Abstract
Background
Experience with zinc in treating symptomatic hepatic Wilson’s disease (WD) is limited.
Aim
To study the efficacy of Penicillamine followed by zinc in treating symptomatic hepatic Wilson’s disease.
Methods
We retrospectively analyzed case records of 31 symptomatic hepatic WD patients for whom disease severity scores (Child’s, model for end-stage liver disease (MELD), Nazer’s, and New Wilson Index (NWI) score) and 24-h urinary copper were compared at 3-time points—baseline at presentation, at transition from penicillamine to zinc and at end of follow up.
Results
Thirty-one patients (median age 11 [5–24] years) with symptomatic hepatic WD were studied; ten had associated neuropsychiatric manifestations of WD. Penicillamine was changed to zinc sulfate either due to financial constraints (28 patients) or due to adverse effects of penicillamine (3 patients). At presentation (baseline), six patients belonged to Child’s class A, five to Child’s B, and 17 to Child’s C. Duration of initial penicillamine chelation therapy was 134 (2–320) weeks, and of subsequent zinc therapy was 363 (35–728) weeks. There was a significant improvement in liver function tests and disease severity scores (Child’s, MELD, Nazer’s, and NWI score) at the transition from penicillamine to zinc compared to baseline. This improvement was maintained until the end of study period with 90% survival at 10 (2–20) years. Fifteen of the 17 Child’s C cirrhotic patients showed significant improvement in disease severity scores from baseline until end of follow up.
Conclusions
Penicillamine followed by zinc may be a safe and effective treatment in resource-constrained setting for symptomatic hepatic WD patients in all grades of baseline disease severity. Some patients with decompensated cirrhosis due to WD may be managed with medical treatment, avoiding liver transplantation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medical treatment to reduce copper overload has improved survival in patients with Wilson’s disease (WD). Most guidelines recommend penicillamine as the first line treatment for WD [1,2,3,4], while zinc is advocated for pre-symptomatic patients or as maintenance therapy [5, 6]. There is a paucity of data about when to change-over from penicillamine to zinc to treat symptomatic hepatic WD [1].
WD is the commonest cause of cirrhosis presenting as portal hypertension in pediatric age group in our center [7]. Our hospital caters mostly to patients from the middle socioeconomic category. In a recent study of portal hypertensive patients at our center, the monthly family income was 4810 to 16,019 Indian rupees [8]. Hence financial constraint in affording treatment is a significant challenge for many patients with chronic diseases like WD.
In our center, we often face two situations when the switch from penicillamine to zinc is considered in WD patients—either a patient is unable to afford the cost of lifelong penicillamine treatment (zinc is much cheaper) or when significant side-effects of penicillamine occur. In WD patients who cannot afford long-term penicillamine, counseling each patient/parent of pediatric patients and after obtaining their consent, we have adopted a policy of initial chelation with penicillamine followed by zinc therapy. We herein report our experience with initial chelation with penicillamine followed by zinc monotherapy in treating symptomatic hepatic WD.
Methods
We performed this retrospective study to evaluate the efficacy of initial penicillamine followed by zinc monotherapy in symptomatic hepatic WD patients. This study was approved by Institutional Review Board. Of the 181 patients from a database of hepatic WD patients managed in Hepatology Department, Christian Medical College, Vellore (from March 1997 to October 2009), we found 31 patients with symptomatic hepatic WD who were treated sequentially with penicillamine followed by zinc, hence they were enrolled in this study (Fig. 1). Online pharmacy records of these patients were retrieved. The reason for switching from penicillamine to zinc was noted. We analyzed follow up data until January 2017.
Patients with asymptomatic Wilson’s disease or those on penicillamine, zinc, or trientene as monotherapy and those on a combination of these drugs were excluded from the study. Patients with atypical copper cirrhosis [9] or presence of any other co-existing liver disease were also excluded from the study.
The diagnosis of WD was made in patients who had two of the following three abnormal test results: reduced serum ceruloplasmin level, raised urinary copper excretion, and the presence of Kayser-Fleischer rings in the cornea on slit lamp examination. In case of diagnostic confusion, liver biopsy, for assessing histology and for dry weight of copper was done.
