Abstract
Purpose
This study assessed novel keratinolytic bacteria for feather valorization to yield feather hydrolysates (FHs) to cultivate vegetables.
Methods
Bacterial degradation of feather was determined through production of keratinase. Corchorus olitorius (Jute mallow), Celosia argentea (Cockscomb), and Amaranthus caudatus (Pendant amaranth) were grown with FHs through soil-less and pot experiments. Nitrogen and amino acid contents of FHs were determined, and the effects on soil microbes and fertility were studied.
Results
Aquamicrobium defluvii and Bacillus safensis degraded feather with keratinolytic activities of 45.2 and 56.7 U/ml, respectively. FHs improved seed germination by 1.29–1.67 folds and vigor index by 2.13–3.87 folds. In pot experiments, 50–100% FHs improved growth over control, while 100% FHs performed better than NPK in C. olitorius and C. argentea. In A. caudatus, NPK performed comparatively with 100% FHs. The FHs had nitrogen contents of 12.60–13.16% with abundance of glycine, alanine and leucine. FHs improved soil nitrogen content (27.91–37.21%) and organic carbon (24.11–29.46%) at 28 days, and stimulated soil microbial growth by 101–102.
Conclusion
The significant plant growth promotion and improved soil fertility demonstrated by FHs suggest their potential application as biofertilizers to promote sustainable agricultural production. To our knowledge, this represents first report of keratinolytic strain of Aquamicrobium defluvii and use of FHs to promote growth of C. olitorius, A. caudatus and C. argentea.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
The two bacterial isolates degraded chicken feather to produce nitrogen and amino acid-rich feather hydrolysates, which enhanced growth of vegetables and improved soil fertility. The keratinolytic Aquamicrobium defluvii being reported here for the first time adds to the growing list of bacteria that can effectively valorize poultry feather in producing feather hydrolysates. The work is impactful as it showed potential applications of the feather hydrolysates to engender sustainable vegetable production. Herein, the use of feather hydrolysates to promote growth of Corchorus olitorius, Celosia argentea, and Amaranthus caudatus is reported for the first time.
Introduction
Agricultural food production currently depends largely on the use of fertilizer for the purpose of enhancing food production to feed the rapidly growing world population, and at the same time to boost the production of healthy foods like fruits and vegetables, whose demand is on the rise over the past two decades [1]. The use of chemical fertilizers is being discouraged in modern agricultural practices due to their high production cost and environmental problems associated with their production and application. Excessive use of chemical fertilizers is not only cost intensive but also leads to their gradual entry into water systems through rain water and seepage to contaminate groundwater, which can induce serious risks to human and animal health [2, 3]. However, replacement of chemical fertilizers with eco-friendly biofertilizers or slow release nitrogenous organic fertilizers could be a better option to increase agricultural productivity [4] within the scope of a more sustainable development.
Several organic wastes have been investigated and utilized as fertilizers and for soil enhancement [5]. Microbiological transformation of organic nitrogenous wastes under controlled conditions yields products of high economic value, some of which are used to improve soil properties for cultivation of plants and as important ingredient for animal feed formulations [6]. Apart from obtaining products of commercial value, the practice is also considered as a suitable approach for waste management and recycling [7, 8], which promotes bio- and circular economy [9, 10]. Among such wastes that can be valorized into high-end products are poultry feathers that are known to be rich in structural proteins [11, 12].
The consumption of chicken in human diet is on the increase in recent times, which has led to the environmental discharge of closely 8.5 million tons of poultry feather wastes annually from the poultry meat production [13]. In Nigeria, about 932.5 metric tons of poultry wastes are discharged annually from various poultry farms. The disposal, utilization and management of poultry wastes have always been a serious concern due to their high recalcitrant keratin content [14]. Feather contains approximately 15% nitrogen on a dry weight basis and its recalcitrant nature results into slow rate of decomposition and nitrogen mineralization in the soil [13]. The stability of keratin and its resistance to microbial degradation are due to the tight packing of the protein chain either in α-helix (hair α-keratin) or β-sheet (feather β-keratin) structures [15, 16].
The traditional physical and chemical methods for the treatment of feathers such as burning, land filling, steam pressure cooking and strong alkali or acid hydrolysis are laborious, expensive, non-ecofriendly and damage their biotechnological values. Hence, the development of ecofriendly method for their degradation that will ensure the production of products of high economic value becomes imperative [17]. Biodegradation of feathers by keratinolytic microorganisms is an efficient, cost-effective, and environmental friendly method for bioconversion of feather wastes into nutritionally rich feather hydrolysates [18] and production of multi-applicable keratinases [11]. The technology has been considered as a biotechnological means of keratinous wastes valorization. Feather hydrolysate (FH) is rich in ammonia, peptides, amino acids and other important products [19]. It can act as slow release nitrogen (N) organic fertilizer to boost agricultural food production [20, 21]. It can stimulate soil microbial activity which will in turn facilitate the assimilation of nutrients by plants for better growth [22].
Vegetables are edible parts of certain plants that include leaf, stem and roots that are usually cooked or eaten raw [23], and are indispensable components of balanced human diet [24] due to their nutritional importance and health-promoting abilities [25]. They are good sources of fiber, minerals and vitamins and WHO has recommended consumption of 400 g/day of fruits and vegetables to prevent non-communicable diseases [26] such as cancer, heart attack, stroke, hypertension, birth defects, cataracts and diabetes [24]. There are different strategies to improve the phytonutrient constituents of vegetables and their bioactivities and these include nanoagriculture and organic farming [6, 25, 27, 28]. Vegetables and fruits are so important that the year 2021 was declared as the international year of fruits and vegetables (IYFV) by General Assembly of the United Nations [29] to raise awareness about the benefits of fruits and vegetables to promote good health and wellbeing towards achieving the SDGs of UN [30].
In Nigeria, among the widely cultivated and consumed vegetables are Corchorus olitorius (Jute mallow), Celosia argentea (Cockscomb), and Amaranthus caudatus (Pendant amaranth). The leaf of jute mallow is the edible portion, while the shoots of cockscomb and pendant amaranth are eaten when cooked. They are annual vegetables that mature in 4–6 weeks. The vegetables are consumed when cooked for their nutritional values and may also be exploited for ethnobotanical purposes. For instance, amaranth can be used to treat diabetes and inflammation, while also possessing immunomodulatory, anthelmintic, anti-androgenic, diuretic and laxative properties [31]. Corchorus olitorius is the most important vegetable in southwestern Nigeria [32] and it is useful in the treatment of fever, anaemia, chronic cystitis, cold and tumours [33]. Celosia argentea also possesses antipyretic, anti-inflammatory and antioxidant activities and often used to treat gastrointestinal disorders [34]. Being part of the staple food in Nigeria, the production of these vegetables needs to be enhanced for their numerous nutritional and medicinal benefits.
