Abstract
Introduction
Oral cancer is one of the most common cancers and it constitutes a major health problem particularly in developing countries. It is one of the leading causes of death. Tobacco and alcohol consumption appears to be the major determinants of oral cancer.
Materials and methods
The literature search was carried out in NCBI Pubmed database using keywords “oral cancer”, “risk factor”, “epidemiology” and “patho*”. Some basic information was also obtained from textbook and medical university websites.
Results
Several risk factors have been well characterized to be associated with oral cancer with substantial evidences. The development of oral cancer is a multistep process involving the accumulation of genetic and epigenetic alterations in key regulatory genes. Experimental pathological studies of oral cancer in animal models and direct molecular genetic analysis of oral cancer subjects in recent times have revealed a substantial amount of knowledge on specific gene alterations or other genetic mechanisms involved in initiation and subsequent progression.
Conclusion
Considering known risk factors, oral cancer appears to be to a certain extent, a preventable disease. Recent development of molecular picture of pathoprogression and molecular genetic tools opens the avenue for easier diagnosis, better prognostication and efficient therapeutic management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral cancer (OC) is the commonest cancer in India, accounting for 50–70% of total cancer mortality and accounts for highest incidence among Asian countries [1]. OC is the sixth most common cancer worldwide [2]. It affects anterior tongue, cheek, floor of mouth, gingiva or any other part of the oral cavity. Worldwide, there is a great variation in the incidence of cancer of the oral cavity. It accounts for less than 5% of all cancers in United States, Western Europe and Australia. India, few pockets in France, Brazil, central and eastern Europe have few of the highest rates of cancer of the oral cavity in the world. The differing social customs are likely to be responsible for regional variations in the disease incidence. The high rate of OC in France and Eastern Europe has historically been linked to the heavy consumption of alcohol and tobacco in these countries. The habit of chewing betel nut leaves rolled with lime and tobacco, a mixture known as pan, results in prolonged contact of the carcinogen with the buccal mucosa, which is thought to be the principal cause of OC in India.
The incidence of OC is directly correlated with age of subjects. Rates rise dramatically after the age of 40–49 years, and reach a plateau around the age of 70–79 years (Fig. 1). Due to increase in ageing population in majority countries world-wide, even the current age-specific rates will be more than sufficient to tilt the balance towards an increase in adult population with higher risk of OC. OC is more frequent in men than women, and depending on its location within the oral cavity, males are two to six times more likely to be affected than females, largely owing to their higher intake of alcohol and tobacco.
Incidence of oral cancer in cases per 100,000 among different age group (Source: Oral Cancer Incidence by Age, Race, and Gender. Data from National Institute of Dental and Craniofacial Research, accessed from http://www.nidcr.nih.gov/)
Etiology and Major risk Factors
Numerous risk factors or possible causative agents for OC have been described. Chemical factors like tobacco and alcohol, biological factors like human papillomavirus (HPV), syphilis, oro-dental factors, dietary deficiencies, chronic candidiasis and viruses have been shown to be significantly associated with OC.
Chemical Factors
Tobacco
There are ample evidences suggesting that tobacco in various forms, including smoking, chewing and in betel quid etc., have carcinogenic impact in oral cavity. The commonest form of tobacco use is smoking. The various forms in which tobacco is used as smoke are- cigarettes, cigars, pipe and bidi etc. Hookah or chillum (a clay pipe used to keep the burning tobacco) are other common forms of smoking in some countries of Asia including India. In some part of India like Mizoram, tobacco smoke is dissolved in water (“smoke on the water”) which is another peculiar form of tobacco use.
Alcohol
Numerous studies have suggested alcohol to be a major risk factor for OC. There is a certain degree of controversy whether alcohol alone may have carcinogenic impact. This is due to simultaneous tobacco and alcohol intake of study subjects in various epidemiological studies. Studies have shown that individuals consuming more than 170 g of whisky daily have ten times higher risk of OC than the light drinkers [3]. Alcohol may have additive effect and it has been suggested that it facilitates the entry of carcinogens into the exposed cells, altering the metabolism of oral mucosal cells [4]. However, the current evidences do not suggest that pure ethanol alone is carcinogen for the development of OC.
