Abstract
Background and aim
The relationship between gastroesophageal reflux disease (GERD) and Helicobacter pylori is controversial. We evaluated endoscopic, 24-h gastric and esophageal acid profile among patients with GERD in relation to H. pylori, as the latter might alter gastric acid secretion.
Methods
Patients with GERD (n = 123), who were not on acid-suppressive drugs, and had not received anti-H. pylori therapy, underwent gastroduodenoscopy and tests for H. pylori detection. Esophageal manometry, 24-h pH metry, serum pepsinogen-I (PG-I), PG-II and gastrin-17 ELISA were done in all these patients. Univariate and multivariate analyses were performed to assess independent predictors for erosive esophagitis (EE).
Results
Of 123 patients (mean age 40.5 [13.1] years, 85 [69.1%] men), 59 (47.9%) had H. pylori infection. EE was more common in H. pylori non-infected than infected (49 vs. 32, p < 0.001). Among patients older than 40 years, absence of H. pylori was associated with lower esophageal pH and longer reflux (p = 0.02 and p < 0.001, respectively). PG-I/PG-II ratio was lower in H. pylori infected subjects (p < 0.001). In patients with higher LA grade of esophagitis, elevated PG-I levels and PG-I/PG-II ratio were associated with more acidic stomach (p = 0.04 and p = 0.01, respectively). Multivariate analyses showed low gastrin-17 (p = 0.016), higher age (p = 0.013), hiatus hernia (p = 0.004) and absence of H. pylori (p = 0.03) were independent predictors for risk of EE.
Conclusion
H. pylori infection is associated with less acidic stomach and less severe GERD. Low gastrin-17, higher age, hiatus hernia and absence of H. pylori were the best predictors for EE risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent data suggest an overall increase in the prevalence and severity of gastroesophageal reflux disease (GERD) in the West [1–3]. Role of various host physiological, dietary and environmental factors have been extensively investigated in the pathogenesis of GERD [4–8]; however, the role of Helicobacter pylori (H. pylori) infection in pathogenesis of GERD is still controversial. H. pylori infection may either increase or decrease gastric acid secretion, thereby increasing or decreasing the severity of GERD [9]. Furthermore, the role of gastric acid in GERD severity is supported by the efficacy of acid suppressive drugs in its treatment.
Several studies suggest a possible protective role of H. pylori in GERD [10–16]. Furthermore, eradication of H. pylori may result both in de novo occurrence and exacerbation of GERD [17]. However, a few studies suggested that H. pylori infection may exacerbate GERD [18–20]. H. pylori may cause (a) antral gastritis leading to more acid secretion, exacerbating GERD, and (b) corpus or pangastritis leading to less acid secretion, causing milder GERD [21]. Thus, severity of GERD in patients with H. pylori infection depends on the site of infection.
Serum pepsinogen-I (PG-I) and PG-II concentration are markers of gastric acid secretory mass; high serum PG-I indicates increased gastric acid secretory capacity [22], whereas high PG-II is associated with reduced gastric acid secretory capacity [22]. PG-I/PG-II ratios are therefore, higher in duodenal ulcer (hyperchlorhydria) and lower in gastric ulcer (hypochlorhydria). These changes in PGs are thus used as a non-invasive marker for assessing the pattern of gastritis. However, there is scarce and contradictory data on the evaluation of PGs in patients with different endoscopic grades of GERD particularly in relation to H. pylori infection [23, 24]. Gastrin is also the most potent endogenous stimulant of gastric acid secretion [25]. A low serum gastrin level is indicative of high gastric acid secretion; whereas, high gastrin levels (like in chronic H. pylori infection and gastric atrophy) is associated with decreased gastric acid secretion [26].
Twenty-four hour pH metry has been widely used in the diagnosis of GERD. However, as H. pylori is known to alter gastric acid secretion, it would be worth evaluating 24-h gastric acid profile (in circadian rhythm) in patients with GERD in relation to H. pylori infection. The limited data available on this issue are contradictory and evaluated the basal and maximal acid output and not 24-h gastric acid profile [27, 28].
We evaluated PG-I, PG-II and gastrin-17, and 24-h gastric and esophageal acid profile in patients with GERD in relation to H. pylori infection. Furthermore, we have assessed whether patients with severe endoscopic grades of GERD have more acidic stomach and have more esophageal acid exposure.