Feather valorization is a continuous biotechnological endeavour, whereby new microorganisms are sought for degradation of the keratin to produce high-end products. Recently, we reported the first demonstration of Bacillus safensis to degrade feather, efficient production of multifunctional keratinase for destaining, dehairing and fabrication of nanoparticles [34,35,36,37,38,39]. Therefore, the present study was aimed at utilizing novel keratinolytic bacterial isolates to degrade feather to produce feather hydrolysates that can be deployed as biofertilizers in the cultivation of some widely consumed vegetables in Nigeria; namely Corchorus olitorius, Celosia argentea and Amaranthus caudatus. Herein, we report the first isolation of keratinolytic strain of Aquamicrobium defluvii that efficiently degraded chicken feather to produce nutritionally rich feather hydrolysate. Till date, there is no report on the evaluation of feather hydrolysates on the growth of Corchorus olitorius, Celosia argentea and Amaranthus caudatus.
Materials and Methods
Preparation of Substrates and Media
Chicken feather wastes and soil samples were obtained from feather dump site of LAUTECH Teaching and Research poultry farm, Ogbomoso, Nigeria. The soil sample was collected in a clean polythene bag and stored under ambient conditions until further use. Feathers were washed using tap water to remove soil and debris, and the clean feathers obtained were dried in hot air oven at 75 °C. The dried feathers were chopped into pieces, milled and then sieved using 60-mesh particle size. The feather powder was kept under ambient conditions for further studies. The minimal medium for the isolation of keratinolytic bacteria was compounded from the keratin powder as follows (g/l): NaNO3, 2; NaCl, 2; KH2PO4, 2; MgSO4, 0.05; FeSO4.7H2O, 0.1; CaCO3, 0.1; keratin substrate, 20; and agar–agar, 20. The medium was sterilized at 121 °C for 15 min, and then fortified with 0.05 g/l of sterile nystatin to inhibit fungal growth [35].
Isolation of Keratinolytic Bacteria
The keratinolytic Bacillus safensis LAU 13 previously reported for the degradation of feather [35] was obtained from the culture collection of Laboratory of Industrial Microbiology and Nanobiotechnology, LAUTECH, Ogbomoso. New keratinolytic bacterial strain was isolated from soil sample collected from the same site where the feather wastes were obtained. One gram of soil sample was dispersed in 9 ml of sterile distilled water. Aliquot of 0.5 ml was inoculated into the minimal medium for the selective growth of bacterial isolates using the pour plate method; the plates were incubated at 37 °C for up to 3 days. Thereafter, distinct colonies observed through morphological features were sub-cultured on yeast extract agar plates to obtain pure cultures. The pure cultures were stored on agar slants of yeast extract agar and minimal selective medium for further use [35].
Biochemical and Molecular Characterization of the Isolate
The biochemical tests, viz., Gram staining, motility, indole production, methyl red, Voges Proskauer, citrate utilization, sugar utilization, spore staining, catalase, oxidase, coagulase, urease, hydrogen sulphide, and hydrolysis of starch was carried out according to the methods of Brenner et al. [40]. The molecular characterization of the best newly isolated bacterium was carried out using 16S rRNA analysis. Genomic DNA was isolated from pure culture of the bacterial isolate using the Quick-DNA™ Fungal/Bacterial Miniprep Kit (Zymo Research, Catalogue No. D6005). The 16S target region was amplified using OneTag® Quick-Load® 2X Master Mix (NEB, Catalogue No. M0486) with 16S-27F (5ˈ-AGAGTTTGATCMTGGCTCAG-3ˈ) and 16S-1492R (5ˈ- CGGTTACCTTGTTACGACTT-3ˈ) as the forward and reverse primers, respectively. The amplification was carried out under the following conditions: 95 °C for 5 min, followed by 25 cycles of 95 °C for 1 min, 50 °C for 30 s and 72 °C for 1.5 min, and finally 72 °C for 5 min. The amplified products were run on a gel to separate the fragments using the Zymoclean™ Gel DNA Recovery Kit (Zymo Research, Catalogue No. D4001). The extracted fragments were sequenced in the forward and reverse direction (Nimagen, BrilliantDye™ Terminator Cycle Sequence Kit V3.1, BRD3-100/1000) and purified (Zymo Research, ZR-96 DNA Sequencing Clean-up Kit™, Catalogue No, D4050). The purified fragment was analyzed on the ABI 3500Xi Genetic Analyzer (Applied Biosystems, ThermoFisher Scientific) and the sequences obtained were browsed in the database of the National Centre for Biotechnological Information (NCBI) (http://blast.ncbi.nlm.nih.gov) via the blastn option [41] for possible matches. This was followed by the deposition of the sequences in the GenBank (www.ncbi.nlm.nih.gov/genbank) under accession number OM281847.1. The phylogenetic tree of the isolate with the closely related species was constructed using MEGA 11.0 [42].
Determination of Keratinolytic Activity of Bacterial Isolates
Activity of keratinase produced by the bacterial isolates was determined by the modified method of Cheng et al. [43]. The pure culture of each of the keratinolytic organisms was used for inoculum development by inoculating into a medium consisting of 1% keratin substrate and 0.2% yeast extract at pH 7.5. The culture was incubated at 37 °C and 100 rpm for 24 h. Feather degradation was carried out in the fermentation medium (minimal medium without agar and nystatin) [35] using 5% inoculum size. The cultures were incubated at 37 °C at 100 rpm for up to 7 days. Crude keratinase (0.5 ml) obtained at 24 h-interval was incubated with 1.5 g of feather powder suspended in 2 ml phosphate buffer (pH 7.5, 40 mM). The control experiment consisted of buffer and feather powder only. The reaction mixture was incubated at 40 °C for 3 h at 100 rpm. At the end of incubation, the reaction was quenched by adding 2 ml of 10% trichloroacetic acid (TCA). Centrifugation was carried out at 5000 rpm for 15 min at room temperature (30 ± 1 °C) to remove precipitated substrate. The increase in absorbance at 280 nm of the filtrate of the test sample relative to that of the control which is a measure of release of protein was converted into keratinase units (1 U = 0.01 absorbance increase for 1 h reaction time [35]. All the tests were performed in replicates.
Bioconversion of Chicken Feather into Feather Hydrolysates
This was investigated by growing the keratinolytic isolates in fermentation medium for 4–5 days on the basis of when highest keratinolytic activities occurred for the two isolates. Feather degradation was carried out in the fermentation medium (minimal medium without agar and nystatin) [35] using 5% inoculum size. The cultures were incubated at 37 °C at 100 rpm for up to 7 days. Thereafter, content of the whole flask was collected, centrifuged at 5000 rpm for 20 min and the supernatant was heated at 60 °C for 30 min to kill the bacterial culture. The non-heated and heated supernatants served as crude keratinase and feather hydrolysate, respectively which were used without further purification. The experiments were performed in triplicates.
Determination of Ammonium Nitrogen and Amino Acid Composition of Feather Hydrolysates
To determine the ammonium nitrogen content, feather hydrolysate of 100 ml was added into 5 ml of borate buffer (pH 9.5) inside distillation flask. The distillate was collected inside conical flask containing 5 ml of indicating boric acid solution. Ammonia in the distillate was titrated against standard sulfuric acid (0.02 N) until the solution turned pale lavender. The blank was titrated in the same way using distilled water.