Biological Factors
Viruses
Role of oncogenic viruses in human cancer is an emerging area of research. Viruses are capable of hijacking host cellular apparatus and modifying DNA and the chromosomal structures and inducing proliferative changes in the cells. HPV [5] and Herpes simplex virus (HSV) have been established in recent years as causative agents of OC.
HPV has been identified in approximately 23.5% of OC cases [6]. The most commonly detected HPV in head and neck squamous cell carcinoma (HNSCC) is HPV-16, which has been demonstrated in 90–95% of all HPV positive HNSCC cases, followed by HPV-18, HPV-31, and HPV-33. The prognostic significance of HPV in pre-cancerous oral lesion is not clear. However, few studies have found improved disease-specific survival and better prognosis for HPV positive OC.
HSV-1 or "oral herpes" is commonly associated with sores around the mouth and lip and has been suggested to be a causative agent of OC [7]. Epidemiological studies showed higher level of IgG and IgM antibodies to OC patients compared to control subjects [8]. Kassim et al [9] also reported oncogenic relationship between HSV-1 and oral squamous cell carcinoma (OSCC). A population based study showed HSV-1 to enhance development of OSCC in HPV infected patients and individuals with history of cigarette smoking [10]. Risk of oral cavity and pharyngeal cancer is two-fold higher among human immunodeficiency virus (HIV) patients indicating a link between HIV and OSCC [11, 12]. Epstein Barr Virus (EBV), human herpesvirus-8 (HHV-8) and cytomegalovirus have also been reported as risk factors of OSCC in different studies [13, 14].
Syphilis
The data on causal association between syphilis and OC is weak. There are reports of 19 [15] and 6% [16] serological positivity for syphilis among tongue cancer patients.
Candida
Candida has been suggested to play a role in initiation of OC. Clinical studies have reported that nodular leukoplakia infected with Candida has a tendency for higher rate of dysplasia and malignant transformation. It has also been shown that epithelium of the chick embryo, when infected with Candida albicans show squamous metaplasia and higher proliferative phenotype [17]. The causal association of Candida infection and OC is still controversial and demands further proof.
Dental Hygiene and Related Factors
There is inverse association between oral hygiene and incidence of OC. Poor oral hygiene and prolonged irritation from sharp teeth have been viewed for their possible role in the development of OC. Poor oral hygiene and dental sepsis is thought to promote carcinogenic action of tobacco [18]. There are several scattered reports on the role of oro-dental factors in the causation of OC, but the hypothesis still lacks major evidence.
Nutritional Factors
Dietary deficiencies are also suggested to play a role in the development of OC. This, however, requires more clinical and experimental evidence for establishment of causal association with the development of OC. Some workers have reported lower risk of OC with higher intake of fruits and vegetables [19].
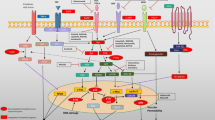
Molecular Pathogenesis of Oral Cancer
Oral carcinogenesis like any other cancer is a progressive disease and normal epithelium passes through stages starting from dysplasia to finally transforming into invasive phenotypes. Although all types of carcinomas are seen in oral cavity, the most common form of OC is squamous cell carcinoma. Use of genetic and proteomic approach in recent years have revealed the molecular pathological picture of OC. There is active search to identify genetic alterations in oncogenes or tumour suppressor genes, role of genomic instability and epigenetic modifications and to generate a gene expression profile in oral oncogenesis [20]. Understanding these genetic changes and gene expression patterns are keys to the understanding of molecular pathogenesis of OC. Though, there are some significant leads achieved, the complete understanding of molecular pathology of OC and its association with causative agent will require another decade of intensive research. We have discussed some of the important updates in this area of active research.
