Abstract
The world generates millions of tons of bioorganic waste in the form of peels annually, thus requiring more sustainable solutions to valorise these bioresources. Therefore, the objectives of the current study are the physicochemical and microbiological characterization of pomegranate (PP), banana (BP), and tangerine (TP) peels and the study of the effects of these peels and their mixtures (PBTP) at each stage of their decomposition in water and soil, as well as the effects of their decomposition water on the germination and growth of Pisum sativum. Based on the physicochemical and microbiological characteristics of PP, BP, and TP (C/N ratio: 29.57 ± 0.51, 23.05 ± 0.38, and 19.28 ± 0.39 respectively), these wastes can be used as bioorganic fertilizers to improve the physicochemical and microbiological properties of the soil. Moreover, their use as bioorganic fertilizers showed positive effects on germination (94.44% by using PBTP without decomposition and 94.44% by using PP after 2 months of decomposition in the soil) and growth of P. sativum (number of pods 8.33 ± 0.58 and 8.67 ± 0.58 by using PP and TP after 2 months of decomposition in the soil). As shown by the obtained results, pomegranate, banana, and tangerine peels can be used as promising and environmentally friendly bioorganic fertilizers that can substitute chemical fertilizers.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
The current study is a new contribution since it assesses the effects of PP, BP, TP, and PBTP at each stage of their decomposition in water and soil, and the water of their decomposition on seed germination and cultivation of P. sativum. Additionally, the present research investigated the physicochemical and microbiological characteristics of PP, BP, and TP in more detail. The current article will be beneficial for academic research and farmers, new scientists, managers of the fruit processing industry, and the public because it shows a more appropriate and suitable solution for the valorisation of PP, BP, and TP to protect the environment and human health.
Introduction
Currently, many farmers use chemical fertilizers to improve the physicochemical properties of soils as well as crop production. However, the increased use of chemical fertilizers disrupts the food chain through eutrophication, pollution of air, surface water, sub-surface water, and soil by incorporating heavy metals, and also presents risks to human health [1, 2]. Therefore, bioorganic fertilizers are the best solution for the preservation of the environment and human health. Yet, organic farming currently provides several fertilizer sources to be used as bioorganic fertilizers in agriculture, thus also increasing the opportunities for cost-effectiveness [2].
The world generates millions of metric tons of organic waste every day [3]. In 2018, the world produced about 34.72 to 46.29 million metric tons of BP. In 2017, the world produced about 1.9 million metric tons of PP. In 2019, the world produced about 12.68 to 15.85 million metric tons of TP [4]. The majority of these wastes are obtained as by-products from the food industry, juice companies, restaurants, as well as household waste [4]. Traditional organic waste disposal strategies are currently insufficient and hurt the environment and human health [5]. Furthermore, to minimize management costs and prevent damage to the environment and human health, several utilizations for tangerine, pomegranate, and banana processing wastes have been evaluated. Some studies have focused on using fruit peels immediately or after drying in the soil to enhance its physicochemical and microbiological properties and to improve plant productivity. In their literature review of recent scientific articles, El Barnossi et al. [4] have discussed that PP, BP, and TP can be successfully used as fertilizers to improve soil fertility and enrich soil microbiota due to their content of minerals necessary for crop growth, whereas BP is a potassium-rich organic waste that could be used as a potassium fertilizer, thus an alternative source of potassium for crop productivity [6]. Also, Anastopoulos et al. [7] have documented that the incorporation of TP and BP into the soil significantly affects the structure of the bacterial community related to nitrous oxide releases. However, the utilization of these wastes as an organic amendment is very poorly documented, and the study of the effects of PP, BP, TP, and PBTP at each stage of their decomposition in water and soil, and the water of their decomposition in comparison with control soil and compost on seed germination and growth of P. sativum particularly and plant crops generally, to the best of our knowledge, has not been studied until now.
The input of these fruit peels into the soil without physicochemical characterization and possible treatment can lead to soil perversion and potential contamination of soil, water, and air. Accordingly, environmental requirements must be adequately accounted for concerning the preservation of the environment. Hence, the most sustainable solutions for the recovery of these peels should include a combination of the highest efficiency measures and sustainable recovery processes, particularly concerning the environment and human health. For the previous reasons, the objectives of the current study are (i) the physicochemical and microbiological characterization of PP, BP, TP, compost, water, and soil used for seed germination and cultivation of P. sativum and (ii) the study of the effect of PP, BP, TP, and PBTP at each stage of their decomposition in water and soil, and the water of their decomposition comparatively with control soil and compost on seed germination and cultivation of P. sativum to investigate their suitability for utilization as promising and environmentally friendly bioorganic fertilizers.