Ammoniacal nitrogen (mg/L) = \(A - B \times \frac{280}{V}\), where A is volume of sulfuric acid used for sample; B is volume of sulfuric acid used for blank, and V is volume of sample taken for test.
The amino acid composition of feather hydrolysates such as tryptophan, cysteine, methionine, phenylalanine, glycine, valine, tyrosine, lysine, leucine, and serine was determined using liquid chromatography. Protein was extracted from the feather hydrolysates using the methods of Saleethong et al. [44], in extraction buffer (30.0 mmol/L Tris–HCl (pH 8.0), 0.1 mmol/L EDTA, 6.0 mmol/L ascorbic acid, 5.0 mmol/L MgCl2, 1% polyvinyl pyrrolidone, 0.02% 2-mercaptoethanol, and 1% glycerol). The extracted protein was hydrolyzed in 6 M HCl under vacuum at 105 °C for 24 h. The amino acid analyses were done by reversed-phase LC (Model Hitachi L-8900) with pre-column derivatization with AccQ-fluor reagent, as indicated by manufactures (Water Corporation, Milford, MA).
Evaluation of Feather Hydrolysates as Biofertilizers for Vegetable Seed Germination in Soil-Less Experiment
The feather hydrolysates (FH) were evaluated for the biofertilizing effects on three leafy vegetables: Corchorus olitorius var. corete potagere, Celosia argentea var. cristata, and Amaranthus caudatus var. hemera in soil-less petri dish experiment. The vegetable seeds were procured from Farm Help Agrostores, Moniya, Ibadan, Oyo State. The hydrolysates were graded into different concentrations (25, 50, 75, and 100%) by diluting the stock with tap water. The experiment involved planting of five vegetable seeds on 1 g of cotton wool placed inside 9 cm-diameter petri dish. Immediately after planting, the petri dish was flooded with 10 ml of each of the graded concentrations. For the control experiment, immediately after sowing, the petri dish was flooded with equal volume of tap water only. The dishes were arranged using completely randomized design (CRD) with four replicates. The experiment was held under ambient conditions where light was accessible and carried out for 7 days. It was thoroughly monitored and irrigation was done on daily basis using 5 ml of each concentration of FH and water for the experiment and the control, respectively.
Determination of Growth Characteristics
The numbers of germinated seeds (minimum radicle length of 2 mm) were noted every day. At the end of 7 days of growth, seedlings were uprooted and parameters such as shoot height, root length, seed germination percentage, seedling vigour index and germination rate index were determined. Metric rule was used to determine the shoot height and root length. The germination rate index was determined according to the method described by Esechi [45]. The vigour index and germination percentage were calculated as follows:
Germination rate index (GRI) = \(\frac{G1}{1} + \frac{G2}{2} + \frac{G3}{3} + \ldots \frac{Gx}{x}\), where G1 is the germination percentage after one day of sowing; G2 is the germination percentage after two days of sowing, G3 is the germination percentage after 3 days of sowing, and Gx is the germination percentage after x days of sowing.
Evaluation of Feather Hydrolysate as Biofertilizer for Vegetables Grown in Pot Experiment
A modified method of Madisa et al. [46] was used in this study. It was carried out at the Botanical Gardens, Department of Pure and Applied Biology, LAUTECH Ogbomoso, Oyo State, Nigeria with geographical coordinates 8° 8ˈ 0 ̎ North, 4° 16ˈ 0 ̎ East between January and March, 2021, where the soil samples were obtained. The soil is sandy loamy which belongs to alfisols and classified locally as Gambari soil series [47]. Pot experiment was set up to evaluate the plant growth promoting effects of feather hydrolysates on the three leafy vegetables Corchorus olitorius, Celosia argentea and Amaranthus caudatus by considering different concentrations (25, 50, 75, and 100%). The experiment was conducted using 1 L capacity transparent polythene bag filled with 250 g of soil sample obtained from the study site. Ten seeds were planted per pot at an average depth of 1–2 cm. Immediately after planting; pots were flooded with 20 ml of each of the graded concentrations of FHs. For positive control experiment, each pot was treated with 2 g of inorganic NPK fertilizer (15:15:15) 5 days before planting, while the negative control pot was free of fertilizer. Both the positive and negative control pots were flooded with equal volume of tap water immediately after planting.
Subsequently, all pots were irrigated with 10 ml of water every day except the test experiment pots that were irrigated with their corresponding FH concentrations every 7 days instead of water to serve as booster doses. Pots were perforated at the base to allow excess water to drain out and they were arranged under a net. The experiment was a randomized complete block design with four replicates. Seedlings were later thinned to maintain three stands per pot two weeks after planting and the vegetables were further grown for two weeks before harvesting. Thereafter, shoot length and root length of harvested vegetables were measured. To determine the fresh weight, the total weight of the shoot portion of plants/pot was taken. The same plants were dried at 60 °C for 8 h to determine the dry weight. The values were expressed as means.
Determination of Ammonium Nitrogen, Total Organic Carbon and Microbial Properties of Soil
Composite soil sample (0–20 cm depth) was taken from three different points of the site and analyzed to determine the ammonium nitrogen using standard laboratory procedures outlined by Mylavapus and Kennelley [48]. Total organic carbon was estimated as described by McLeod [49]. One gram of soil sample was added with 10 mL 1 N K2Cr2O7, the flask was swirled gently to disperse the soil in the solution. Twenty milliliter of concentrated H2SO4 was added to the mixture and swirled immediately. The flask contents was heated until 135 °C for 30 s after cooling in a fume cupboard, it was diluted to 200 mL with deionised water. Twenty five milliliter of the sample was added with 3 drops of ferroin indicator and titrated with 0.4 N FeSO4. As the end point was approached, the solution turned greenish and final changed to a dark green. At this point, ferrous sulphate was added drop wisely until the colour changed sharply from blue-green to reddish-grey. Two blanks (without soil) were run similarly to standardise the FeSO4 solution. The total organic carbon was calculated thus:
where N = Normality of K2Cr2O7 solution; T = Volume of FeSO4 used in sample titration (mL); S = Volume of FeSO4 used in blank titration (mL); W = Weight of sample used (g).
Microbial loads of the composite soil sample before (day 0) and after the experiments (day 28) were enumerated. The soil sample was serially diluted in sterile water, and then inoculated into nutrient agar and potato dextrose agar plates followed by 24 h and 48 h incubation for the enumeration of bacterial and fungal load, respectively.
Statistical Analysis
Data obtained were analyzed using SPSS statistical package version 20. Values were expressed as means ± SD, and evaluated for significant difference through ANOVA (p < 0.05) and Duncan’s multiple range test.