Genetic Susceptibility
It is now established that up to 10% of all cancers have a strong hereditary component. Role of genetic component in the development of OC is being suggested by several studies showing familial clustering [21]. A clustering of OC has been seen in certain ethnic groups, like Askenazi group in Israel; with incidence being double as compared to other Jewish population in that country. However, the basis of this genetic susceptibility is not well understood, as yet.
Evaluation of specific genetic polymorphism in key genes involved in oral carcinogenesis has been the major area of study. Glutathione S-transferase M1 (GSTM1) null genotype appears to be the most consistent polymorphic susceptibility marker for head and neck cancer including OC. Meta-analyses by Tripathy and Roy showed that the GSTM1 null genotype conferred a 20–50% significantly increased HNSCC risk [22].
The variant val allele of the CYP1A1 (Cytochrome P450, family 1, member A1) polymorphism is another fairly consistent susceptibility marker with a 35% increased risk in a meta- analysis of 12 studies [21]. The studies on many other gene polymorphisms have been inconclusive. Brennan et al. [23] found that ALDH1B and ALDH2 (Aldehyde dehydrogenase 2) genes were associated with HNSCC and showed significant correlation with alcohol consumption.
Proto-oncogenes, Oncogenes and Genetic Alterations:
Genetic alterations define molecular basis of carcinogenesis which includes point mutations, amplifications, rearrangements, and deletions. Several oncogenes have also been implicated in oral carcinogenesis [20]. Aberrant expression of epidermal growth factor receptor (EGFR), K-ras, c-myc, int-2, Parathyroid adenomatosis 1 (PRAD-1) and B-cell lymphoma (bcl) like oncogenes have been reported in OC development [24]. Over expression and amplification of cellular oncogene EGFR have been reported in a 7,12-Dimethylbenz(a)anthracene (DMBA) induced hamster cheek pouch malignant OC model [25]. Transforming growth factor-alpha (TGF-α) is known to promote neovascularization and mitogenesis. It has been shown to be aberrantly expressed in human OC and in hamster oral tumor [26].
Tumor Suppressor Genes
More than 50% of all primary HNSCC harbour p53 mutation [27]. Inactivation of p53 represents the most common genetic change in all human cancers [28]. The most commonly deleted region in HNC is located at chromosome 9p21–22 [29]. Loss of chromosome 9p21 occurs in the majority of invasive tumors in head and neck cancer [30]. Homozygous deletions in this region are frequent and represent one of the most common genetic changes identified. p16 (CDKN2) present in this deleted region, is a potent inhibitor of cyclin D1 [31]. Loss of p16 protein has been observed in most advanced pre-malignant lesions also [32]. Mayo et al. [33] have identified an alternative RNA transcript for p16 termed as Alternative Rating Frame (ARF; or p16β). Introduction of p16 or p16ARF into HNC cell lines result in potent growth suppression [34].
Loss of chromosome 17p is also frequent in most human cancer including OC. It is seen in approximately 60% of invasive lesions. Although p53 inactivation correlates closely with loss of 17p in invasive lesions, p53 mutations are quite rare in early lesions that contain 17p loss. Loss of chromosome arm 10 and 13q are also noted in primary tumors [35].
Many other regions of chromosomal loss have been seen in OC. Further, fine mapping of these critical genes within these areas may provide important information in the understanding of genomic instability leading to the development of this neoplasm.
Genomic Instability
Genomic instability such as loss of hetrozygosity (LOH) and microsatellite instability (MSI) are frequently observed in cancer and such instability has been investigated and several reports are available in OC. Chromosome 9p21 containing p16 tumor suppressor gene is frequently lost in HNSCC and oral preneoplastic lesions [30, 36]. Chromosome 3p14 contains the tumor suppressor gene fragile histidine triad (FHIT) as well as a common fragile site, FRA3B which is also found to be frequently deleted in early tumorigenesis [37] and its deletion is associated with exposure to cigarette smoke [38]. Loss of chromosome 17p13 harboring p53 tumour suppressor gene is also common in multistep head and neck tumorigenesis [39]. Loss of function of the tumour suppressor p53 can result in uncontrolled cell division and progressive genomic instability [40]. The increased frequency of LOH in invasive tumours at the 9p21 locus is also reported and may suggest that the region, probably the p16 gene is important in early malignant progression. Several studies have demonstrated these by using microsatellite markers. Alterations in certain regions of chromosomes 3p, 9p, 17p and 18q are associated with the development of HNSCC [41]. Nunes et al. [42] performed a microsatellite analysis of cells sampled from the oral cavity of oral and oro-pharyngeal cancer patients and observed LOH in 84% of samples. In another study, Spafford et al. [43] identified genomic alterations in all of the malignant lesions of the oral cavity included in their sample.