Methods
Study subjects
In this prospective study, patients with heartburn of more than two months duration, referred to the Gastrointestinal Pathophysiology and Motility Laboratory of the Department of Gastroenterology of our center from April 2005 to September 2008 were evaluated for the presence of GERD by fulfilling at least two of these criteria: 1) Carlsson-Dent score of >6 [29], 2) presence of endoscopic GERD, 3) significant reflux on 24-h pH metry (% time esophageal pH <4 for ≥5% of recorded time) [30], 4) histological assessment of esophagitis [31], and 5) response to omeprazole 20 mg/day [32, 33].
All patients were off acid suppressive drugs and prokinetics at least one month before inclusion, and none had received anti-H. pylori therapy in the past. Patients were allowed to take antacids if they had intolerable symptoms, till one week before pH metry. Informed consent was taken from each patient and the protocol was approved by the Institutional Ethics Committee. Patients, who could not remain off PPI for one month, were excluded from the study.
Investigations
Esophagogastroduodenoscopy was performed using a forward-viewing endoscope (Olympus video endoscope). Esophagitis, if present, was graded using Los Angeles (LA) classification [34]. Patients without any erosion in esophagus were classified as endoscopy negative reflux disease (ENRD). Barrett’s esophagus (BE) was diagnosed by the criteria as described previously [35]. Hiatus hernia was defined as a distance of >2 cm between squamocolumnar junction and the impression of the crural diaphragm.
Six biopsies of 3–5 mm were obtained (three each from antrum and corpus) during the procedure. Of these, two biopsies each from antrum and corpus were used for histological examination and rest two were used for H. pylori detection.
H. pylori infection was diagnosed using rapid urease test (RUT), histology and anti-H. pylori IgG enzyme linked immunoabsorbent assay (ELISA), diagnostic criteria being any two of the three given tests positive. RUT was performed using an in-house RUT solution, the sensitivity and specificity of which have been validated previously [36]. Gastric biopsies were stained with hematoxylin and eosin, and Giemsa, to evaluate H. pylori. ELISA was done for IgG antibodies (H. pylori-IgG ELISA) using commercially available kit (Genesis Diagnostics, Cambridgeshire, UK). This has been validated previously in our population [37].
Serum PG-I (n = 81), PG-II (n = 81) and gastrin-17 (n = 76) were performed using commercially available ELISA kit (Biohit Oyj, Finland).
Histological examination of gastric biopsies was performed in 74 patients with GERD. Two biopsies (3–5 mm) per site (antrum and corpus) were assessed by a single expert pathologist for the presence and grading of gastritis according to the updated Sydney system (1994). The pathologist was unaware about the endoscopic findings. When the scores between the two biopsies were different, the more severe scores were selected.
Twenty-four hour dual channel pH metry was performed in subset of patients who gave consent for this procedure. Eighty-three patients underwent 24-h dual channel pH metry after an overnight fast using a pH meter (Naik-II, RedTech, CA, USA) and antimony pH probes (the two sensors placed 15 cm apart) as per the protocol described previously [38]. Prior to pH metry, esophageal manometry was performed using an eight-channel (4 radial and 4 concentric ports) water perfusion system (RedTech, CA, USA) to localize and measure lower esophageal sphincter (LES) pressure and to study esophageal body motility. In three patients, in whom esophageal manometry could not be performed, the pH probe was placed 5 cm above the change in pH of the proximal sensor from acidic to alkaline. After 24-h, pH data was downloaded and analyzed for esophageal acid exposure and gastric acid profile using the Naik-II software from RedTech, CA, USA [30, 38].
Statistical analysis
Patients were categorized on the basis of presence and absence of H. pylori infection and on different grades of esophagitis (ENRD, LA-A, and LA grades B-D). Inter-group comparison between two or more than two continuous variables was performed by Mann-Whitney U or Kruskal Wallis tests, respectively. Variables found significant by latter analysis were subjected to post-hoc analysis by Mann-Whitney U test. Categorical variables were compared using Chi-squared test with Yates’ correction as applicable. P-values < 0.05 were considered significant. Pearson correlation coefficient (CC) was calculated to assess the degree of association between the two variables.
Categorical variables found significant in univariate analysis were subjected to multivariate analysis by binary logistic regression. Presence and grades of esophagitis were taken as dependent variable and forward LR method was chosen.