Protocol for switching from penicillamine to zinc
After initial penicillamine treatment, patients were changed over to zinc either because of financial constraints or adverse effects of penicillamine. In the early stages of implementing this policy, patients with financial constraints were given penicillamine for at least 6 months and once clinical improvement was noted, they were switched over to zinc. Thus, the change from penicillamine to zinc was based on clinical improvement (and not based on any laboratory end-points). The patients were initially treated with penicillamine for a variable duration based on treating clinician. The switch was made by gradually tapering penicillamine to a minimum dose of 250 mg once a day (depending on the dose of penicillamine an individual was getting) and then stopping it completely. The duration of tapering down the dose of penicillamine was two or more months. Zinc was started from the next day after completely stopping penicillamine. Over the subsequent years, we have decreased the duration of penicillamine treatment, before switching to zinc, as mentioned later under Results.
We studied clinical response to treatment and survival in the study patients. We analyzed the clinical (both hepatic and neurological signs and symptoms) and laboratory parameters at 3-time points: at baseline (when WD was diagnosed and penicillamine was initiated), at the transition from penicillamine to zinc, and at the end of the study period (while on zinc therapy). The clinical and laboratory details of the patients at each visit were documented and analyzed retrospectively from our computerized hospital records. Similarly, disease severity was assessed at these 3-time points using Child’s score, model for end-stage liver disease (MELD) score [10], Nazer’s score [11], and New Wilson Index (NWI) score [12]. NWI (range 0–20) incorporates total white blood cell count and serum albumin in addition to all variables needed for Nazer’s score (serum bilirubin, prothrombin time, and aspartate aminotransferase). Outcome variables studied were-survival, clinical course of the liver or neurological disease, liver function tests, disease severity scores, urinary copper levels, and adverse effects of treatment. Socioeconomic scoring of patients or family was not done. The normal values of bilirubin, albumin, and 24-h urinary copper in our study were 0.5–1 mg/dL, 3.5–5 g/dL, and < 100 μg/L respectively.
Statistical analysis
Data analysis was done using SPSS version 17. Generalized estimating equations (GEE) was used to compare data at baseline vs. that at the transition from penicillamine to zinc and baseline vs. that at end of follow up. In patients who had paired data available, Wilcoxon signed-rank test (non-parametric) was used to compare data for each variable at the transition from penicillamine to zinc to data at end of follow up (while on zinc treatment). We set the level of statistical significance at p < 0.05. The survival curves were plotted using Kaplan-Meier methods.
Results
Baseline characteristics of study patients
A total of 31 patients were included in this study. Study patients were from southern India (23), eastern India (7), and Bangladesh (1). The baseline demographics, presentation, and WD diagnostics are described in Table 1.
Twenty-four-hour urinary copper values included in this study were baseline values without any penicillamine challenge. All 31 patients had at least two of the three tests abnormal (ceruloplasmin, Kayser-Fleisher ring, 24-h urinary copper). Liver function tests at baseline are given in Table 2. Data on liver biopsy was retrievable in three patients, and raised copper was noted in all (400, 454, and 400 μg/g of liver tissue dry weight). These three patients had bridging fibrosis/cirrhosis and stainable copper deposits on liver biopsy.
Disease severity scores at baseline presentation
Child’s grade at presentation (calculated in 28 patients) were as follows—A, 6 patients; B, 5 patients; and C, 17 patients. The three patients, in whom Child’s grade could not be calculated (due to missing prothrombin time value) did not have any ascites and had normal serum bilirubin and serum albumin. MELD score, Nazer’s score, and NWI score at baseline are given in Table 2. Incomplete data in some patients meant that disease severity scores could not be calculated in all 31 study patients at all 3-time points.
Details of de-coppering treatment
The dose of penicillamine prescribed varied from 250 mg once a day to 500 mg three times per day (19.8 ± 6.2 mg/kg/day). The dose of zinc sulfate varied from 140 mg twice a day to 140 mg thrice a day, i.e. 100–150 mg/day of elemental zinc.