Materials and Methods
Collection and Decomposition of Pomegranate Peel, Banana Peel, Tangerine Peel, and Their Mixtures in Water and Soil
Pomegranate, banana, and tangerine peels were collected from fruit juice sellers in the city of Fez, Morocco, these wastes were received in sterile polyethylene bags. For the decomposition in water, the collected peels were put in mesh bags (in polyethylene, 10 cm wide and 15 cm long) in the order of 150 ± 1.00 g, and then were introduced separately in 20 L polyethylene buckets, filled with 15 ± 0.01 L of well water from the Faculty of Sciences Dhar El Mahraz in Fez, Morocco (see characteristics in the results section, Table 2). For the decomposition in soil, all peels were dried at room temperature (between 15 and 35 °C), then cut up and put into pots containing 5 ± 0.01 kg of garden soil of the Faculty of Sciences Dhar El Mahraz Fez, Morocco (see characteristics in the results section, Figs. 1 and 2; Tables 3 and 4) in the order of 50 ± 1.00 g in each pot. The decomposed peels in the soil were irrigated periodically with well water to maintain a favourable moisture content for the decomposition. Two parameters were monitored to maintain constant moisture content, soil moisture, and temperature to determine the volume of water to be used for irrigation to maintain a favourable and constant soil moisture content for peel decomposition.
Physicochemical and Microbiological Characterization of Pomegranate Peel, Banana Peel, Tangerine Peel, Compost, Water, and Soil Used for the Growth of P. sativum
Physicochemical Characterization
pH, temperature, electrical conductivity, turbidity, DO, COD, BOD5, NH4+, NO3−, NO2−, PO43−, SO42− and salinity were measured according to the standard methods of Rodier et al. [8]. Moisture was determined by the method used by Ghanem et al. [9]. The alcohol-solubles were determined in accordance with Stevenson [10]. Ether-solubles are extracted with diethyl ether, in the same way as alcohol-solubles, from the residues remaining from the alcoholic extraction. To determine the total water-solules, each residue, freed from the alcohol and ether-soluble faction, is suspended in 20 ml of distilled water, then autoclaved for 1 h at 120 °C to obtain, after centrifugation and filtration, two fractions; one containing the water-solubles (supernatant + filtrate), and the other containing the insoluble (pellet). Water-soluble sugars were determined by the standard method of Dubois et al. [11]. The water-soluble proteins were determined by the standard technique of Lowry et al. [12]. Water-soluble phenols were determined using the “2–6-dibromoquinone-chloroimide” technique described by Rodier et al. [13]. The cellulose and the hemicellulose were evaluated according to David and Fornasier, [14]. Total Kjeldahl nitrogen was estimated according to Awasthi et al. [15]. Total organic carbon was determined by wet digestion according to the method of Heanes, [16].
Microbiological Characterization
Global Microbial Activities
To assess the global microbial activity, three activities were studied. The release of CO2 was monitored according to the standard method described by Crawford et al. [17]. The dehydrogenase activity was determined by using TTC (2,3,5-triphenyl-tetrazolium-chloride) as substrate, according to the method adopted by Sardar et al. [18]. The global hydrolytic activity was determined by using FDA (fluorescein diacetate) as substrate, according to the method described by Chergui and Legssyer, [19].
Systematic and Functional Microbial Groups
Systematic groups were enumerated by using elective culture media modified by enrichment with 7% (v/v) peel extracts and by using well water [20]. The abundance of total bacteria, moulds, yeasts, and actinomycetes was estimated on their respective media: nutrient broth medium, agar-malt medium, YPG (yeast, peptone, and glucose) medium, and actinomycetes medium by utilization of the suspension-dilution method; 0.1 ml of each dilution (10–1 to 10–8) prepared from the homogenate (10 g of peel + 90 ml of sterile spring water) was deposited separately in the centre of the medium in 3 Petri dishes of 90 mm diameter, after spreading on the surface and incubation at 28 °C for 7 days, the colonies that appeared were counted for each dilution, and then the average number of CFU (colony-forming units) per gram of dry weight was calculated for each peel [20]. For the microbiological analysis of the well water, total and faecal coliforms were enumerated using BGBLB (Brilliant Green Bile Lactose Broth) medium, according to the standard method of Raugel, [21]. While for the quantification of functional groups, enumeration of amylolytic, aerobic nitrogen fixers, ammonifiers, denitrifiers, and nitrifiers, 1 ml in three replicates of each dilution (10–1 to 10–8) corresponding to each type of peel was added separately to the tubes containing the elective culture media for each microbial group, after inoculation, the tubes were incubated at 28 °C for 7 days and the determination of the microbial load was done by determining the most probable number of germs (MPN) using the Mac Crady table [20].
Study of the Effect of Pomegranate Peel, Banana Peel, Tangerine Peel, and Their Mixtures Decomposing in Water and Soil and Water of Their Decomposition on Germination and Growth of P. sativum
Plant Material
In the present study, we evaluated the effect of different peels in comparison with compost and control soil for seed germination and growth of pea. The species Pisum sativum L (smooth pea) was procured from farmers in the region of Fez, Morocco. P. sativum is one of the most important legume species in the world. Due to its high sensitivity to inorganic and organic contaminants, it has been used as an indicator plant in various studies [22].