Results
One hundred twenty-three patients (mean age 40.5 [13.1] years; 85 [69.1%] men) fulfilled the criteria for diagnosis of GERD; 95/106 (89.6%) had Carlsson-Dent score ≥6.0, 88/123 (75.5%) had erosive esophagitis (EE); 120/123 (97.5%) responded to omeprazole, 50/83 (60.2%) had significant reflux on 24-h pH metry, and 68/74 (91.9%) had histological evidence of esophagitis.
Of 123 patients with GERD, 59 (47.96%) had H. pylori infection. Patients with and without H. pylori infection were comparable in respect to age, gender and Carlsson-Dent score (Table 1).
Patients with H. pylori infection more often had ENRD as compared to those without it (p = 0.022; Table 1). GERD LA-A was more common in H. pylori non-infected patients (p = 0.013). Frequency of GERD LA-B and higher LA (>LA-A) grades were comparable among patients with and without H. pylori infection. EE was more common in patients without H. pylori infection than those with it. Frequency of LA-C, LA-D, peptic stricture and BE were 1 (1.7%), 1 (1.7%), 2 (3.4%), and 3 (5.2%), respectively in patients with H. pylori infection whereas it was 1 (1.6%), 0 (0%), 1 (1.6%), and 0 (0%), respectively in those without H. pylori infection. Twenty-two of 54 (40.7%) patients with H. pylori infection and 33/62 (53.2%) patients without H. pylori infection had hiatus hernia. Men more often had EE and higher LA grades as compared to women (EE: 77.1% vs. 54.3%, p = 0.02; higher LA: 47% vs. 20%, p = 0.01). Patients with EE were older than those with ENRD (median age 42.7 [14–74] y vs. 37.2 [19–66] y, p = 0.02).
At manometry, patients with GERD with and without H. pylori infection had comparable LES pressure (12 [4–52] vs. 13 [4–63] mmHg, p = 0.31), average amplitude of contraction in proximal (30.2 [8–88] vs. 38.5 [6.7–100.5] mmHg, p = 0.23) and distal esophageal body (58.7 [15–206.5] vs. 65.7 [10.5–203] mmHg, p = 0.24).
The average gastric pH, % time gastric pH <4, <3, <2 were comparable among the two groups; percentage of time gastric pH <1.5 was higher in patients without H. pylori infection (Table 1, Fig. 1).
Gastric and esophageal acid profile among H. pylori infected and non-infected patients with GERD. % time gastric pH < 1.5 was higher in patients without H. pylori infection than the other group (b); Esophageal acid profile was however comparable among patients with and without H. pylori infection (c, d, e and f)
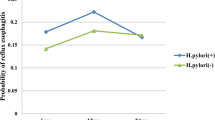
Gastric and esophageal acid profile among elderly (>40 y) H. pylori infected and non-infected patients with GERD. % time gastric pH < 1.5 was higher in patients without H. pylori infection than the other group (b); average esophageal pH was lower and longest reflux (in min) was higher in patients without H. pylori infection than those with it (c and f); % reflux time and time esophageal pH < 4.0 (in hours) was however comparable among the two groups (d and e)
Esophageal acid exposure was abnormal in 24/42 (57.1%) patients with H. pylori infection and 23/41 (56.1%) without it (p = NS). Patients with GERD with and without H. pylori infection had comparable esophageal pH metry findings. Patients older than 40 years without H. pylori infection had lower average esophageal pH and longer reflux time in minutes than those with H. pylori infection (Table 1, Fig. 2).
Esophageal acid exposure was higher among men than in women (4.2 [0.01–65] vs. 2.3 [0–76.15], p = 0.04). Twenty-four hour gastric acid profile was comparable among male and female patients. Males showed a trend towards longer time with esophageal pH <4 (in h) than females (0.8 [0–15.6] vs. 0.5 [0–18.2], p = 0.05).
Patients with higher LA had more esophageal acid exposure than those with ENRD and LA-A both (Table 2), the gastric acid profile was comparable among them.
Serum PG-I was comparable (135 [64–422] vs. 123 [36–440] μg/L, p = 0.09), PG-II levels were higher (10.3 [5–44.2] vs. 7.1 [3–61] μg/L, p < 0.0001) and PG-I/PG-II ratio was lower (12.5 [6.6–23.5] vs. 15.4 [6.4–42.3], p = 0.003) in H. pylori infected than in non-infected patients. Patients with GERD with H. pylori infection tended to have increased serum gastrin-17 levels than those without it (7.2 [0.05–51] vs. 2.7 [0.01–53] pmol/L, p = 0.06).