Epigenetic Alterations
The major epigenetic modification in tumours is methylation. Changes in the methylation patterns can play an important role in tumorigenesis. Epigenetic modifications are frequently connected with the loss of genetic expression and important for the multiple indispensable genetic events during carcinogenesis. Malignant progression takes place because these alterations can inactivate DNA repairing genes.
Methylation patterns of p16, methylguanine-DNA methyltransferase (MGMT) and Death-associated protein kinase (DAP-K) genes in smears of patients suffering from head and neck cancer showed abnormal hypermethylation patterns by a methylation specific polymerase chain reaction (PCR) [43].
Molecular Progression Model
Califano et al. [41] tested ten most common allelic events in a large number of primary pre-invasive lesions and invasive HNSCC to develop a molecular progression model. It involves inactivation of many putative suppressor gene loci. Chromosomes 9p and 3p appear to be lost early, closely followed by loss of 17p. Mutations in p53 gene are seen in the progression of pre-invasive to invasive lesions. Many other genetic events occur later during progression. Other genetic events, such as amplification of cyclin D1 and inactivation of p16 have been tested predominantly in invasive lesions, but their precise order in the model was not determined [44].
Molecular Epidemiology
The pattern of specific gene mutation in OC patient may give a clue to the aetiology of that particular tumor. Brennan et al. [27] analyzed the pattern of p53 mutation in HNSCC. They found that the incidence of p53 mutation was much higher in patients who were exposed to both tobacco and alcohol versus non-users.
It has been suggested that alcohol appears to augment the effect of smoking due to an increase in the absorbance of carcinogens contained within the cigarette smoke. Several epidemiologic evidences suggest that abstinence from cigarette smoking may decrease the overall incidence of HNSCC [45].
HPV positive oral and oro-pharyngeal cancer comprise a distinct clinico-pathological entity. They are less likely to occur among heavy smokers and drinkers, have lesser likelihood of p53 mutation and have better cancer-specific survival. It has been suggested that HPV positive tumours may have better prognosis by inactivating retinoblastoma (Rb) [46].
Conclusions
Extensive case-control and longitudinal studies have implicated tobacco and alcohol as major risk factor for OC. That is why widespread prevention and control programs are being designed. Efforts are also going on to understand the initiation and progression of OC by designing laboratory models. Increasing knowledge of molecular genetic alterations in OC has led to a better understanding of molecular pathways in the development of OC. This new knowledge is expected to generate new lead for prevention, early diagnosis and devising new therapy for OC. A robust progress has been made in our understanding of molecular basis of oral carcinoma, but there is lot more to be understood.
