestations and impact on outcomes in UC. A population-based study from a European cohort suggested that the presence of vitamin D deficiency did not predispose to the occurrence of IBD [29]. However, the presence of vitamin D deficiency in patients with UC is likely to increase the risk of relapse in these patients [30]. Furthermore, supplementation of vitamin D in patients with UC resulted in reduction in inflammatory markers like the erythrocyte sedimentation rate and C-reactive protein levels suggesting that vitamin D levels bear a relationship with the disease activity [30,31,32,33,34,35].
Vitamin D deficiency in patients with UC is likely to exacerbate the underlying colitis through one or more of the following mechanisms. Vitamin D plays an important role in development of self-tolerance by regulating T-helper cells and dendritic cell function while inducing regulatory T cell function [4]. The net effect of vitamin D is reduction in TH1 driven autoimmune responses and reduction in mediators such as TNFα, IL-2, and INF-γ (interferon-gamma) [2, 4]. The mucosa in patients with UC is primarily dominated by TH2 cells rather than TH1 cells [4]. Vitamin D also has a role in promoting anti-bacterial properties of lymphocytes, macrophages, and Paneth cells, thus reducing the infection-associated disease activity [2,3,4,5].
The current study, however, has a few limitations. It is well known that vitamin D levels have seasonal variations [28]. We did not particularly study this aspect. The second limitation of the study was that the control population underwent 25-hydroxy vitamin D3 estimation using a chemiluminescence immunoassay whereas ELISA was employed in the cases due to certain logistic reasons. However, the estimation characteristics of the assays were similar. Moreover, the wide difference noticed between the cases and the controls cannot be accounted by minor difference in the assay characteristics.
The recommended vitamin D dose for healthy adults is 1000 IU a day [10]. Patients with UC possibly need higher doses of oral vitamin D than healthy individuals in view of poor absorption and higher GI losses. Oral daily vitamin D in a dose of 2000 IU has been found to be safe and well-tolerated [10, 36]. In a recent study, Jorgensen et al. showed that vitamin D supplementation in a dose 1200 IU daily for 3 months increased the mean serum levels of vitamin D from 29.6 to 38.4 ng/mL that lowered the relapse rate of UC from 29% to 13% (p = 0.06). It was found to be safe and well-tolerated [37].
We recommend that patients with UC should be assessed for vitamin D levels and appropriately supplemented as part of long-term management [38, 39]. On the basis of this study, which shows highly prevalent vitamin D deficiency in patients with UC time is ripe for an interventional trial to study the effect of supplementation of vitamin D on disease activity and disease course in these patients.
In conclusions, vitamin D deficiency is highly prevalent in patients with UC and is related to disease severity, extent, and duration. Patients with UC should be assessed for their serum 25 (OH) vitamin D levels and appropriately supplemented in order to prevent vitamin D–related systemic morbidity and aid in the restoration of the gut mucosal immune homeostasis.
References
Bernstein CN, Leslie WD, Leboff MS. AGA technical review of osteoporosis in gastrointestinal diseases. Gastroenterology. 2003;124:795–841.
Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004;80 6 Suppl:1717S–20S.
Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. FASEB J. 2001;15:2579–85.
Cantorna MT. Vitamin D and its role in immunology: multiple sclerosis, and inflammatory bowel disease. Prog Biophys Mol Biol. 2006;92:60–4.
Veldman CM, Cantorna MT, DeLuca HF. Expression of 1,25-dihydroxyvitamin D3 receptor in the immune system. Arch Biochem Biophys. 2000;374:334–8.
Gerd Bouma WS. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–33.
Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol. 2003;17:2386–92.
Froicu M, Zhy Y, Cantorna MT. Vitamin D receptor is required to control gastrointestinal immunity in IL-10 knockout mice. Immunology. 2006;117:310–8.
Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000;130:2648–52.
Pappa HM, Grand RJ, Gordon CM. Report on the vitamin D status of adult and pediatric patients with inflammatory bowel disease and its significance for bone health and disease. Inflamm Bowel Dis. 2006;12:1162–74.
Vogelsang H, Klamert M, Resch H, Ferenci P. Dietary vitamin D intake in patients with Crohn’s disease. Wien Klin Wochenschr. 1995;107:578–81.
Jowett SL, Seal CJ, Phillips E, Gregory W, Barton JR, Welfare MR. Dietary beliefs of people with ulcerative colitis and their effect on relapse and nutrient intake. Clin Nutr. 2004;23:161–70.
Bartram SA, Peaston RT, Rawlings DJ, Francis RM, Thompson NP. A randomized controlled trial of calcium with vitamin D, alone or in combination with intravenous pamidronate, for the treatment of low bone mineral density associated with Crohn’s disease. Aliment Pharmacol Ther. 2003;18:1121–7.
Kornbluth A, Sachar DB, Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology, Practice Parameters Committee. Am Gastroenterol. 2004;99:1371–85.
Schroeder KW, Tremaine WJ, Illstrup DM. Coated oral 5ASA therapy for mildly to moderately active disease. N Engl J Med. 1987;317:1625–9.
Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805–6.
Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22:142–6.
Lips P. Which circulating level of 25-hydroxy vitamin D is appropriate? J Steroid Biochem Mol Biol. 2004;89-90:611–4.
Leslie WD, Miller N, Rogala L, Bernstein CN. Vitamin D status and bone density in recently diagnosed inflammatory bowel disease: the Manitoba IBD cohort study. Am J Gastroenterol. 2008;103:1451–9.