Results and Discussion
Isolation and Characterization of Keratinolytic Bacteria
The previously isolated Bacillus safensis LAU 13 (KJ461434) displayed remarkable keratin degrading ability as earlier documented [35]. It produced the most potent keratinolytic activity as the feather substrate was almost completely degraded. In addition, among the newly isolated bacteria from the screened feather dump site in the present study, a particular isolate designated as FH 20 displayed high feather degradation. It was a short rod, gram negative, motile, non-spore forming and aerobic bacterium. It was positive to catalase and oxidase but negative to coagulase test. The novel keratinolytic strain was identified as Aquamicrobium defluvii FH 20 with accession number OM281847.1 (Fig. 1). It has 97.60% homology with Aquamicrobium defluvii TLA-7 (KU163265.1) isolated from landfill stabilization pond in Greece [50].
Keratinolytic microorganisms are found in the environment, and several authors have reported the isolation of multitude of keratinolytic bacterial species. However, larger proportion of them are found to be confined to Gram-positive bacteria including Bacillus, Lysobacter, and a few strains of Gram-negative bacteria, viz., Vibrio and Xanthomonas [7, 15, 51]. However, there is no evidence of keratin degradation by a member of the genus Aquamicrobium. This study therefore represents the first reference on the keratin degrading ability of a member of the genus Aquamicrobium.
Species of Aquamicrobium are gram negative and aerobic bacteria capable of degrading thiophene-2-carboxylate, petroleum, polychlorinated biphenyls, biphenyl, and cyhalofop-butyl. Their isolation from polluted environments such as sewage treated factory, active sewage sludge and biological filters have been reported [52]. Aquamicrobium defluvii is an hydrocarbon degrading bacterium that was first isolated from activated sewage sludge in Germany [53]. The first petroleum-degrading strain Aquamicrobium defluvii W13Z1 which was isolated from petroleum-contaminated drill cuttings was reported by Wang et al. [54]. Members of the genus Aquamicrobium have potential applications in livestock wastewater treatment facilities to degrade organic pollutants [55], herbicide [56], dye and bioelectricity generation [57]. However, few species have been recently reported from pathological investigations, such as Aquamicrobium lusatiense [58], Aquamicrobium terrae [59]. However, this is the first report of the isolation of keratinolytic Aquamicrobium defluvii for the treatment of keratinous waste.
Keratinolytic Activities and Course of pH of the Bacterial Growth
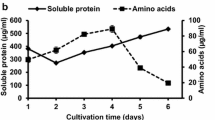
The keratinolytic activities (KA) of the two bacterial strains, Aquamicrobium defluvii FH 20 and B. safensis LAU 13 cultivated on feather substrate through submerged fermentation are as shown in Fig. 2. Aquamicrobium defluvii FH 20 produced KA in the range of 19.5–45.2 U/ml, whereas the KA values recorded for Bacillus safensis LAU 13 were in the range of 29.4–56.7 U/ml. The latter produced its maximum KA (56.7 U/ml) at 96 h of fermentation, while the maximum KA value (45.2 U/ml) obtained for the former organism was achieved at 120 h. The course of pH during the hydrolysis of feathers by the two bacterial species rose from the initial value of 6.0 to reach maximum value of 8.3 during 120 h of fermentation.
In many previous studies, similar KA values of some species of bacterial have been reported. For instance, Lateef et al. [35] reported KA of 50.4 U/ml for B. safensis LAU 13 isolated from a feather dump site using feather waste as keratin substrate. Using casein as the substrate, Alahyaribeik et al. [60] reported maximum KA of 50.41 and 35.41 U/ml after 48 h of fermentation by strains of B. licheniformis and B. pumilus respectively using feathers as substrate. The shift in pH during keratinase production corresponds with the report of Gupta and Ramnani [15], that environment with pH of 6 to 9 favors keratin degradation by most microorganisms. The rise in alkalinity of the fermentation medium could be attributed to the release of ammonium through deamination reaction that occurs during keratin hydrolysis [61]. Authors have previously reported pH 6.0–8.2 for Bacillus safensis [35] and pH 7.5–9.7 for Bacillus cereus [7] during the degradation of chicken feather.
Ammonium Nitrogen Content and Amino acids Composition of the Feather Hydrolysate
The ammonium nitrogen content of liquid FH resulted from the feather degradation by Bacillus safensis LAU 13 and Aquamicrobium defluvii FH 20 were 13.16 ± 0.22 and 12.60 ± 0.15% (w/v), respectively. Analysis of amino acids profile of the FHs revealed the presence of essential and non-essential amino acids (Table 1). This reflects the proteinaceous nature of the FHs and also indicates the hydrolysis of feather substrate by the microorganisms. The FH resulted from feather degradation by B safensis LAU 13 is richer in glycine (3.86 mg/ml), alanine (3.45 mg/ml), and leucine (2.52 mg/ml) than cysteine, tryptophan, and methionine. In the same vein, the FH obtained through Aquamicrobium defluvii FH 20 have some similarities in amino acids compositions. It consisted of leucine (4.20 mg/ml) and glycine (3.80 mg/ml) in larger quantities than phenylalanine, cysteine, lysine, tyrosine, and tryptophan that were present in very small amounts.
The nitrogen contents of the feather hydrolysates in the range of 12.60–13.16% corroborate the findings of Gurav et al. [28] that reported the presence of 8.03% Kjeldhal nitrogen content in the feather hydrolysate resulted from degradation by Chryseobacterium sp. RBT, whereas 15.36% (w/v) nitrogen content was obtained for the FH of Bacillus sp. CL18 [6]. Calin et al. [62] reported 15.5 and 15.0% (w/w) for ammonium nitrogen feather hydrolysates of Trichoderma atroviridiae and Trichoderma asperellum, respectively. The richness of FHs in amino acids is supported by evidences in the literature. Recently, Hendrick et al. [63] reported amino acid contents of valorized chicken feather by Chryseobacterium cucumeris FHN1 as arginine (3.44%), phenylalanine (3.59%), glycine (3.68%), leucine (4.41%), histidine (4.54%), valine (4.63%), aspartic acid (4.75%), serine (5.19%), glutamic acid (5.86%), and proline (6.08%). Others included hydroxyproline (0.01%), methionine (0.43%), tryptophan (0.86%), cysteine (1.11%), and lysine (1.13%). The differences in types and amounts of amino acids might be attributed to the differences in the keratin degrading ability of the isolates. Authors have reported that the richness in amino acids of keratin hydrolysates potentiates their application as biofertilizer to boost agricultural crop production [11, 64]. They enhance soil fertility and microbial activity which in turn stimulate plant growth and metabolism [65].
Effects of FHs on the Seed Germination and Seedling Growth of Vegetables in Petri dish
The feather hydrolysates enhanced the germination characteristics and seedling growth of Corchorus olitorius, Amaranthus caudatus and Celosia argentea seeds (Fig. 3) after seven days of cultivation. B. safensis FH at all concentrations demonstrated potent growth-promoting activities on the vegetable seeds by obtaining highest values for their growth characteristics such as shoot height (SH), root length (RL), germination percentage (GP), vigor index (VI), and germination rate index (GRI) over their respective controls. Optimal performances were produced at dose of 25% FH with germination rate of 100, 86.67 and 50% for C. olitorius, A. caudatus and C. argentea, respectively representing 1.67, 1.63 and 1.57-fold improvement compared to control (Table 2). Conversely, significant lower germination rates of 60, 53.3 and 26.67% were recorded in the control experiments for C. olitorius, A. caudatus and C. argentea, respectively. Seeds treated with 25% FH of B. safensis demonstrated improvement in vigour index of C. olitorius, A. caudatus and C. argentea by 3.87, 3.40 and 2.33-fold, respectively compared to the control experiments.