Germination Test of P. sativum Seeds
P. sativum seeds were sterilized with a 4% sodium hypochlorite solution for 30 min on a magnetic stirrer and then rinsed with sterile distilled water [22]. After the sterilization of the seed surface, 20 seeds were sown in each pot containing the different types of decomposing peels in water and soil or irrigated with water from their decompositions in comparison with the control soil and compost. After 5 days the germination rate (%) was calculated using the following equation [22]:
Monitoring the Growth Parameters of P. sativum Crop
The seeds were sown in pots containing the different types of peels (PP, BP, TP, and PBTP) decomposed in water and soil or irrigated with water from their decompositions in comparison with the control soil and compost; before decomposition, after 2 months, 4 months, and 6 months of decomposition between November 2020 and July 2021. The crops were irrigated with well water of the Faculty of Sciences Dhar El Mahraz Fez, Morocco (See characteristics in the results section, Table 2). The soil used is slightly alkaline (pH 7.77 ± 0.047), with low carbon and nitrogen availability (See characteristics in the section results, Figs. 1 and 2; Tables 3 and 4). The data regarding the fresh weight of the aerial part, dry weight of the aerial part, stem height of aerial part, number of nodes, number of leaves, number of flowers, number of pods, and number of seeds. Were determined after 30, 60, and 90 days of sowing, as per Newman protocol [23].
Statistical Analyses
Quantitative results were expressed as means of triplicate experiments ± SD (standard deviation). The significance of the difference between the means was tested by analysis of variance (one-way and two way-ANOVA). Tukey’s multiple range tests and Dunnett’s multiple range tests at p < 0.05 were performed using GraphPad Prism 8.0.1 (Graph Pad Software Inc., San Diego, United States). Principal component analyses were performed using Minitab 19.1.1 software (Minitab Inc., State College, USA).
Results and Discussion
Physicochemical and Microbiological Characteristics of Pomegranate Peel, Banana Peel, Tangerine Peel, Compost, Water, and Soil Used for Germination and Growth of P. sativum
Main Constituents of Pomegranate, Banana, and Tangerine Fruits
The pomegranate fruit consists of the peel, pulp, and seeds (Table 1), 49.95 ± 3.636% of the fresh weight of this fruit consists in the peel. Our result is similar to Jalal et al. [24] who also showed that about 50% of the weight of pomegranate consists in the peel. The tangerine fruit also consists of the peel, pulp, and seeds (Table 1), 42.75 ± 1.47% of the fresh weight of this fruit consists in the peel. Our result is in line with the study of Kashyap et al. [25] which has shown that the tangerine fruit consists of between 40 and 50% peel. The banana fruit consists only of the peel and the pulp (Table 1), 33.20 ± 2.044% of the fresh weight of this fruit consists in the peel. Our result is in line with Albarelli et al. [26] who have been shown that the banana fruit consists of between 30 and 40% peel. Returning to the worldwide production of these three fruits, and the percentage of peels in each fruit, the world generates millions of metric tons of peels of these fruits each year as a result of the large production and consumption of these fruits worldwide, which requires finding optimal and sustainable recovery pathways to change the traditional strategies of dumping these wastes in landfills and thus protecting the environment and human health.
Physicochemical and Microbiological Characteristics of Well Water Used for Peels Decomposition and P. sativum Crop Irrigation
The well water used for the decomposition of the studied peels and the irrigation of the P. sativum crop was characterized physicochemically and microbiologically (Table 2) to show the quality of the water used. The water used is characterized by a slightly alkaline pH, with very low concentrations of Dissolved oxygen, Chemical oxygen demand, Biochemical oxygen demand for 5 days, Ammonia, Nitrate, Nitrite, Orthophosphate, Sulphate, and Total Kjeldahl nitrogen. Concerning the microbiological characteristics, the well water used contains very low densities of microorganisms especially coliforms, it contains only 4.66 ± 1.15 Cells/ml of total coliforms, and it does not contain faecal coliforms. Based on the current results of the physicochemical and microbiological characteristics of the well water used, and based on these comparisons with the standard norms [27, 28], we can conclude that the well water used is of good quality, so it does not have any harmful effect on the physicochemical and microbiological properties of the soil and also on the growth of the plant studied particularly and the plants generally.
Physicochemical and Microbiological Characteristics of Pomegranate Peel, Banana Peel, Tangerine Peel, Compost, and the Soil Used for Germination and Growth of P. sativum
Physicochemical Characteristics
The physicochemical characteristics of the peels used for germination and growth of P. sativum show a statistically significant difference between TP, PP, BP, compost, and the soil used, for total water-soluble, water-soluble phenols, cellulose, total Kjeldahl nitrogen, and total organic carbon (Table 3). While they show a statistically no significant difference for the other physicochemical characteristics examined (Table 3). TP, PP, and BP have good C/N ratios (< 40), so they are in the optimal range because reports have shown that a C/N ratio of about 25 to 30 is optimal, while a higher C/N ratio slows down the rate of decomposition of organic matter and a lower C/N ratio leads to a loss of nitrogen, generally, net immobilization of nitrogen occurs when the C/N ratio is above 20 [29]. Furthermore, TP and PP and BP have a reliable pH of 4.83 ± 0.091, 4.24 ± 0.057, and 5.74 ± 0.13 respectively, which shows that alkaline soils are the best soils that can be used for crops when using these wastes as an organic amendment to reach the optimal pH of the pea crop which is between 6 and 7 [30]. In addition, soil pH levels near 7 are optimal for overall nutrient availability, crop tolerance, and soil micro-organism activity [31]. However, the physicochemical composition of TP, PP, and BP depends on several factors, such as the method of cropping, the time of harvesting, the stage of maturation, and the various juice extraction techniques applied to the fruit [32]. But thanks to these physicochemical characteristics summarized in Table 3, TP, PP, and BP can be used as an organic amendment to improve the physicochemical properties of the soil and consequently the yield of crops.