After initial penicillamine treatment, patients were changed over to zinc either because of financial constraints (in 28 patients; after 143 [34–320] weeks, median [range]) or adverse effects of penicillamine (in three patients; after 2, 8, and 120 weeks). Duration of initial penicillamine therapy in the 31 patients was 134 (2–320) weeks (median [range]), whereas duration of subsequent zinc therapy was 363 (35–728) weeks (Fig. 2).
Overall compliance was excellent with both the drugs except for one patient who stopped penicillamine for 2 weeks and three patients who stopped zinc for 3, 6, and 12 months during a follow up of 9, 13, and 5 years respectively.
We did not advise any specific dietary restriction.
Follow up
The 31 patients were followed up for 506 (112–1030) weeks (median [range]) (Fig. 2, Fig. 3). Twenty-seven patients were followed up for > 5 years. The 31 study patients attended our unit for 10 (2–42) visits while on initial penicillamine treatment and for 18 (2–67) visits while on maintenance zinc treatment.
Clinical course of liver disease while on treatment
While most patients steadily improved with treatment, there were cirrhosis-related complications in a few patients. Complications while on penicillamine were spontaneous bacterial peritonitis (4 patients); esophageal variceal bleed (4 patients), and bleed from portal hypertensive colopathy (1 patient).
Complications while on zinc maintenance therapy were esophageal variceal bleed (4 patients), fundal variceal bleed (1 patient), spontaneous bacterial peritonitis (1 patient), hepatic encephalopathy (1 patient), and acute-on-chronic liver failure (1 patient). All these patients had improved disease severity scores at end of follow up.
The ten patients with additional neurological WD had an overall improvement of neurological manifestations. Five patients had some worsening of neurological manifestations while on zinc maintenance therapy (dysphonia/dysarthria (4), limb dystonia (2). After 86 (24–152) months from diagnosis, they showed improvement with continued medical management. Three of these patients were found to be temporarily not compliant with zinc therapy (for 3, 6, and 12 months) prior to worsening of neurological manifestations.
One patient who was switched over to zinc after 72 weeks of penicillamine therapy had an asymptomatic unexplained rise in AST levels after 186 weeks of zinc maintenance. He was switched back to penicillamine, following which, his AST levels improved.
Serial liver function tests and disease severity scores on treatment
There was a statistically significant improvement in serum bilirubin, serum albumin, and prothrombin time and in disease severity scores (Child’s, MELD, Nazer’s, and NWI scores) at the transition from penicillamine to zinc compared to baseline (Table 2), and this improvement was maintained until the end of follow up (Table 2).
Urinary copper levels on treatment
The median level of urine copper at end of the study (on maintenance zinc) was significantly lesser than at the time of transition from penicillamine to zinc (Table 2).
Patients with decompensated liver disease at baseline
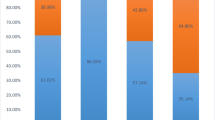
Seventeen of the 31 study patients were in Child’s grade-C at baseline presentation. They received penicillamine for 111 (2–320) weeks followed by zinc maintenance therapy for 344 (41–652) weeks. Two patients (patient numbers 2, 5) died after 284 and 112 weeks respectively, as mentioned below. Remaining 15 patients improved with significantly improved disease severity scores at transition which was maintained until the end of follow up (Fig. 4).
Wilson’s disease (WD) severity scores (at presentation, at transition, and at end of follow-up) of patients with decompensated cirrhosis (Child class C) (n = 17) who were on sequential penicillamine followed by zinc therapy (MELD model for end-stage liver disease, NS Nazer’s score, NWI New Wilson Index score)
Patients treated with penicillamine for less than a year
Ten patients (age, 11 (5–24) years; 6 males) were shifted to zinc-after 35 (2–52) weeks, median (range) of penicillamine; eight of these patients were alive at follow up of 378 (138–704). Two patients (patient numbers 2, 5) died, as mentioned above.