Similar results on growth promotion were obtained on the vegetable seeds treated with feather hydrolysate of Aquamicrobium defluvii FH 20 (Fig. 4). In C. olitorius, the best treatment (FH at 50%) boosted the seed germination (93%) against control (80%), and also produced 2.34 and 1.26-fold improvement in vigour index and germination rate index, respectively (Table 3). However, it was observed that 25% FH performed optimally on A. caudatus and C. argentea with germination rate of 66.67 and 60%, respectively corresponding to 1.67 and 1.29-fold improvement when compared with the control experiments. Also, the 25% FH treatment produced 3.38 and 2.13-fold improvement in vigour index for A. caudatus and C. argentea, respectively.
At optimal levels, vigour index of 157.50–425.00, and 205.34–331.32 were obtained in vegetables treated with feather hydrolysates of B. safensis and A. defluvii, respectively compared to 60.00–145.61 obtained in the control experiments (Tables 2 and 3). Authors have reported improved seedling growth of tomato [62] upon treatment with feather hydrolysates. However, Kshetri et al. [66] reported improved vigour index of 458.7–677.3 for garden pea treated with 5–100 × dilution of FH produced by Streptomyces sp RCM-SSR-6 compared to 343.07 recorded for the control experiment. The undiluted and 2 × dilution FHs produced vigour index of 217.3 and 247.5, respectively. The study concluded that high concentrations of FH inhibited vigour index of garden pea due to concentration effects. Furthermore, Gurav et al. [28] reported positive impact of FH produced by Chryseobacterium sp. RBT on the seed and seedling growth of Solanum melongena and Capsicum annuum.
The increase in germination and other agronomic parameters by the FH may be due to the absorption and utilization of nitrogen and other nutrients by the seeds [65]. These potentiate the application of FH as a sustainable and alternative tool to promote and improve organic farming [6]. While the use of protein-rich hydrolysates including feather hydrolysates in sustainable horticulture has been advocated [67], there is paucity of information on the evaluation of effects of feather hydrolysates on Corchorus olitorius, Amaranthus caudatus and Celosia argentea until now. In addition, the feather hydrolysates can find useful applications in soil-less cultivation of vegetables. Feather hydrolysates have been considered as superior biofertilizer in agro-industrial enterprise [22].
Effects of Feather Hydrolysates as Biofertilizer on Vegetables Grown in Pot Experiment
The feather hydrolysates generated after the degradation of feather by both B. safensis and A. defluvii enhanced the growth of the vegetables (Fig. 4) after 28 days of cultivation. Plants treated with 100% FH of B. safensis displayed higher shoot height (SH), root length (RL), fresh weight (FW) and dry weight (DW) compared to other treatments except plants treated with NPK fertilizer that showed similar growth characteristics (Table 4). It was observed that the plant growth promoting activity of the FH is dose dependent as the optimum activity was obtained on plant treated with highest FH concentration. More so, 100% FH of A. defluvii produced positive effects on all the vegetables (Table 5). Noteworthy that it was in A. caudatus alone that the application of NPK fertilizer performed comparatively or higher than the 100% FH of both B. safensis and A. defluvii (Tables 4 and 5).
The application of FH produced by A. defluvii at 75 and 100% significantly increased fresh weight of C. olitorius compared with the negative control and NPK application. However, in B. safensis FH, application only at 100% was significantly higher than the NPK application. In C. argentea, 100% application of A. defluvii FH produced significant higher fresh weight than NPK application, while at the same concentration; FH of B. safensis produced similar result with NPK application. Generally, the applications of FH at 25, 50, 75 and 100% produced better fresh weight than the negative control (water) in all the vegetables except for 25% applications of FH of B. safensis on A. caudatus and C. argentea (Tables 4 and 5).
Authors have documented quite a number of findings reporting positive effect of feather hydrolysates on the growth and yield performance of some agronomic crops. For instance, Sobucki et al. [6] reported a significant improvement in the growth of lettuce plant cultivated in plastic pot with FH over the urea treated lettuce plant cultivated under the same conditions. Similarly, Kucinska et al. [68] reported a positive plant growth promoting effect of feather hydrolysate on cucumber plant cultivated inside plastic pot. Moreover, application of FH had been reported to facilitate seed germination and plant growth through rapid nutrient uptake [65]. The contributions of feather hydrolysates in terms of nutrient provision stimulate plant growth. Hence, the feather hydrolysates as obtained in this study have potential application as organic fertilizer for the cultivation of agronomic crops for sustainable agricultural food production. To the best of our knowledge, these reports are the pioneering efforts at evaluating the impacts of feather hydrolysates on C. olitorius, A. caudatus and C. argentea that are widely consumed in Nigeria.
Effect of Feather Hydrolysates on the Soil Nutrients and Microbiota
The total organic carbon and nitrogen contents of the soil used for the cultivation were found to be 2.24 and 0.43%, respectively while the results of the microbial analysis before (day 0) and after applications of FH and NPK (day 28) are as shown in Table 6. Application of B. safensis LAU 13 and A. defluvii FH 20 feather hydrolysates at 100% improved soil fertility both in terms of total organic carbon and nitrogen content. The treatment with B. safensis FH enhanced the total organic carbon and nitrogen contents to 2.90 and 0.59%, representing 29.46 and 37.21% improvement, respectively. Similarly, A. defluvii FH increased total organic carbon and nitrogen content of the soil to 2.78 and 0.55% with improvement of 24.11 and 27.91%, respectively. However, NPK fertilizer application increased the initial value of total organic carbon and nitrogen content of the soil to 2.37 and 0.61%, respectively. The increased total organic carbon might be due to booster application of FHs and contribution of microbial biomass in the confined pot environment.
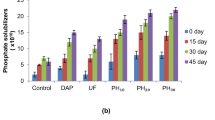
Applications of FH and NPK fertilizer considerably improved the soil microbial loads. For instance, at 100% application, B. safensis FH used for Corchorus olitorius cultivation increased the bacterial and fungal loads in the range of 4.85 × 102—1.14 × 104 cfu/g and 1.15 × 103—1.13 × 104 cfu/g, respectively while the FH of Aquamicrobium defluvii used for the cultivation of Celosia argentea increased the bacterial and fungal loads in the range of 4.29 × 102–2.51 × 103 cfu/g and 1.39 × 103—1.33 × 104 cfu/g, respectively. Generally, FHs application increased microbial growth by 10–100 folds.