Microbiological Characteristics
For the microbiological characterization of TP, PP, BP, compost, and the control soil, the overall microbial activities (Fig. 1), the systematic microbial groups (Fig. 2), and the functional microbial groups (Table 4) were studied to show the safety of using these peels as an organic amendment. Concerning the overall microbial activity, Fig. 1A illustrates that CO2 release is high in BP compared to other peels, compost, and the control soil, Fig. 1B shows that hydrolytic activity is high in all three peels and also in compost and the control soil, and Fig. 1C indicates that dehydrogenase activity is high in PP compared to BP, TP, compost, and the control soil. The results of the overall microbial activity show statistically no significant differences between TP, PP, BP, compost, and the control soil for hydrolytic activity. But they show statistically significant differences for CO2 release and dehydrogenase activity, especially for BP compared to other substrates in the case of CO2 release, and for PP compared to other substrates in the case of dehydrogenase activity. As for the results of the systematic microbial group’s study, we found significant differences for some microbial groups and no significant differences for the other groups between TP, PP, BP, compost, and the control soil (Fig. 2), PP has the highest amount of bacteria and moulds compared to other peels, compost, and control soil (Figs. 2A and B), TP and PP have a high yeast amount compared to BP, compost, and control soil (Fig. 2C), and the control soil has the highest level of actinomycetes than other substrates (Fig. 2D), thus showing that the density of systematic microbial groups depends on the microbial groups studied (Bacteria, yeasts, moulds, and actinomycetes) on the one hand, and the substrates used (TP, PP, BP, compost, and the control soil) on the other. Concerning the functional microbial groups, we found an absence of aerobic nitrogen fixers for all substrates studied except for compost, and an absence of nitrifiers for all substrates. However, for the remaining microbial groups, we found densities that differ from one functional microbial group to another and from one substrate to another (Table 4).
Global microbial activity of PP, BP, TP, compost, and control soil used for germination and growth of P. sativum. A CO2 release; B Hydrolytic activity and C Dehydrogenase activity. Means (± SD, n = 3) denoted by the same letter indicate no significant difference according to Tukey’s multiple range tests at p < 0.05
Systematic microbial groups of PP, BP, TP, compost, and the control soil used for germination and growth of P. sativum. A Bacteria; B Moulds; C Yeasts and D Actinomycetes. Means (± SD, n = 3) denoted by the same letter indicate no significant difference according to Tukey’s multiple range tests at p < 0.05
The microorganisms associated with TP, PP, BP, compost, and control soil are varied according to the varieties, the study period, the study conditions, and the study methods. In the published research, many scientific studies have concentrated on the microbiological study of TP, PP, and BP in the context of compost or vermicompost preparation [33]. Yet, the study of the global microbial activities, systematic and functional microbial groups of TP, PP, and BP is very poorly documented until now. There are only a few research studies that focus on the study of these microorganisms, such as the study of El Barnossi et al. [20] which showed a quantitative difference for the systematic and functional microbial groups associated with decomposing PP and BP in water and soil, and also the study of El Barnossi et al. [34] which reported that the CO2 release of the microflora associated with the decomposition of TP and PP in water and soil evolves according to a sigmoid model over time, and an increase in the dehydrogenase activity and hydrolytic activity as a function of time of the decomposition of TP and PP in water and soil. Based on these microbiological characteristics and based on the comparison of these characteristics with those of compost and control soil. TP, PP, and BP could be used as bioorganic fertilizers to improve the microbiological properties of soils without any harmful effects on the environment and human health.
Effect of Pomegranate Peel, Banana Peel, Tangerine Peel, and Their Mixtures at Each Stage of Their Decomposition in Water and Soil, and the Water of Their Decomposition on Seed Germination and Growth of P. sativum
Germination Rates
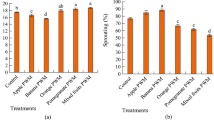
The germination rates of P. sativum by the application of PP, BP, PBTP decomposing in water and soil, and the water of their decompositions in comparison with compost and control soil show a promising result which is summarised in Fig. 3. For the peels used without decomposition; BP, PBTP, and compost show significant results compared to the control soil (p < 0.05), were respectively, 83.33 ± 16.67, 94.44 ± 9.62, and 94.44 ± 9.62% (Fig. 3A). For peels used after 2 months of decomposition in water and soil, Fig. 3B illustrates that PP/Soil, BP/Soil, PP/Water, TP/Water, Water/PBTP, and compost presented the highest germination rates compared to the other peels and the control soil (p < 0.05). For peels used after 4 months of decomposition in water and soil, Fig. 3C indicates that only PBTP/Soil and compost showed high germination rates compared to the control soil (p < 0.05). For the peels used after 6 months of decomposition in water and soil, Fig. 3D illustrates that PP/Soil, PP/Water, BP/Water, PBTP/Water, and compost present the highest germination rates compared to the control soil (p < 0.05). The current results indicate that the germination rate of P. sativum seeds varies according to the substrates used as organic amendments and also according to the time of decomposition in water or soil. Furthermore, these results show that PP, BP, and TP decompose in water and soil, and the water from their decompositions could be used as promising bioorganic fertilizer for the germination of P. sativum seeds particularly and for the germination of other plants seeds generally.