References
Khandekar SP, Bagdey PS, Tiwari RR (2006) Oral cancer and some epidemiological factors: a hospital based study. Indian J Community Med 31(3):157–159
Boring CC, Squires TS, Tong T (1992) Cancer statistics, 1992. CA Cancer J Clin 42(1):19–38
Wynder EL, Bross IJ, Feldman RM (1957) A study of the aetiological factors in cancer of the mouth. Cancer 10(6):1300–1323
McCoy GD (1978) A biochemical approach to the aetiology of alcohol related cancers of the head and neck. Laryngoscope 88:59–62
Ha PK, Califano JA (2004) The role of human papillomavirus in oral carcinogenesis. Crit Rev Oral Biol Med 15(4):188–196
Kreimer AR, Clifford GM, Boyle P, Franceschi S (2005) Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 14(2):467–475
Schildt EB, Eriksson M, Hardell L, Magnuson A (1998) Oral infections and dental factors in relation to oral cancer: a Swedish case—control study. Eur J Cancer Prev 7(3):201–206
Shillitoe EJ, Greenspan D, Greenspan JS, Hansen LS, Silverman S Jr (1982) Neutralising antibody to Herpes simplex virus type 1 in patients with oral cancer. Cancer 49(11):2315–2320
Kassim KH, Daley TD (1988) Herpes simplex virus type 1 proteins in human oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol 65(4):445–448
Starr JR, Daling JR, Fitzgibbons ED, Madeleine MM, Ashley R, Galloway DA, Schwartz SM (2001) Serologic evidence of herpes simplex virus 1 infection and oropharyngeal cancer risk. Cancer Res 61(23):8459–8464
Chidzonga MM (2003) HIV/AIDS orofacial lesions in 156 Zimbabwean patients at referral oral and maxillofacial surgical clinics. Oral Dis 9(6):317–322
Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM (2007) Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 370(9581):59–67
Al Moustafa AE, Chen D, Ghabreau L, Akil N (2009) Association between human papillomavirus and Epstein-Barr virus infections in human oral carcinogenesis. Med Hypotheses 73(2):184–186
Atula T, Grénman R, Klemi P, Syrjänen S (1998) Human papillomavirus, Epstein-Barr virus, human herpesvirus 8 and human cytomegalovirus involvement in salivary gland tumours. Oral Oncol 34(5): 391–395
Trieger N, Ship II, Taylor GW, Weisberger D (1958) Cirrhosis and other predisposing factors in carcinoma of the tongue. Cancer 11(2):357–362
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics-2002. CA Cancer J Clin 55(2):74–108
Cawson RA, Binnie WH (1980) Candida, leukoplakia and carcinoma: a possible relationship. In: Mackenzie IC, Dabelsteen E, Squier CA (eds) Oral premalignancy, 1st edn. University of Iowa Press, Iowa city, pp 59–66
Wahi PN, Kehar U, Lahiri B (1965) Factors influencing oral and oropharyngeal cancer in India. Br J Cancer 19(4):642–660
Notani PN, Sanghvi LD (1976) Role of diet in the cancer of the oral cavity. Indian J Cancer 13(2):156–160
Williams HK (2000) Molecular pathogenesis of oral squamous carcinoma. Mol Pathol 53(4):165–172
Jefferies S, Eeles R, Goldgar D, A’Hern R, Henk JM, Gore M et al (1999) The role of genetic factors in predisposition to squamous cell cancer of the head and neck. Br J Cancer 79(5–6):865–867
Tripathy CB, Roy N (2006) Meta analysis of glutathione S-transeferase M1 genotype and risk toward head and neck cancer. Head Neck 28(3):217–224
Brennan P, Lewis S, Hashibe M, Bell DA, Botteffa D, Bouchardy C et al (2004) Pooled analysis of alcohol dehydrogenase genotypes and head and neck cancer—review. Am J Epidemiol 159(1):1–16
Sidransky D (1995) Molecular genetics of head and neck cancer. Curr Opin Oncol 7:229–233
Wong DT, Biswas DK (1987) Expression of c-erb B proto-oncogene during dimethylbenzanthracene induced tumorigenesis in hamster cheek pouch. Oncogene 2(1):67–72
Wong DT, Gallagher GT, Gertz R, Chang ALC, Shklar G (1988) Transforming growth factor-α in chemically transformed hamster oral keratinocytes. Cancer Res 48(11):3130–3134