Disease severity scores of these ten patients significantly improved at transition, and this improvement was maintained until end of follow up. Child’s score reduced from 12 (7–13) to 7 (5–12) at transition [p < 0.01] and did not worsen at the end of follow up (8 [5–15], p = 0.32). MELD score reduced from 18 (13–20) at baseline to 14 (8–18) at transition (p = 0.02) and did not worsen at the end of follow up (11 [6–20], p = 0.58). Nazer’s score reduced from 3 (2–5) at baseline to 1 (0–4) at transition (p < 0.01) and did not worsen at the end of follow up (1 [0–3], p = 1.0). New Wilson Index score reduced from 9 (5–11) at baseline to 4 (0–7) at transition (p < 0.01) and did not worsen at the end of follow up (3 [0–10], p = 0.79).
Details of “on-treatment mortalities”
Three patients died during follow up (Fig. 2, Fig. 3).
Patient number 2 (baseline Child’s score 5), was started on zinc after 8 weeks of penicillamine (stopped due to febrile neutropenia). She had developed transient self-limiting proteinuria (renal histology showed diffuse proliferative glomerulonephritis with mesangial sclerosis) at 197 weeks. She died at 284 weeks of sudden onset short duration worsening liver functions and sepsis.
Patient number 5 (baseline Child’s score 13) died of E. coli sepsis (spontaneous bacterial peritonitis, infected pleural effusion), after 112 weeks into WD treatment (35 weeks on penicillamine and 77 weeks on zinc).
Patient number 10 (baseline Child’s score 11) died of spontaneous bacterial peritonitis, hepatic hydrothorax, and septic shock after 437 weeks into WD treatment (70 weeks on penicillamine and 367 weeks on zinc).
Reducing the duration of initial chelation with penicillamine over the years
We have steadily reduced the duration of penicillamine treatment over years (Fig. 5).
Discussion
To our knowledge, this is the largest reported series of symptomatic hepatic WD patients, treated sequentially with penicillamine followed by zinc. We used different scores (Child score, MELD score, Nazer’s score, and NWI score) to objectively assess disease severity in the study patients. As more than half (55%) of the study patients had advanced liver disease (17 patients with Child’s grade-C at presentation), this low-cost treatment regime led to the 2-year survival of 100%. Over a follow up period of 500 (112–1030) weeks, median (range), only three of the 31 study patients died suggesting 90.3% survival over 10 (2–20) years.
We found statistically significant improvement in liver function tests and disease severity scores in the study patients at the time of transition from penicillamine to zinc, compared to baseline (Table 2). This improvement persisted at the end of the study period (on zinc) (Table 2).
The urinary copper levels remained high at the time of transition to zinc. Urinary copper levels at the end of the study period (on zinc) were significantly lesser than at the time of transition from penicillamine to zinc (Table 2). This may reflect reduced enteral absorption of copper with zinc therapy and/or decreasing whole body copper overload status after initial treatment with penicillamine.
Two patients (patient numbers 8 and 15) with very high disease severity scores at baseline improved with medical treatment alone, without liver transplantation. They had NWI score ≥ 11 (which predicted death without liver transplantation in 93% patients) [12]. Zinc alone or with copper chelators have been reported to be life-saving in WD patients with liver failure with advanced disease severity scores [13, 14].
A study by Dhawan et al. [12] suggested that patients with NWI score > 6 do not improve with medical treatment alone (specificity 91%). In our report where 14 patients with baseline NWI score > 6 improved when maintained on zinc (follow up of 440 (112–850) weeks). Encouraged by our initial experience with this treatment regime, we have steadily reduced the duration of penicillamine treatment over the years.
While three patients on penicillamine had cytopenias needing stopping of penicillamine, adverse effects were minimal with zinc sulfate. Of the different zinc salts, the study patients received zinc sulfate, as this was easily available during the study period.
In India, zinc sulfate is much cheaper than penicillamine. Five years’ treatment with zinc sulfate (at 140 mg, three times a day) currently costs Rs 5475 (US$ 82), while penicillamine at 250 mg three times a day for 5 years’ costs Rs 87,648 (US$ 1315), i.e. savings of Rs 82,173 (US$ 1233) per patient over a 5-year period.