Germination rates of P. sativum seeds by using PP, BP, TP, and PBTP decomposed in water and soil. A peels without decomposition; B peels after 2 months of decomposition; C peels after 4 months of decomposition and D peels after 6 months of decomposition. Means (± SD, n = 3) denoted by stars indicate a significant difference in comparison to the control soil according to Dunnett’s multiple range tests at p < 0.05
Few scientific research studies have focused mainly on the use of fruit peels for the seed germination of crops. But after extensive research in the scientific literature, we were lucky to find some researches that are useful for the discussion of the present results. As Balliu and Sallaku, [35] have shown that environmental temperature is a key factor involved in seed germination of P. sativum L., and also Švubová et al. [36] have demonstrated that seed germination is the first critical moment in the life cycle of every plant and is strongly influenced by many biotic and abiotic factors. Numerous other studies have indicated that the improvement of seed germination can be attributed to the role of some important microorganisms that influence the availability of nitrogen, phosphorus, and potassium in the soil, which improves the metabolic activity of the cells and increases germination rates [37]. Our result is similar to Kamboj et al. [38] who have found that application of vermicompost in P. sativum crop significantly affected seed germination. However, our result is in contradiction with the study of Adam and Ramdhani, [39] who showed that leachate from biowaste of selected invasive alien plant species did not have significant effects on seed germination of maize and pea.
Growth Parameters of P. sativum
The variation of different growth parameters of P. sativum through the application of PP, BP, TP, and PBTP in water and soil decomposition and the water of their decompositions according to decomposition times and in comparison with compost and control soil are summarised in Tables 5 and 6. For the various types of peels without decomposition (Table 5), the results obtained show that these peels present positive effects on the various parameters of the growth of P. sativum, especially the number of seeds, all the peels and the compost present statistically no significant differences between each other’s (p < 0.05). But they show statistically significant differences compared to the seeds obtained by using control soil (p > 0.05). For the results of the effects of different types of peels decomposed in water and soil and the water of their decompositions (Table 6), these peels have improved significantly the growth parameters such as seed germination rate, number of leaves, number of flowers, number of pods, number of seeds, number of nodes, stem height of aerial part, fresh weight of the aerial part, and dry weight of aerial part of P. sativum as well as the effects on the growth of P. sativum are varied depending on the time of decomposition and the type of peels used as an organic amendment. Moreover, these peels after 2 months of decomposition have proved the best effects on the improvement of the growth parameters of P. sativum among all other decomposition times (at p < 0.05).
As for the results of the statistical correlations (Fig. 4), the principal component analysis of PP, BP, TP, and PBTP decomposing in water and soil, the water of their decompositions, compost, and control soil (Fig. 4A) show that the effect of TP/Soil, Water/PBTP, and PP/soil are positively correlated with each other, and also the compost, BP/Soil, PP/Water and PBTP/Soil are positively correlated with each other concerning all other types of peels decomposing in water and soil, water from their decompositions and control soil. According to these results, the different decomposing peels in the soil are the most successful organic amendments for improving the growth parameters of P. sativum. The statistical correlations of the different growth parameters of P. sativum through principal component analysis in the C1–C2 plane (Fig. 4B), show that nearly all parameters are positively correlated with the first plane. This means that all parameters studied according to time and peel types are positively correlated with each other on the one hand and they have direct effects on the growth of P. sativum on the other hand. The current results clearly show that PP, BP, TP decomposed in water and soil, and also the water of their decompositions could be used as the best bioorganic fertilizers to improve the physicochemical and microbiological properties of the soil, as well as the yield of the P. sativum crop specifically and the yield of crops generally.