Histology of gastric biopsies
Of 74 patients with GERD, 40 (54.1%) had H. pylori infection. Thirty-four (45.9%) had antral gastritis (mild: 27, moderate: 6, severe: 0), 17 (23.0%) had pangastritis (mild: 8, moderate: 7, severe: 2) and 23 (31.1%) had normal gastric mucosa.
Gastric acid profile (% time gastric pH <1.5) showed a trend from pangastritis < normal gastric mucosa < antral gastritis. Patients with antral gastritis had more acidic stomach than those with pangastritis (Table 3). Gastric acid profile was comparable in patients with normal gastric mucosa than those with antral or pangastritis. Patients with pangastritis had lower PG-I/PG-II ratio than those with normal gastric mucosa (Table 3). Patients with normal gastric mucosa had lower gastrin-17 levels than those with antral gastritis as well as pangastritis. Patients with H. pylori-positive gastritis had less acidic stomach, lower PG-I/PG-II ratio and higher gastrin-17 levels than those with gastritis who were H. pylori-negative.
Histology of esophageal biopsies
Fifty-six of 74 patients (75.7%) had mild esophagitis, nine (12.2%) had moderate and three (4.1%) had severe esophagitis; six patients (8.1%) had normal esophageal mucosa on histopathology.
Among patients with ENRD, 4 (22.2%), 13 (72.2%), 1 (5.6%) and 0 (0%) had normal esophageal mucosa, mild, moderate and severe esophagitis, respectively; among patients with LA-A grade, the corresponding values were 2 (9.1%), 18 (81.8%), 2 (9.1%) and 0 (0%), respectively, and in those with higher LA grades, the values were 0 (0%), 25 (73.5%), 6 (17.6%) and 3 (8.8%), respectively.
Relationship between gastric acid and pepsinogen levels
Gastric acidity correlated with PG-I levels and PG-I/PG-II ratio (Table 4) irrespective of the presence or absence of H. pylori infection. However, gastric acidity correlated with PG-I and PG-II levels among patients with H. pylori infection, and with PG-I/PG-II ratio among patients without H. pylori infection. Gastric acidity correlated with PG-I levels and PG-I/PG-II ratio among patients with higher LA grades.
Among patients with mild esophagitis on histopathology, gastric acidity was positively correlated with esophageal acid exposure (CC = 0.3, p = 0.03), longest reflux in min (CC = 0.3, p = 0.03) and number of reflux episodes >5 min (CC = 0.31, p = 0.02).
Multivariate analysis
Parameters found significant on univariate analysis were entered into a multivariate model. Multivariate analysis showed that serum gastrin-17 ≤ 10 pg/L, presence of hiatus hernia and age >40 years were independently associated with higher risk of GERD (Table 5). Patients having these parameters had 80.3% correct prediction for having EE. Removal of above three independent parameters from multivariate analysis showed an independent association of absence of H. pylori infection with presence of EE. Patients without H. pylori infection had 73.2% correct prediction for having EE.
Discussion
The present study shows that (a) EE was more common in patients without H. pylori infection, (b) absence of H. pylori was associated with more acidic stomach, (c) among patients >40 years old, absence of H. pylori was associated with higher esophageal acid exposure, (d) though gender did not have any effect on gastric acid profile, males had higher esophageal acid exposure and higher grades of GERD, (e) PG-I/PG-II ratio was lower in H. pylori infected patients than non-infected patients, (f) in patients without H. pylori infection, higher acidity was associated with elevated PG-I/PG-II ratio, (g) in patients with higher LA grades, elevated PG-I levels and PG-I/PG-II ratio were associated with more acidic stomach, (h) esophageal motility parameters were not different among the two groups, (i) low gastrin-17, higher age, hiatus hernia and absence of H. pylori were associated with risk of EE.
Present study showed that H. pylori infection was associated with milder grades of GERD. Secondly, the LES and esophageal motility parameters were comparable among patients with and without H. pylori infection; this finding is supported by the results of previous studies [39, 40].