The application of feather hydrolysates performed better at increasing the organic carbon of the soil for soil amendment. Jain et al. [69] described feather hydrolysate produced by Streptomyces sampsonii GS 1322 as an excellent soil amendment strategy to improve the fertility of barren soil for growth of wheat. Nurdiawati et al. [20] have posited that feather hydrolysate being rich in organic carbon represents a potent way of enriching soil organic matter. The increased microbial loads recorded for soils treated with NPK fertilizer was almost equivalent to the FH treated soils due to available inorganic nutrients. The improved microbial loads of the FH-treated soils by factor of 101–102 are attributable to improved levels of organic matter in the treated soil which stimulates soil microbial activities. Jain et al. [69] reported improved microbial activities in a barren soil that was amended with feather hydrolysate. The nutrients supplied by FH serve as stimulant for microbial growth in the amended soil [22], and various reports have shown the improved growth of beneficial microbes such as phosphate solubilizers, nitrogen fixers and siderophore producers following the application of feather hydrolysates [2, 21]. Keratin hydrolysates are nutrient-rich organic materials that can be exploited as slow release nitrogen fertilizer and as biostimulant capable of increasing microbial activities in the soil [64]. Similarly, Bhari et al. [22] reported that the application of protein hydrolysates as organic fertilizer improved soil ecosystem and microbial populations. In the same vein, the findings of Kaur et al. [21] indicated the enhancement of growth of mung beans, improvement of soil fertility and microbial activity upon soil treatment with feather hydrolysate of Bacillus aerius NSMk2.
Conclusion
In this study, Bacillus safensis LAU 13 and a newly isolated Aquamicrobium defluvii FH 20 displayed potent keratinolytic activities, and degraded chicken feather to produce feather hydrolysates that were rich in amino acids. The application of FHs in soil-less petri dish experiments involving Corchorus olitorius, Celosia argentea, and Amaranthus caudatus seeds enhanced seed germination, germination percentage, vigour index and other growth characteristic over the control. Similarly, the growth of the vegetables in pot experiment was positively affected by FHs as they displayed higher shoot height, root length, fresh weight and dry weight over the fertilizer and water treated seedlings in C. olitorius and C. argentea particularly at 100% dose. The fertilizer only performed better than 100% FH in A. caudatus. The treatment with the FHs improved nitrogen content, organic carbon and microbial activity in the soil. Further research will be needed to determine the long-term benefits and limitations of FHs applications on the production cycle of plants and soil characteristics for large scale adoption.
The remarkable poultry feather bioconversion displayed by novel Aquamicrobium defluvii FH 20 and Bacillus safensis LAU 13 represents a biotechnological means of keratinous wastes valorization. Hence, the significant plant growth-promoting ability demonstrated by the FHs herein indicates their potential application as organic nitrogen-rich fertilizer representing eco-friendly viable substitute to synthetic chemical fertilizer. The study can contribute immensely to the implementation of strategies to enhance sustainable vegetable production to meet the targets of UN on the production and consumption of fruits and vegetables for promotion of good health.
Availability of Data and Material
Data for the work are available with the authors.
Code Availability
Not applicable.
References
Kyriacou, M.C., Rouphael, Y., Colla, G., Zrenner, R., Schwarz, D.: Vegetable grafting: the implications of a growing agronomic imperative for vegetable fruit quality and nutritive value. Front. Plant. Sci. 8, 741 (2017)
Bhange, K., Chaturvedi, V., Bhatt, R.: Ameliorating effects of chicken feathers in plant growth promotion activity by a keratinolytic strain of Bacillus subtilis PF1. Bioresour. Bioprocess. 3, 13 (2016)
Srivastav, A.L.: Chemical fertilizers and pesticides: role in groundwater contamination. In: Agrochemicals Detection, Treatment and Remediation, pp 143–159. Butterworth-Heinemann (2020)
Chatzistathis, T., Kavvadias, V., Sotiropoulos, T., Papadakis, I.E.: Organic fertilization and tree orchards. Agriculture 11(8), 692 (2021)
Sharma, B., Vaish, B., Singh, U.K., Singh, P., Singh, R.P.: Recycling of organic wastes in agriculture: an environmental perspective. In.t J. Environ. Res. 13(2), 409–429 (2019)
Sobucki, L., Ramos, R.F., Gubiani, E., Brunetto, G., Kaiser, D.R., Daroit, D.J.: Feather hydrolysate as a promising nitrogen-rich fertilizer for greenhouse lettuce cultivation. Int. J. Recyc. Org. Waste Agric. 8(1), S493–S499 (2019)
Lateef, A., Oloke, J.K., Gueguim-Kana, E.B., Sobowale, B.O., Ajao, S.O., Bello, B.Y.: Keratinolytic activities of a new feather-degrading isolate of Bacillus cereus LAU 08 isolated from Nigerian soil. Int. Bioremed. Biodegrad. 64(2), 162–165 (2010)
Alvarenga, P., Mourinha, C., Farto, M., Santos, T., Palma, P., Sengo, J., Cunha-Queda, C.: Sewage sludge, compost and other representative organic wastes as agricultural soil amendments: benefits versus limiting factors. Waste Manag. 40, 44–52 (2015)
Mahjoub, B., Domscheit, E.: Chances and challenges of an organic waste-based bioeconomy. Curr. Opin. Green Sustain. Chem. 25, 100388 (2020)
Elegbede, J.A., Ajayi, V.A., Lateef, A.: Microbial valorization of corncob: novel route for biotechnological products for sustainable bioeconomy. Environ. Technol. Innov. 24, 102073 (2021)
Adelere, I.A., Lateef, A.: Degradation of keratin biomass by different microorganisms. In: Sharma S, Kumar A (Eds.) Keratin as a Protein Biopolymer. Springer Series on Polymer and Composite Materials. https://doi.org/10.1007/978-3-030-02901-2_5. Springer, Cham. ISBN 978-3-030-02900-5, 2019;123–162.