Statistical correlations. A Principal component analysis of PP, BP, TP, and PBTP decomposed in water and soil, and the water of their decompositions. B Principal components analysis in the C1–C2 plane presenting the correlations of different studied parameters of P. sativum cultivation. FW fresh weight, DW dry weight, SH stem height, NN The number of nodes, NL The number of leaves, NF The number of flowers, NP The number of pods, NS The number of seeds. A After 2 months of decomposition; B After 4 months of decomposition; C After 6 months of decomposition; 30. Observation after 30th day; 60. Observation after 60th day and 90. Observation after 90th day
Tangerine, pomegranate and, banana peels are very high in the most nutritious minerals. BP biochar is highly potassium content that is suitable for application as potassium fertilizer, thus providing an important source of potassium for crop yields [40]. BP, when decomposed in the soil increases the phosphorus content, suggesting that incorporation of this peel might enhance the soil with phosphorus [41]. Many research reports have documented that the utilization of TP, PP, and BP as biofertilizers for soil enrichment has other advantages, for example, the research by Cao et al. [42] which has demonstrated that biochar based on PP has a significant capacity as an adsorbent for Cu (II) disposal in the soil, and the research of Sial et al. [43] which has shown the reduction of greenhouse gas releases through the use of BP and its biochar. Also, Anastopoulos et al. [7] have been demonstrated that the incorporation of TP and BP into the soil is known to have a substantial impact on the bacterial community pattern. The usage of TP, PP, and BP or their biochar also helps plants to resist diseases [4]. Numerous scientists such as Maji et al. [44] and Singh et al. [22] have shown that plant growth is directly proportional to the increase in parameters such as seed germination rate, number of leaves, number of flowers, number of pods, number of seeds, number of nodes, height, fresh weight, and dry weight. Our conclusion fits very well with the previous report of Maji et al. [44] who found that the growth parameters showed an increase in total height, fresh weight, and dry weight by uses of organic fertilizers, such as manure, compost, and vermicompost. Many other reports show that the addition of fresh organic matter, such as green manures, mulch, composts, and vermicompost intensifies the mineralization of soil organic matter [45]. The explanation for the better improvement of growth parameters obtained by application of different peels after 2 months of decomposition might be due to the time of decomposition, and our conclusion is in line with that of Frink et al. [46] who found that the nutrients present in organic fertilizers can be released only when their decomposition takes a long time. Moreover, of the essential nutrients nitrogen, phosphorus, and potassium needed by crop plants, nitrogen is most often deficient in tropical soils and is widely available through the decomposition of organic matter [47]. The present results are similar to Chinthapalli et al. [48] who have studied the effect of organic and inorganic fertilizers on the agronomic performance of faba bean (Vicia faba L.) and pea (P. sativum L.). And to Kamboj et al. [38] who have shown that organic fertilizer was good for pea production. Also, to Khan et al. [49] who have demonstrated distinct differences between vermicompost, put compost, and garden soil (control) in terms of nutrient content and effect on P. sativum growth. Furthermore, Eid et al. [50] have reported that root, shoot and pod length, biomass, and the number of leaves and pods of P. sativum increased with the different amendments used. However, our results are opposite to those of Adam and Ramdhani [39] who have shown that composts of biowaste of selected invasive alien plant species have not significantly improved the growth of maize and pea compared to the commercial compost.
Conclusions
According to the physicochemical and microbiological characteristics of PP, BP, and TP, these wastes could be the best bioorganic fertilizers to improve the physicochemical and microbiological properties of soils, as well as the productivity of crops. In the greenhouse experiments, parameters such as seed germination rate, number of leaves, number of flowers, number of pods, number of seeds, number of nodes, stem height, fresh weight, and dry weight of P. sativum were significantly improved by the application of these peels. In conclusion, the different peels studied could be used as promising and environmentally friendly bioorganic fertilizers.
Data Availability
The data was not deposited in public repositories.
Abbreviations
- BP/soil:
-
Banana peel decomposing in the soil
- BP/water :
-
Banana peel decomposing in water
- BP:
-
Banana Peel
- DW:
-
Dry weight
- FW:
-
Fresh weight
- No:
-
Number
- OD:
-
Optical Density
- P. sativum :
-
Pisum sativum
- PBTP/soil:
-