The 24-h gastric acid profile showed that absence of H. pylori infection was associated with more acidic stomach. H. pylori infection was shown to decrease gastric acid secretion in healthy persons [41]. The difference in gastric acid profile among H. pylori infected and non-infected patients with GERD could be important in understanding the relationship of H. pylori with GERD. The difference in severity of GERD among patients with and without H. pylori infection is not due to impairment in esophageal motility parameters, but possibly due to the difference in gastric acid profile among these groups.
Esophageal acid exposure was comparable among patients with GERD with and without H. pylori infection; this has also been shown in previous studies [28, 42]. However, among patients >40 years old, absence of H. pylori infection was associated with higher esophageal acid exposure. A previous study on healthy volunteers showed that advancing age had no influence on gastric acid secretion in H. pylori-negative subjects [43]. Gastric acid secretion decreases with age in H. pylori-positive subjects because of the increasing prevalence of atrophic gastritis [26, 43]. We found that gastric acid profile was similar in males and females, in contrast to the studies on healthy population [44]. Male patients had higher esophageal acid exposure and higher endoscopic grades of GERD than females, probably due to more exposure to dietary and environmental factors than women [21, 45].
Our study showed comparable PG-I levels, higher PG-II levels and lower PG-I/PG-II ratio in patients with H. pylori infection than those without it, indicating low gastric acid secretion by H. pylori infected subjects. Gastrin-17 levels tended to be lower in patients without H. pylori infection indicating high acid output and therefore is associated with increased risk of GERD and BE [46]. We also found a correlation of serum PG-I levels and PG-I/PG-II ratio with gastric acidity among patients with GERD irrespective of presence or absence of H. pylori infection. In patients with H. pylori infection, higher PG-I and PG-II levels were associated with higher acidity of the stomach. The PG-I/PG-II ratio did not correlate with gastric acidity; this might be related to the fact that our study population included patients with antral (high acid) and pangastritis (reduced acid) both among H. pylori infected group, thus balancing the effect of each other. Secondly, presence of H. pylori itself increases the gastric pH probably due to inflammation of the stomach and buffering of acid because of ammonia [47, 48]. However, among patients without H. pylori infection, higher PG-I/PG-II ratio correlated with gastric acidity.
Patients with antral gastritis had more acidic stomach than those with pangastritis, as expected. After categorizing patients based on H. pylori status and gastritis pattern, the trend for gastric acidity and PG-I/PG-II ratio was H. pylori-negative antral gastritis > H. pylori-positive antral gastritis > H. pylori-positive pangastritis. This further demonstrated that presence of H. pylori may be associated with less acidic stomach and lower PG-I/PG-II ratio.
Our data showed that patients with normal gastric mucosa had low gastrin-17 levels as compared to those with antral or pangastritis. The latter two groups had comparable gastrin-17 levels. This was probably due to inclusion of both H. pylori-positive as well as H. pylori-negative patients. After categorizing the patients with gastritis based on H. pylori positivity, we found that patients with H. pylori-negative antral gastritis had lower gastrin-17 levels than those with H. pylori-positive antral and H. pylori-positive pangastritis. The trend for gastin-17 level was H. pylori-negative antral gastritis < H. pylori-positive antral gastritis < H. pylori-positive pangastritis, suggesting the trend for gastric acidity as H. pylori-negative antral gastritis > H. pylori-positive antral gastritis > H. pylori-positive pangastritis. In patients with higher grades of EE, elevated serum PG-I levels and PG-I/PG-II ratio were associated with increased acidity of the stomach. Thus, they are likely to have more esophageal acid exposure.
Interestingly, our data also demonstrated that among patients with mild esophagitis, increase in gastric acid was associated with increased esophageal acid exposure. This phenomenon was found only among patients with mild esophagitis probably, due to sufficient number of cases in this group.
Multivariate analysis showed that presence of low serum gastrin-17 levels, age >40 years, and presence of hiatus hernia were the best predictors for diagnosis of EE. Removal of these three variables from the analysis showed absence of H. pylori as an independent predictor for risk of EE. Hence, higher acid output might lead to higher esophageal acid exposure leading to severe GERD. Presence of hiatus hernia is an independent risk factor for EE [49–51]. This functional impairment of the gastroesophageal junction might lead to increased esophageal acid exposure. In this study, presence of H. pylori might have had an additive effect on the serum gastrin-17 levels in risk of EE. Positive H. pylori status has been shown to be associated with a lower risk of EE [45]. One study showed that H. pylori eradication was associated with presence of EE [51]; other studies showed contradictory findings [52, 53]. Our study though performed in a subset of patients considered acid related as well as motility parameters. Hence, our study better supports the role of gastrin-17, higher age, hiatus hernia and absence of H. pylori infection as independent predictors for EE.