Fontoura, R., Daroit, D.J., Corrêa, A.P.F., Moresco, K.S., Santi, L., Beys-da-Silva, W.O., Yates, J.R., III., Moreira, J.C.F., Brandelli, A.: Characterization of a novel antioxidant peptide from feather keratin hydrolysates. New Biotechnol. 49, 71–76 (2019)
Kumari, M., Kumar, J.: Chicken feather waste degradation by Alternaria tenuissima and its application on plant growth. J. Appl. Nat. Sci. 12(3), 411–414 (2020)
Kolade, M.O., Adewumi, B.A., Dairo, O.U., Adejuyigbe, C.O., Adejumo, O.A.: Waste to wealth-conversion of poultry litter from raw form to pelleted organic fertilizer. Lautech J. Eng. Technol. 12(2), 10–15 (2018)
Gupta, R., Ramnani, P.: Microbial keratinases and their prospective applications: an overview. Appl. Microbiol. Biotechnol. 70(1), 21–33 (2006)
Mazotto, A.M., Coelho, R.R.R., Cedrola, S.M.L., de Lima, M.F., Couri, S., Paraguai de Souza, E., Vermelho, A.B.: Keratinase production by three Bacillus spp. using feather meal and whole feather as substrate in a submerged fermentation. Enzyme Res. Article ID 523780 (2011)
Adelere, I.A., Lateef, A.: Keratinases: emerging trends in production and applications as novel multifunctional biocatalysts. Kuwait J. Sci. 43(3), 118–127 (2016)
Chaturvedi, V., Agrawal, K., Verma, P.: Chicken feathers: a treasure cove of useful metabolites and value-added products. Environ. Sustain. 4, 231–243 (2021)
Bokveld, A., Nnolim, N.E., Digban, T.O., Okoh, A.I., Nwodo, U.U.: Chryseobacterium aquifrigidense keratinase liberated essential and nonessential amino acids from chicken feather degradation. Environ. Technol. (2021). https://doi.org/10.1080/09593330.2021.1969597
Nurdiawati, A., Suherman, C., Maxiselly, Y., Akbar, M.A., Purwoko, B.A., Prawisudha, P., Yoshikawa, K.: Liquid feather protein hydrolysate as a potential fertilizer to increase growth and yield of patchouli (Pogostemon cablin Benth) and mung bean (Vigna radiata). Int. J. Recycl. Org. Waste Agric. 8(3), 221–232 (2019)
Kaur, M., Bhari, R., Singh, R.S.: Chicken feather waste-derived protein hydrolysate as a potential biostimulant for cultivation of mung beans. Biologia 76(6), 1807–1815 (2021)
Bhari, R., Kaur, M., Singh, R.S.: Chicken feather waste hydrolysate as a superior biofertilizer in agroindustry. Curr. Microbiol. 78, 2212–2230 (2021)
Omale, J., Emmanuel, U.C.: Comparative studies on the protein and mineral composition of some selected Nigerian vegetables. Afr. J. Food Sci. 5(1), 22–25 (2011)
Ramya, V., Patel, P.: Health benefits of vegetables. Int. J. Chem. Stud. 7(2), 82–87 (2019)
Azeez, L., Lateef, A., Adebisi, S.A.: Silver nanoparticles (AgNPs) biosynthesized using pod extract of Cola nitida enhances antioxidant activity and phytochemical composition of Amaranthus caudatus Linn. Appl. Nanosci. 7(1–2), 59–66 (2017)
Frank, S.M., Webster, J., McKenzie, B., Geldsetzer, P., Manne-Goehler, J., Andall-Brereton, G., Houehanou, C., Houinato, D., Gurung, M.S., Bicaba, B.W., Jaacks, L.M.: Consumption of fruits and vegetables among individuals 15 years and older in 28 low-and middle-income countries. J. Nutr. 149(7), 1252–1259 (2019)
Azeez, L., Lateef, A., Wahab, A.A., Rufai, M.A., Salau, A.K., Ajayi, I.O., Ajayi, E.M., Maryam, A.K., Adebisi, B.: Phytomodulatory effects of silver nanoparticles on Corchorus olitorius: its antiphytopathogenic and hepatoprotective potentials. Plant Physiol. Biochem. 136, 109–117 (2019)
Gurav, R., Nalavade, V., Aware, C., Vyavahare, G., Bhatia, S.K., Yang, Y.H., Bapat, V., Jadhav, J.: Microbial degradation of poultry feather biomass in a constructed bioreactor and application of hydrolysate as bioenhancer to vegetable crops. Environ. Sci. Pollut. Res. 27(2), 2027–2035 (2020)
UN. Resolution adopted by the General Assembly on 19 December 2019: 74/244. International year of fruits and vegetables, 2021. United Nations General Assembly. 2020; https://undocs.org/en/A/RES/74/244.
FAO. Fruit and vegetables – your dietary essentials. The International Year of Fruits and Vegetables, 2021. 2020; https://doi.org/10.4060/cb2395en.
Ogwu, M.C. (2020). Value of Amaranthus (L.) species in Nigeria. In (Ed.), Nutritional Value of Amaranth. IntechOpen. https://doi.org/10.5772/intechopen.86990.
Arowosegbe, S., Olanipekun, M.K., Adeloye, I.A.: Ethnobotanical survey of indigenous leafy vegetable consumed in Ekiti state, Nigeria. Eur. J. Biol. Med. Sci. Res. 6(1), 7–14 (2018)
Oboh, G., Raddatz, H., Henle, T.: Characterization of the antioxidant properties of hydrophilic and lipophilic extracts of Jute (Corchorus olitorius) leaf. Int. J. Food Sci. Nutr. 60(Suppl 2), 124–134 (2009)
Kanu, C.L., Owoeye, O., Imosemi, I.O., Malomo, A.O.: A review of the multifaceted usefulness of Celosia argentea Linn. Eur. J. Pharm. Med. Res. 4(10), 72–79 (2017)
Lateef, A., Adelere, I.A., Gueguim-Kana, E.B.: Bacillus safensis LAU 13: a new source of keratinase and its multi-functional biocatalytic applications. Biotechnol. Biotechnol. Equip. 29(1), 54–63 (2015)
Lateef, A., Adelere, I.A., Gueguim-Kana, E.B.: The biology and potential biotechnological applications of Bacillus safensis. Biologia 70(4), 411–419 (2015)
Lateef, A., Adelere, I.A., Gueguim-Kana, E.B., Asafa, T.B., Beukes, L.S.: Green synthesis of silver nanoparticles using keratinase obtained from a strain of Bacillus safensis LAU 13. Int. Nano Lett. 5(1), 29–35 (2015)
Lateef, A., Ojo, S.A., Oladejo, S.M.: Anti-candida, anti-coagulant and thrombolytic activities of biosynthesized silver nanoparticles using cell-free extract of Bacillus safensis LAU 13. Process Biochem. 51(10), 1406–1412 (2016)
Ojo, S.A., Lateef, A., Azeez, M.A., Oladejo, S.M., Akinwale, A.S., Asafa, T.B., Yekeen, T.A., Akinboro, A., Oladipo, I.C., Gueguim-Kana, E.B., Beukes, L.S.: Biomedical and catalytic applications of gold and silver-gold alloy nanoparticles biosynthesized using cell-free extract of Bacillus safensis LAU 13: antifungal, dye degradation, anti-coagulant and thrombolytic activities. IEEE Trans. NanoBiosci. 15(5), 433–442 (2016)
Brenner DJ, Krieg NR, Staley JT. Bergey’s manual of systematic bacteriology. 2nd ed., Part B. New York, NY: Springer; 2004. p. 323–358.
Zhang, Z., Schwartz, S., Wagner, L., Miller, W.: A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 7(1–2), 203–214 (2000)
Tamura, K., Stecher, G., Kumar, S.: MEGA 11: molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. (2021). https://doi.org/10.1093/molbev/msab120
Cheng, S.W., Hu, H.M., Shen, S.W., Takagi, H., Asano, M., Tsai, Y.C.: Production and characterization of keratinase of a feather-degrading Bacillus licheniformis PWD-1. Biosci. Biotechnol. Biochem. 59(12), 2239–2243 (1995)
Saleethong, P., Roytrakul, S., Kong-Ngern, K., Theerakulpisut, P.: Differential proteins expressed in rice leaves and grains in response to salinity and exogenous spermidine treatments. Rice Sci. 23(1), 9–21 (2016)
Esechie, H.A.: Interaction of salinity and temperature on the germination of sorghum. J. Agron. Crop Sci. 172(3), 194–199 (1994)
Madisa, M.E., Mathowa, T., Mpofu, C., Stephen, N., Machacha, S.: Effect of chicken manure and commercial fertilizer on performance of jute mallow (Corchorus olitorius). Agric. Biol. J. N. Am. 4(6), 617–622 (2013)
] Smyth, A.J., Montgomery, R.F.: Soils and land use in central western Nigeria. Government Printer, Ibadan, p 250 (1962)
Mylavarapu, R.S., Kennelley, E.D.: UF/IFAS extension soil testing laboratory (ESTL) analytical procedures and training manual. EDIS, University of Florida, Circular 1248;2002.