A mixture of decaying pomegranate, banana, and tangerine peels in soil
- PBTP/water:
-
A mixture of decaying pomegranate, banana, and tangerine peels in water
- PBTP:
-
Pomegranate, banana, and tangerine peel mixtures
- PP/soil:
-
Pomegranate peel decomposing in the soil
- PP/water:
-
Pomegranate peel decomposing in water
- PP:
-
Pomegranate Peel
- TP/soil:
-
Tangerine peel decomposing in the soil
- TP/water:
-
Tangerine peel decomposing in water
- TP:
-
Tangerine Peel
- Water/BP:
-
Water from the decomposition of banana peel
- Water/PBTP:
-
Water from the decomposition of a mixture of pomegranate, banana, and tangerine peels
- Water/PP:
-
Water from the decomposition of pomegranate peel
- Water/TP:
-
Water from the decomposition of tangerine peel
References
Guo, J.K., Muhammad, H., Lv, X., Wei, T., Ren, X.H., Jia, H.L., Atif, S., Hua, L.: Prospects and applications of plant growth-promoting rhizobacteria to mitigate soil metal contamination: a review. Chemosphere 246, 125823 (2020). https://doi.org/10.1016/j.chemosphere.2020.125823
Ugulu, I., Ahmad, K., Khan, Z.I., Munir, M., Wajid, K., Bashir, H.: Effects of organic and chemical fertilizers on the growth, heavy metal/metalloid accumulation, and human health risk of wheat (Triticum aestivum L.). Environ. Sci. Pollut. Res. 28, 12533–12545 (2021). https://doi.org/10.1007/s11356-020-11271-4
Wei, Y., Wang, N., Lin, Y., Zhan, Y., Ding, X., Liu, Y., Zhang, A., Ding, G., Xu, T., Li, J.: Recycling of nutrients from organic waste by advanced compost technology—a case study. Bioresour. Technol. (2021). https://doi.org/10.1016/j.biortech.2021.125411
El Barnossi, A., Moussaid, F., Iraqi Housseini, A.: Tangerine, banana, and pomegranate peels valorization for sustainable environment: a review. Biotechnol. Rep. 29, e00574 (2021). https://doi.org/10.1016/j.btre.2020.e00574
Tenodi, S., Krčmar, D., Agbaba, J., Zrnić, K., Radenović, M., Ubavin, D., Dalmacija, B.: Assessment of the environmental impact of sanitary and unsanitary parts of a municipal solid waste landfill. J. Environ. Manag. 258, 1–15 (2020). https://doi.org/10.1016/j.jenvman.2019.110019
Kalemelawa, F., Nishihara, E., Endo, T., Ahmad, Z., Yeasmin, R., Tenywa, M.M., Yamamoto, S.: An evaluation of aerobic and anaerobic composting of banana peels treated with different inoculums for soil nutrient replenishment. Bioresour. Technol. 126, 375–382 (2012). https://doi.org/10.1016/j.biortech.2012.04.030
Anastopoulos, I., Omirou, M., Stephanou, C., Oulas, A.: Valorization of agricultural wastes could improve soil fertility and mitigate soil direct N2O emissions. J. Environ. Manag. 250, 109389 (2019). https://doi.org/10.1016/j.jenvman.2019.109389
Rodier, J., Legube, B., Merlet, N.: L’analyse de l’eau. Dunod, Paris (2016)
Ghanem, N., Mihoubi, D., Kechaou, N., Mihoubi, N.B.: Microwave dehydration of three citrus peel cultivars: effect on water and oil retention capacities, color, shrinkage and total phenols content. Ind. Crops Prod. 40, 167–177 (2012). https://doi.org/10.1016/j.indcrop.2012.03.009
Stevenson, F.J.: Gross chemical fractionation of organic matter. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. (2016). https://doi.org/10.2134/agronmonogr9.2.c43
Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A., Smith, F.: Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356 (1956). https://doi.org/10.1038/168167a0
Lowry, O.H., Roserough, N.J., Farr, A.L., Randall, R.J.: Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 (1951). https://doi.org/10.1016/s0021-9258(19)52451-6
Rodier, J., Bazin, C., Broutin, J., Chambon, P., Champsaur, H., Rodier, L.: L’Analyse de l’Eau. Dunod, Paris (1996)
David, C., Fornasier, R.: Hydrolysis of cellulose and hemicellulose: valorization of lignin. A new pretreatment for lignocellulosic substrates. In: Ferrero, G.L., Ferrante, M.P., Naveau, H. (eds.) Anaerobic digestion and carbohydrate hydrolysis of waste, pp. 69–82. Elsevier, London (1984)
Awasthi, M.K., Pandey, A.K., Bundela, P.S., Khan, J.: Co-composting of organic fraction of municipal solid waste mixed with different bulking waste: characterization of physicochemical parameters and microbial enzymatic dynamic. Bioresour. Technol. 182, 200–207 (2015). https://doi.org/10.1016/j.biortech.2015.01.104
Heanes, D.L.: Determination of total organic-c in soils by an improved chromic acid digestion and spectrophotometric procedure. Commun. Soil Sci. Plant Anal. 15, 1191–1213 (1984). https://doi.org/10.1080/00103628409367551
Crawford, D.L., Doyle, J.D., Wang, Z., Hendricks, C.W., Bentjen, S.A., Bolton, H., Fredrickson, J.K., Bleakley, B.H.: Effects of a lignin peroxidase-expressing recombinant, Streptomyces lividans TK23.1, on biogeochemical cycling and the numbers and activities of microorganisms in soil. Appl. Environ. Microbiol. 59, 508–518 (1993). https://doi.org/10.1128/aem.59.2.508-518.1993
Sardar, K., Qing, C., Abd El-Latif, H., Yue, X., Ji-zheng, H.: Soil enzymatic activities and microbial community structure with different application rates of Cd and Pb. J. Environ. Sci. 19, 834–840 (2007)