This study had certain limitations such as we considered Carlsson-Dent score and response to omeprazole as the diagnostic criteria in addition to other invasive tests. These parameters have been previously used as diagnostic criteria for GERD [29, 54–56]. Secondly, 24-h gastric and esophageal acid profile and histological assessment were not performed in all patients. Furthermore, estimation of gastric and esophageal acid profile after H. pylori eradication would have given a clearer effect of role of H. pylori in GERD.
In conclusion, our study shows that presence of H. pylori in patients with GERD was associated with less acidic stomach and milder esophagitis. Patients without H. pylori infection and higher age especially males are at a higher risk of developing EE.
References
El-Serag HB, Sonnenberg A. Opposing time trends of peptic ulcer and reflux disease. Gut. 1998;43:327–33.
Falk GW. GERD and H. pylori: is there a link? Semin Gastrointest Dis. 2001;12:16–25.
Holtmann G. Reflux disease: the disorder of the third millennium. Eur J Gastroenterol Hepatol. 2001;13 Suppl 1:S5–11.
Kahrilas PJ, Lee TJ. Pathophysiology of gastroesophageal reflux disease. Thorac Surg Clin. 2005;15:323–33.
Terry P, Lagergren J, Wolk A, Nyren O. Reflux-inducing dietary factors and risk of adenocarcinoma of the esophagus and gastric cardia. Nutr Cancer. 2000;38:186–91.
Holtmann G, Adam B, Liebregts T. Review article: the patient with gastro-oesophageal reflux disease–lifestyle advice and medication. Aliment Pharmacol Ther. 2004;20 Suppl 8:24–7.
El-Serag HB, Graham DY, Satia JA, Rabeneck L. Obesity is an independent risk factor for GERD symptoms and erosive esophagitis. Am J Gastroenterol. 2005;100:1243–50.
Ho KY, Cheung TK, Wong BC. Gastroesophageal reflux disease in Asian countries: disorder of nature or nurture? J Gastroenterol Hepatol. 2006;21:1362–5.
El-Omar EM. Mechanisms of increased acid secretion after eradication of Helicobacter pylori infection. Gut. 2006;55:144–6.
Falk GW. The possible role of Helicobacter pylori in GERD. Semin Gastrointest Dis. 2001;12:186–95.
Garrido Serrano A, Lepe Jimenez JA, Guerrero Igea FJ, Perianes Hernandez C. Helicobacter pylori and gastroesophageal reflux disease. Rev Esp Enferm Dig. 2003;95:788–90.
Fallone CA, Barkun AN, Friedman G, et al. Is Helicobacter pylori eradication associated with gastroesophageal reflux disease? Am J Gastroenterol. 2000;95:914–20.
Gisbert JP, Pajares JM, Losa C. Helicobacter pylori and gastroesophageal reflux disease: friends or foes? Hepatogastroenterology. 1999;46:1023–9.
Haruma K. Review article: influence of Helicobacter pylori on gastro-oesophageal reflux disease in Japan. Aliment Pharmacol Ther. 2004;20 Suppl 8:40–4.
Richter J. Do we know the cause of reflux disease? Eur J Gastroenterol Hepatol. 1999;11 Suppl 1:S3–9.
Wu JC, Sung JJ, Ng EK, et al. Prevalence and distribution of Helicobacter pylori in gastroesophageal reflux disease: a study from the East. Am J Gastroenterol. 1999;94:1790–4.
Cremonini F, Di Caro S, Delgado-Aros S, et al. Meta-analysis: the relationship between Helicobacter pylori infection and gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2003;18:279–89.
El-Omar EM, Penman ID, Ardill JE, Chittajallu RS, Howie C, McColl KE. Helicobacter pylori infection and abnormalities of acid secretion in patients with duodenal ulcer disease. Gastroenterology. 1995;109:681–91.
Vicari J, Falk GW, Richter JE. Helicobacter pylori and acid peptic disorders of the esophagus: is it conceivable? Am J Gastroenterol. 1997;92:1097–102.