When should we consider zinc therapy in WD? Treatment recommendations for WD are based on case series rather than randomized control trials. The utility of zinc monotherapy to treat pre-symptomatic WD [15] or symptomatic neurological WD [16,17,18] has been documented in the literature. American Association for the Study of Liver Diseases suggests that in patients with hepatic WD, the change to zinc can be considered after initial chelation with penicillamine for 1–5 years [1]. However, there is limited data regarding the timing of this change-over in treatment [1, 19, 20]. At our center, we consider penicillamine as the drug of first choice hence given to 119 patients of a total of 177 patients with symptomatic hepatic WD (Fig. 1).
There are two reports of ≥ 10 patients with symptomatic hepatic WD, treated with zinc [21, 22]. Brewer et al. [21] reported 21 patients with symptomatic hepatic WD who had initial penicillamine (or molybdenum) followed by zinc. These 21 patients were part of a cohort of 141 WD patients, 30 of whom had pre-symptomatic WD and 90 had neurological WD. Of the total 141 patients who were treated for 4.8 years, six died. Linn et al. [22] reported the use of zinc monotherapy from the start of treatment in 12 patients with symptomatic hepatic WD for 14 (2–30 years), of whom none died, while two needed liver transplant. Our current report of 31 symptomatic hepatic WD patients treated with zinc adds to this experience. It has been hypothesized that symptomatic WD is caused by toxic non-ceruloplasmin bound (free) copper in the blood, whereas accumulated copper and copper bound to ceruloplasmin or metallothionein is not toxic [23]. Thus, treatment of symptomatic WD is aimed at normalizing free copper concentration in blood which is achieved safely and effectively with zinc therapy. Zinc induces metallothionein, a detoxification protein that binds copper. Oral zinc therapy leads to storage of metallothionein-bound copper in the gut mucosa which is then shed along with feces. Some guidelines caution against the use of chelating agents as an initial treatment as they may aggravate copper intoxication and cause iatrogenic deterioration [23].
Some of the limitations of our study are its retrospective design and incomplete data in some patients which prevented us from calculating the disease severity scores in all study patients at the different time points. We have not identified any objective parameter to guide the timing of switchover from penicillamine to zinc.
In conclusion, while we recommend penicillamine as the drug of the first choice to all patients with symptomatic hepatic WD patients, in resource-constrained setting sequential penicillamine followed by zinc therapy may be safe and effective across all degrees of baseline disease severity. We found that switch from penicillamine to zinc can be made once there is a clinical improvement. Our data also suggests that some patients with decompensated cirrhosis due to WD may be managed with medical treatment, avoiding liver transplantation.
References
Roberts EA, Schilsky ML. Diagnosis and treatment of Wilson’s disease: an update. Hepatology. 2008;47:2089–111.
European Association for Study of Liver. EASL clinical practice guidelines: Wilson’s disease. J Hepatol. 2012;56:671–85.
Ala A, Walker AP, Ashkan K, Dooley JS, Schilsky ML. Wilson’s disease. Lancet. 2007;369:397–408.
Ferenci P. Review article: Diagnosis and current therapy of Wilson’s disease. Aliment Pharmacol Ther. 2004;19:157–65.
Ranucci G, Di Dato F, Spagnuolo MI, Vajro P, Iorio R. Zinc monotherapy is effective in Wilson’s disease patients with mild liver disease diagnosed in childhood: a retrospective study. J Rare Dis. 2014;9:41.
Mizuochi T, Kimura A, Shimizu N, Nishiura H, Matsushita M, Yoshino M. Zinc monotherapy from time of diagnosis for young pediatric patients with presymptomatic Wilson disease. J Pediatr Gastroenterol Nutr. 2011;53:365–7.
Simon EG, Joseph AJ, George B, et al. Aetiology of paediatric portal hypertension - experience of a tertiary care centre in South India. Trop Doct. 2009;39:42–4.
Goel A, Madhu K, Zachariah U, et al. A study of aetiology of portal hypertension in adults (including the elderly) at a tertiary centre in southern India. Indian J Med Res. 2013;137:922–7.