Joseph AJ, George B, Pulimood AB, Seshadri MS, Chacko A. 25 (OH) vitamin D level in Crohn’s disease: association with sun exposure and disease activity. Indian J Med Res. 2009;130:133–7.
Harinarayan CV, Ramalakshmi T, Prashad UV, et al. High prevalence of low dietary calcium, high phytate consumption, and vitamin D deficiency in healthy south Indians. Am J Clin Nutr. 2007;85:1062–7.
Harinarayan CV, Joshi SR. Vitamin D status in India – its implications and remedial measures. J Assoc Physicians India. 2009;57:40–8.
Goswami R, Gupta N, Goswami D, Marwaha RK, Tandon N, Kochupillai N. Prevalence and significance of low 25-hydroxyvitamin D concentrations in healthy subjects in Delhi. Am J Clin Nutr. 2000;72:472–5.
Goswami R, Mishra SK, Kochupillai N. Prevalence and potential significance of vitamin D deficiency in Asian Indians. Indian J Med Res. 2008;127:229–38.
Agarwal KS, Mughal MZ, Upadhyay P, Berry JL, Mawer EB, Puliyel JM. The impact of atmospheric pollution on vitamin D status of infants and toddlers in Delhi. India Arch Dis Child. 2002;87:111–3.
Saitoh O, Matsumoto H, Sugimori K, et al. Intestinal protein loss and bleeding assessed by fecal hemoglobin, transferrin, albumin, and alpha-1-antitrypsin levels in patients with colorectal diseases. Digestion. 1995;56:67–75.
Haddad JG. Plasma vitamin D-binding protein (Gc-globulin): multiple tasks. J Steroid Biochem Mol Biol. 1995;53:579–82.
Sahu M, Bhatia V, Aggarwal A, et al. Vitamin D deficiency in rural girls and pregnant women despite abundant sunshine in northern India. Clin Endocrinol. 2009;70:680–4.
Opstelten JL, Chan SSM, Hart AR, et al. Prediagnostic serum vitamin D levels and the risk of Crohn's disease and ulcerative colitis in European populations: a nested case-control study. Inflamm Bowel Dis. 2018;24:633–40.
Gubatan J, Mitsuhashi S, Zenlea T, Rosenberg L, Robson S, Moss AC. Low serum vitamin D during remission increases risk of clinical relapse in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2017;15:240–246.e1.
Sharifi A, Hosseinzadeh-Attar MJ, Vahedi H, Nedjat S. A randomized controlled trial on the effect of vitamin D3 on inflammation and cathelicidin gene expression in ulcerative colitis patients. Saudi J Gastroenterol. 2016;22:316–23.
Chatu S, Chhaya V, Holmes R, et al. Factors associated with vitamin D deficiency in a multicultural inflammatory bowel disease cohort. Frontline Gastroenterol. 2013;4:51–6.
Del Pinto R, Pietropaoli D, Chandar AK, Ferri C, Cominelli F. Association between inflammatory bowel disease and vitamin D deficiency: a systematic review and meta-analysis. Inflamm Bowel Dis. 2015;21:2708–17.
Frigstad SO, Høivik M, Jahnsen J, et al. Vitamin D deficiency in inflammatory bowel disease: prevalence and predictors in a Norwegian outpatient population. Scand J Gastroenterol. 2017;52:100–6.
Zheng SZ, Zhang DG, Wu H, et al. The association between vitamin D receptor polymorphisms and serum 25-hydroxyvitamin D levels with ulcerative colitis in Chinese Han population. Clin Res Hepatol Gastroenterol. 2017;41:110–7.
Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D and fluoride. Washington, DC: The National Academies Press; 1997.
Jorgensen SP, Agnholt J, Glerup H, et al. Clinical trial : vitamin D3 treatment in Crohn’s disease – a randomized double-blind placebo-controlled study. Aliment Pharmacol Ther. 2010;32:377–83.
Papaioannou A, Ferko NC, Adachi JD. All patients with inflammatory bowel disease should have bone density assessment. Inflamm Bowel Dis. 2001;7:158–62.
Khandgawat R, Makharia GK, Puri K. Evaluation of bone mineral density among patients with inflammatory bowel disease in a tertiary care setting in India. Indian J Gastroenterol. 2008;27:103–6.
Acknowledgments
We would like to acknowledge the efforts of Dr. Sharonjeet Kaur, Anu Sharma, and Neha Thakur for their assistance in data compilation and preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
ADL, UD, RK, CV, SK, TN, SB, and KS declare that they have no conflict of interest.
Ethical clearance
The authors declare that the study was performed in a manner conforming to the Helsinki declaration of 1975, as revised in 2000 and 2008 concerning human and animal rights, and the authors followed the policy concerning informed consent as shown on Springer.com.
Disclaimer
The authors are solely responsible for the data and the content of the paper. In no way, the Honorary Editor-in-Chief, Editorial Board Members, or the printer/publishers are responsible for the results/findings and content of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Law, A.D., Dutta, U., Kochhar, R. et al. Vitamin D deficiency in adult patients with ulcerative colitis: Prevalence and relationship with disease severity, extent, and duration. Indian J Gastroenterol 38, 6–14 (2019). https://doi.org/10.1007/s12664-019-00932-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12664-019-00932-z