Vicari JJ, Peek RM, Falk GW, et al. The seroprevalence of cagA-positive Helicobacter pylori strains in the spectrum of gastroesophageal reflux disease. Gastroenterology. 1998;115:50–7.
Chourasia D, Ghoshal UC. Pathogenesis of gastro-oesophageal reflux disease: what role do Helicobacter pylori and host genetic factors play? Trop Gastroenterol. 2008;29:13–9.
Samloff IM, Stemmermann GN, Heilbrun LK, Nomura A. Elevated serum pepsinogen I and II levels differ as risk factors for duodenal ulcer and gastric ulcer. Gastroenterology. 1986;90:570–6.
Monkemuller K, Neumann H, Nocon M, et al. Serum gastrin and pepsinogens do not correlate with the different grades of severity of gastro-oesophageal reflux disease: a matched case-control study. Aliment Pharmacol Ther. 2008;28:491–6.
Kwon JH, Chung IS, Son HS, et al. The relationship of gastrin, pepsinogen, and Helicobacter pylori in erosive reflux esophagitis. Korean J Gastroenterol. 2008;51:159–66.
Dockray GJ. Clinical endocrinology and metabolism. Gastrin. Best Pract Res Clin Endocrinol Metab. 2004;18:555–68.
Katelaris PH, Seow F, Lin BP, Napoli J, Ngu MC, Jones DB. Effect of age, Helicobacter pylori infection, and gastritis with atrophy on serum gastrin and gastric acid secretion in healthy men. Gut. 1993;34:1032–7.
Abe Y, Ohara S, Koike T, et al. The prevalence of Helicobacter pylori infection and the status of gastric acid secretion in patients with Barrett's esophagus in Japan. Am J Gastroenterol. 2004;99:1213–21.
Grande M, Cadeddu F, Villa M, et al. Helicobacter pylori and gastroesophageal reflux disease. World J Surg Oncol. 2008;6:74.
Ando T, El-Omar EM, Goto Y, et al. Interleukin 1B proinflammatory genotypes protect against gastro-oesophageal reflux disease through induction of corpus atrophy. Gut. 2006;55:158–64.
Saraswat VA, Dhiman RK, Mishra A, Naik SR. Correlation of 24-hr esophageal pH patterns with clinical features and endoscopy in gastroesophageal reflux disease. Dig Dis Sci. 1994;39:199–205.
Collins BJ, Elliott H, Sloan JM, McFarland RJ, Love AH. Oesophageal histology in reflux oesophagitis. J Clin Pathol. 1985;38:1265–72.
Carlsson R, Dent J, Bolling-Sternevald E, et al. The usefulness of a structured questionnaire in the assessment of symptomatic gastroesophageal reflux disease. Scand J Gastroenterol. 1998;33:1023–9.
Bilgen C, Ogut F, Kesimli-Dinc H, Kirazli T, Bor S. The comparison of an empiric proton pump inhibitor trial vs. 24-hour double-probe pH monitoring in laryngopharyngeal reflux. J Laryngol Otol. 2003;117:386–90.
Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172–80.
Campos GM, DeMeester SR, Peters JH, et al. Predictive factors of Barrett esophagus: multivariate analysis of 502 patients with gastroesophageal reflux disease. Arch Surg. 2001;136:1267–73.
Ghoshal UC, Ghosh TK, Ghoshal U, Shujaatullah F, Banerjee PK, Mazumder DN. In-house rapid urease test kit and commercial kit: which is better? Indian J Gastroenterol. 1999;18:183.
Ghoshal UC, Tiwari S, Dhingra S, et al. Frequency of Helicobacter pylori and CagA antibody in patients with gastric neoplasms and controls: the Indian enigma. Dig Dis Sci. 2008;53:1215–22.
Ghoshal UC, Chourasia D, Tripathi S, Misra A, Singh K. Relationship of severity of gastroesophageal reflux disease with gastric acid secretory profile and esophageal acid exposure during nocturnal acid breakthrough: a study using 24-h dual-channel pH-metry. Scand J Gastroenterol. 2008;43:654–61.
Gisbert JP, de Pedro A, Losa C, Barreiro A, Pajares JM. Helicobacter pylori and gastroesophageal reflux disease: lack of influence of infection on twenty-four-hour esophageal pH monitoring and endoscopic findings. J Clin Gastroenterol. 2001;32:210–4.