Ramakrishna B, Date A, Kirubakaran C, Raghupathy P. Atypical copper cirrhosis in Indian children. Ann Trop Paediatr. 1995;15:237–42.
Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–70.
Nazer H, Ede RJ, Mowat AP, Williams R. Wilson’s disease: clinical presentation and use of prognostic index. Gut. 1986;27:1377–81.
Dhawan A, Taylor RM, Cheeseman P, De Silva P, Katsiyiannakis L, Mieli-Vergani G. Wilson’s disease in children: 37-year experience and revised King’s score for liver transplantation. Liver Transpl. 2005;11:441–8.
Lee VD, Northup PG, Berg CL. Resolution of decompensated cirrhosis from Wilson’s disease with zinc monotherapy: a potential therapeutic option? Clin Gastroenterol Hepatol. 2006;4:1069–71.
Askari FK, Greenson J, Dick RD, Johnson VD, Brewer GJ. Treatment of Wilson’s disease with zinc. XVIII. Initial treatment of the hepatic decompensation presentation with trientine and zinc. J Lab Clin Med. 2003;142:385–90.
Marcellini M, Di Ciommo V, Callea F, et al. Treatment of Wilson’s disease with zinc from the time of diagnosis in pediatric patients: a single-hospital, 10-year follow-up study. J Lab Clin Med. 2005;145:139–43.
Hoogenraad TU, van Hattum J, Van den Hamer CJ. Management of Wilson’s disease with zinc sulphate. Experience in a series of 27 patients. J Neurol Sci. 1987;77:137–46.
Czlonkowska A, Gajda J, Rodo M. Effects of long-term treatment in Wilson’s disease with D-penicillamine and zinc sulphate. J Neurol. 1996;243:269–73.
Sinha S, Taly AB. Withdrawal of penicillamine from zinc sulphate-penicillamine maintenance therapy in Wilson’s disease: promising, safe and cheap. J Neurol Sci. 2008;264:129–32.
Shimuzu N, Fujiwara J, Ohnishi S, et al. Effects of long-term zinc treatment in Japanese patients with Wilson’s disease: efficacy, stability, and copper metabolism. Transl Res. 2010;156:350–7.
Arnon R, Calderon JF, Schilsky M, Emre S, Shneider BL. Wilson disease in children: serum aminotransferases and urinary copper on triethylene tetramine dihydrochloride (trientine) treatment. J Pediatr Gastroenterol Nutr. 2007;44:596–602.
Brewer GJ, Dick RD, Johnson VD, Brunberg JA, Kluin KJ, Fink JK. Treatment of Wilson's disease with zinc: XV long-term follow-up studies. J Lab Clin Med. 1998;132:264–78.
Linn FH, Houwen RH, van Hattum J, van der Kleij S, van Erpecum KJ. Longterm exclusive zinc monotherapy in symptomatic Wilson disease: experience in 17 patients. Hepatology. 2009;50:1442–52.
Hoogenraad TU. Paradigm Shift in treatment of Wilson’s disease: zinc therapy now treatment of choice. Brain and Development. 2006;28:14–8.
Acknowledgements
We acknowledge the Fluid research funds (Christian Medical College, Vellore, India) for funding this study.
Funding
Fluid research funds, Christian Medical College, Vellore - 632 004, Tamil Nadu, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
PG, MC, AG, UZ, KGS, JR, GC, GK, GR, and CEE declare that they have no conflict of interest.
Ethics statement
The authors declare that the study was performed in a manner conforming to the Helsinki declaration of 1975, as revised in 2000 and 2008 concerning human and animal rights, and the authors followed the policy concerning informed consent as shown on Springer.com.
Rights and permissions
About this article
Cite this article
Gupta, P., Choksi, M., Goel, A. et al. Maintenance zinc therapy after initial penicillamine chelation to treat symptomatic hepatic Wilson’s disease in resource constrained setting. Indian J Gastroenterol 37, 31–38 (2018). https://doi.org/10.1007/s12664-018-0829-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12664-018-0829-x