McLeod, S.: Studies on wet oxidation procedures for the determination of organic carbon in soils. Notes on soil techniques, pp. 73–79 (1973)
Ntougias, S.: Phylogenetic identification and enzyme activities of indigenous bacteria from a landfill stabilization pond. Environ. Process. 3(2), 341–352 (2016)
Yue, X., Zhang, B., Zou, J., Chen, W., Yang, N.: Characterization of a new bacterium with high alkaline keratinase activity from Calotes versicolor feces. J. Biotechnol. Res. 8, 83 (2017)
Ji, J., Zheng, Y., Gao, T., Wu, C.: Isolation and identification of Aquamicrobium strains from a fecal contaminated sludge sample. MEDS Public Health Prev. Med. 1(1), 18–22 (2021)
Bambauer, A., Rainey, F.A., Stackebrandt, E., Winter, J.: Characterization of Aquamicrobium defluvii gen. nov. sp. Nov., a thiophene-2-carboxylate-metabolizing bacterium from activated sludge. Arch. Microbiol. 169(4), 293–302 (1998)
Wang, X., Jin, D., Zhou, L., Zhang, Z.: Draft genome sequence of Aquamicrobium defluvii strain W13Z1, a psychrotolerant halotolerant hydrocarbon-degrading bacterium. Genome Announc. 3(4), e00984-e1015 (2015)
Kim, D.H., Yun, H.S., Kim, Y.S., Kim, J.G.: Pollutant-removing biofilter strains associated with high ammonia and hydrogen sulfide removal rate in a livestock wastewater treatment facility. Sustainability 13(13), 7358 (2021)
Wang, C., Qiu, J., Yang, Y., Zheng, J., He, J., Li, S.: Identification and characterization of a novel carboxylesterase (FpbH) that hydrolyzes aryloxyphenoxypropionate herbicides. Biotechnol. Lett. 39(4), 553–560 (2017)
Xu, Q., Sun, J., Hu, Y.Y., Chen, J., Li, W.J.: Characterization and interactions of anodic isolates in microbial fuel cells explored for simultaneous electricity generation and Congo red decolorization. Bioresour. Technol. 142, 101–108 (2013)
Taubenslag, K.J., Gangaputra, S., Kim, S.J.: Chronic endophthalmitis from Aquamicrobium lusatiense. Am. J. Ophthalmol. Case Rep. 22, 101067 (2021)
Venkat, A.G., Abraham, J.R., Bessette, A., Karthik, N., Baynes, K., Lowder, C.Y., Srivastava, S.: A case report of chronic endophthalmitis secondary to Aquamicrobium terrae. Reti Cases Brief Rep. (2021). https://doi.org/10.1097/ICB.0000000000001003
Alahyaribeik, S., Sharifi, S.D., Tabandeh, F., Honarbakhsh, S., Ghazanfari, S.: Bioconversion of chicken feather wastes by keratinolytic bacteria. Process. Saf. Environ. Prot. 135, 171–178 (2020)
Riffel, A., Lucas, F., Heeb, P., Brandelli, A.: Characterization of a new keratinolytic bacterium that completely degrades native feather keratin. Arch. Microbiol. 179(4), 258–265 (2003)
Calin, M., Raut, I., Arsene, M.L., Capra, L., Gurban, A.M., Doni, M., Jecu, L.: Applications of fungal strains with keratin-degrading and plant growth promoting characteristics. Agronomy 9, 543 (2019)
Hendrick, Q., Nnolim, N.E., Nwodo, U.U.: Chryseobacterium cucumeris FHN1 keratinolytic enzyme valorized chicken feathers to amino acids with polar, anionic and non-polar imino side chain characteristics. Biocatal. Agric. Biotechnol. 35, 102109 (2021)
de Menezes, C.L.A., do Couto Santos, R., Santos, M.V., Boscolo, M., da Silva, R., Gomes, E., da Silva, R.R.: Industrial sustainability of microbial keratinases: production and potential applications. World J. Microbiol. Biotechnol. 37(5), 1–17 (2021)
Tamreihao, K., Mukherjee, S., Khunjamayum, R., Devi, L.J., Asem, R.S., Ningthoujam, D.S.: Feather degradation by keratinolytic bacteria and biofertilizing potential for sustainable agricultural production. J. Basic Microbiol. 59(1), 4–13 (2019)
Kshetri, P., Roy, S.S., Sharma, S.K., Singh, T.S., Ansari, M.A., Sailo, B., Singh, S., Prakash, N.: Feather degrading, phytostimulating, and biocontrol potential of native actinobacteria from North Eastern Indian Himalayan Region. J. Basic Microbiol. 58(9), 730–738 (2018)
Colla, G., Nardi, S., Cardarelli, M., Ertani, A., Lucini, L., Canaguier, R., Rouphael, Y.: Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 196, 28–38 (2015)
Kucinska, J.K., Magnucka, E.G., Oksinska, M.P., Pietr, S.J.: Bioefficacy of hen feather keratin hydrolysate and compost on vegetable plant growth. Compost. Sci. Util. 22(3), 179–187 (2014)
Jain, R., Jain, A., Rawat, N., Nair, M., Gumashta, R.: Feather hydrolysate from Streptomyces sampsonii GS 1322: A potential low cost soil amendment. J. Biosci. Bioeng. 121(6), 672–677 (2016)
Acknowledgements
AL appreciates the authority of Ladoke Akintola University of Technology, Ogbomoso, Nigeria for provision of some facilities used in this study. AIA is grateful to Tertiary Education Trust Fund (TETFund) for PhD sponsorship, and the authority of Federal University of Technology, Minna, Nigeria for the study leave granted to undertake this study. Farm Help Agrostores, Moniya, Ibadan, Oyo State is deeply appreciated for the gift of vegetable seeds.
Funding
This work received funding from Tertiary Education Trust Fund (TETFund), Nigeria.
Author information
Authors and Affiliations
Contributions
AL conceived, supervised, interpreted the results, wrote part of the manuscript and edited the manuscript; AIA carried out the entire laboratory and field investigations, collected, analyzed and interpreted the data and wrote draft of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that there is no conflict of interest.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Adelere, I.A., Lateef, A. Valorization of Feather by Bacillus safensis and Aquamicrobium defluvii for Growth Promotion in Leafy Vegetables. Waste Biomass Valor 14, 723–737 (2023). https://doi.org/10.1007/s12649-022-01904-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-022-01904-9