Brennan JA, Boele JO, Koch WM, Goodman SN, Hruban RH, Eby YJ et al (1995) Association between cigarette smoking and mutation of the p53 gene in head and neck squamous cell carcinoma. N Engl J Med 332(11):712–717
Hollstien M, Sidransky D, Volgelstein B, Harris CC (1991) p53 mutations in human cancer. Science 253(5015):49–53
Nawroz H, van der Riet P, Hruban RH, Koch W, Ruppert JM, Sidransky D (1994) Allelo type of head and neck squamous cell carcinoma. Cancer Res 54(5):1152–1155
Van der Riet P, Nawroz H, Hruban RH, Coria R, Tokino K, Koch W et al (1994) Frequent loss of chromosome 9p21–22 in head and neck cancer progression. Cancer Res 54:1156–1158
Kamb A, Gruis NA, Weavar-Feldhaus J, Liu Q, Harshman K, Tavtigian SV et al (1994) A cell cycle regulator potentially involved in genesis of many tumor types. Science 264(5157):436–440
Papadimitrakopoulou V, Izzo J, Lippman SM, Lee JS, Fan YH, Clayman G et al (1997) Frequent inactivation of p16ink4a in oral premalignant lesions. Oncogene 14(15):1799–1803
Mao L, Merlo A, Bedi G, Shapiro GI, Edwars CD, Rollins BJ et al (1995) A noval p16 INK 4A transcript. Cancer Res 55(14):2995–2997
Ligget WH Jr, Sewell DA, Rocco J, Ahrendt SA, Koach W (1996) Sidransky. P16 and p16beta are potent growth suppressors of head and neck squamous carcinoma cells in vitro. Cancer Res 56(18):4119–4123
Shao X, Tandon R, Samara G, Kanki H, Yano H, Close LG et al (1998) Mutational analysis of PTEN in head and neck squamous cell carcinoma. Int J Cancer 77(5):684–688
Bishop JM (1987) The molecular genetics of cancer. Science 235(4786):305–311
Mao L, Lee JS, Fan YH, Ro JY, Batsakis JG, Lippman S et al (1996) Frequent microsatellite alterations at chromosome 9p21 and 3p14 in oral premalignant lesions and their value in cancer risk assessment. Nat Med 2(6):682–685
Tseng JE, Kemp BL, Khuri FR, Kurie J, Lee JS, Zhou X et al (1999) Loss of Fhit is frequent in stage I non small cell lung cancer and in the lung of chronic smokers. Cancer Res 59(19):4798–4803
Boyle JO, Hakim J, Koch W, van der Riet P, Hruban RH, Roa RA et al (1993) The incidence of p53 mutations increases with progression of head and neck cancer. Cancer Res 53(19):4477–4480
Kirsch DG, Kastan MB (1998) Tumor-suppressor p53: implications for tumor development and prognosis. J Clin Oncol 16(9):3158–3168
Califano J, Van der Riet P, Westra W, Nawroz H, Clayman G, Piantadosi S et al (1996) Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res 56(11):2488–2492
Nunes DN, Kowalski LP, Simpson AJ (2000) Detection of oral and oropharyngeal cancer by microsatelite analysis in mouth washes and lesions brushings. Oral Oncol 36(6):525–528
Spafford MF, Koch WM, Reed AL, Califano JA, Xu LH, Eisenberger CF et al (2001) Detection of head and neck squamous cell carcinoma among exfoliated oral mucosal cells by microsatellite analysis. Clin Cancer Res 7(3):607–612
Rosas SL, Koch W, da Costa Carvalho MG, Wu L, Califano J et al (2001) Promoter hypermethylation patterns of p16, O6-methylguanine-DNA methyltransferase, and death-associated protein kinase in tumors and saliva of head and neck cancer patients. Cancer Res 61(3):939–942
Devita VT Jr, Lawrence TS, Rosenberg SA (eds) (2008) Cancer principals and practice of oncology, vol 1, 8th edn. Lippincott-Williams and Wilkins, Philadelphia, pp 799–808
Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L et al (2000) Evidence of a causal association between Human papilloma virus and a subset of head and neck cancers. J Natl Cancer Inst 92(9):709–720
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ram, H., Sarkar, J., Kumar, H. et al. Oral Cancer: Risk Factors and Molecular Pathogenesis. J. Maxillofac. Oral Surg. 10, 132–137 (2011). https://doi.org/10.1007/s12663-011-0195-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12663-011-0195-z