Zerbib F, Bicheler V, Leray V, Joubert M, Bruley des Varannes S, Galmiche JP. H. pylori and transient lower esophageal sphincter relaxations induced by gastric distension in healthy humans. Am J Physiol Gastrointest Liver Physiol. 2001;281:G350–6.
Goldschmiedt M, Barnett CC, Schwarz BE, Karnes WE, Redfern JS, Feldman M. Effect of age on gastric acid secretion and serum gastrin concentrations in healthy men and women. Gastroenterology. 1991;101:977–90.
Moschos J, Kouklakis G, Lyratzopoulos N, Efremidou E, Maltezos E, Minopoulos G. Gastroesophageal reflux disease and Helicobacter pylori: lack of influence of infection on oesophageal manometric, 3-hour postprandial pHmetric and endoscopic findings. Rom J Gastroenterol. 2005;14:351–5.
Haruma K, Kamada T, Kawaguchi H, et al. Effect of age and Helicobacter pylori infection on gastric acid secretion. J Gastroenterol Hepatol. 2000;15:277–83.
Derakhshan MH, El-Omar E, Oien K, et al. Gastric histology, serological markers and age as predictors of gastric acid secretion in patients infected with Helicobacter pylori. J Clin Pathol. 2006;59:1293–9.
Labenz J, Jaspersen D, Kulig M, et al. Risk factors for erosive esophagitis: a multivariate analysis based on the ProGERD study initiative. Am J Gastroenterol. 2004;99:1652–6.
Sipponen P, Vauhkonen M, Helske T, Kaariainen I, Harkonen M. Low circulating levels of gastrin-17 in patients with Barrett's esophagus. World J Gastroenterol. 2005;11:5988–92.
Dixon MF. Pathophysiology of Helicobacter pylori infection. Scand J Gastroenterol Suppl. 1994;201:7–10.
Richter JE, Falk GW, Vaezi MF. Helicobacter pylori and gastroesophageal reflux disease: the bug may not be all bad. Am J Gastroenterol. 1998;93:1800–2.
Kahrilas PJ. GERD revisited: advances in pathogenesis. Hepatogastroenterology. 1998;45:1301–7.
Kahrilas PJ. The role of hiatus hernia in GERD. Yale J Biol Med. 1999;72:101–11.
Kim N, Lee SW, Cho SI, et al. The prevalence of and risk factors for erosive oesophagitis and non-erosive reflux disease: a nationwide multicentre prospective study in Korea. Aliment Pharmacol Ther. 2008;27:173–85.
Tsukada K, Katoh H, Miyazaki T, et al. Factors associated with the development of reflux esophagitis after Helicobacter pylori eradication. Dig Dis Sci. 2006;51:539–42.
Moon A, Solomon A, Beneck D, Cunningham-Rundles S. Positive association between Helicobacter pylori and gastroesophageal reflux disease in children. J Pediatr Gastroenterol Nutr. 2009;49:283–8.
Schenk BE, Kuipers EJ, Klinkenberg-Knol EC, et al. Omeprazole as a diagnostic tool in gastroesophageal reflux disease. Am J Gastroenterol. 1997;92:1997–2000.
Fass R, Fennerty MB, Johnson C, Camargo L, Sampliner RE. Correlation of ambulatory 24-hour esophageal pH monitoring results with symptom improvement in patients with noncardiac chest pain due to gastroesophageal reflux disease. J Clin Gastroenterol. 1999;28:36–9.
Chourasia D, Achyut BR, Tripathi S, Mittal B, Mittal RD, Ghoshal UC. Genotypic and functional roles of IL-1B and IL-1RN on the risk of gastroesophageal reflux disease: the presence of IL-1B-511*T/IL-1RN*1 (T1) haplotype may protect against the disease. Am J Gastroenterol. 2009;104:2704–13.
Acknowledgements
This work was supported by an extramural research grant from Department of Biotechnology (No. BT/PR10141/MED/30/65/2007), Govt. of India to Dr Uday C Ghoshal.
Dipti Chourasia received a fellowship from Indian Council of Medical Research (ICMR), New Delhi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chourasia, D., Misra, A., Tripathi, S. et al. Patients with Helicobacter pylori infection have less severe gastroesophageal reflux disease: a study using endoscopy, 24-hour gastric and esophageal pH metry. Indian J Gastroenterol 30, 12–21 (2011). https://doi.org/10.1007/s12664-010-0078-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12664-010-0078-0