Chergui, H., Legssyer, B.: Variation in the activity of micro-organisms during the decomposition of Salix pedicellata leaves in the Basse Moulouya network, eastern Morocco. Ann. Limnol. 31, 287–298 (1995). https://doi.org/10.1051/limn/1995026
El Barnossi, A., Moussaid, F., Iraqi, H.A.: Quantitative research of systematic and functional microbial groups associated with decaying solid green household waste in water and soil. Polish J. Environ. Stud. 29, 2631–2639 (2020). https://doi.org/10.15244/pjoes/112365
Raugel, P.: 3M microbiology. In: Rapid food analysis and hygiene monitoring, pp. 614–648. Springer, Berlin, Heidelberg (1999)
Singh, S., Kumar, V., Sidhu, G.K., Datta, S., Dhanjal, D.S., Koul, B., Janeja, H.S., Singh, J.: Plant growth promoting rhizobacteria from heavy metal contaminated soil promote growth attributes of Pisum sativum L. Biocatal. Agric. Biotechnol. 17, 665–671 (2019). https://doi.org/10.1016/j.bcab.2019.01.035
Goubran, F.H., Richards, D.: The estimation of root length in samples and sub-samples—comparison of a visual and an automatic method. Plant Soil. 52, 77–83 (1979). https://doi.org/10.1007/BF02197734
Jalal, H., Pal, M.A., Ahmad, S.R., Rather, M., Andrabi, M., Hamdani, S.: Physico–chemical and functional properties of pomegranate peel and seed powder. Pharma Innov. J. 7, 1127–1131 (2018)
Kashyap, K., Kashyap, D., Nitin, M., Ramchiary, N., Banu, S.: Characterizing the nutrient composition, physiological maturity, and effect of cold storage in Khasi Mandarin (Citrus reticulata Blanco). Int. J. Fruit Sci. 20, 521–540 (2020). https://doi.org/10.1080/15538362.2019.1666334
Albarelli, J.Q., Rabelo, R.B., Santos, D.T., Beppu, M.M., Meireles, M.A.A.: Effects of supercritical carbon dioxide on waste banana peels for heavy metal removal. J. Supercrit. Fluids. 58, 343–351 (2011). https://doi.org/10.1016/j.supflu.2011.07.014
Mastoi, G.M., Shah, S.G.S., Khuhawar, M.Y.: Assessment of water quality of Manchar Lake in Sindh (Pakistan). Environ. Monit. Assess. 141, 287–296 (2008). https://doi.org/10.1007/s10661-007-9895-8
Hashmat, S., Shahid, M., Tanwir, K., Abbas, S., Ali, Q., Niazi, N.K., Akram, M.S., Saleem, M.H., Javed, M.T.: Elucidating distinct oxidative stress management, nutrient acquisition and yield responses of Pisum sativum L. fertigated with diluted and treated wastewater. Agric. Water Manag. 247, 106720 (2021). https://doi.org/10.1016/j.agwat.2020.106720
Chen, Y., Li, X., Li, S., Xu, Y.: Effect of C/N ration on disposal of pig carcass by co-composting with swine manure: experiment at laboratory scale. Environ. Technol. (2020). https://doi.org/10.1080/09593330.2020.1760358
Havlin, J., Beaton, J., Tisdale, S., Nelson, W.: Soil fertility and fertilizers. Prentice-Hall, Upper Saddle River (1999)
McCauley, A., Jones, C., Jacobsen, J.: Soil pH and organic matter. Nutr. Manag. Modul. 8, 1–12 (2009)
Sharma, K., Mahato, N., Cho, M.H., Lee, Y.R.: Converting citrus wastes into value-added products: economic and environmentally friendly approaches. Nutrition 34, 29–46 (2017). https://doi.org/10.1016/j.nut.2016.09.006
Cerda, A., Artola, A., Font, X., Barrena, R., Gea, T., Sánchez, A.: Composting of food wastes: status and challenges. Bioresour. Technol. 248, 57–67 (2018). https://doi.org/10.1016/j.biortech.2017.06.133
El Barnossi, A., Moussaid, F., Iraqi, H.A.: Decomposition of tangerine and pomegranate wastes in water and soil: characterisation of physicochemical parameters and global microbial activities under laboratory conditions. Int. J. Environ. Stud. 76, 456–470 (2019). https://doi.org/10.1080/00207233.2019.1579584
Balliu, A., Sallaku, G.: The environment temperature affects post-germination growth and root system architecture of pea (Pisum sativum L.) plants. Sci. Hortic. (Amsterdam) 278, 109858 (2021). https://doi.org/10.1016/j.scienta.2020.109858
Švubová, R., Kyzek, S., Medvecká, V., Slováková, Ľ, Gálová, E., Zahoranova, A.: Novel insight at the effect of cold atmospheric pressure plasma on the activity of enzymes essential for the germination of Pea (Pisum sativum L. cv. Prophet) seeds. Plasma Chem. Plasma Process. 40, 1221–1240 (2020). https://doi.org/10.1007/s11090-020-10089-98
Ram, M., Dawari, R., Sharma, N.: Effect of organic manures on basmati rice (Oryza sativa L.) under organic forming of rice-wheat cropping system. Int. J. Agric. Crop Sci. 3, 76–84 (2011)
Kamboj, V., Bharti, M., Sharma, S.: Productive assessment of Pisum sativum L. by using organic fertilizers. Environ. Conserv. J. 17, 79–83 (2016)
Adam, Y., Ramdhani, S.: Maize and pea germination and seedling growth responses to compost generated from biowaste of selected invasive alien plant species. Compost. Sci. Util. 24, 30–41 (2016). https://doi.org/10.1080/1065657X.2015.1051633
Islam, M., Halder, M., Siddique, M.A.B., Razir, S.A.A., Sikder, S., Joardar, J.C.: Banana peel biochar as alternative source of potassium for plant productivity and sustainable agriculture. Int. J. Recycl. Org. Waste Agric. 8, 407–413 (2019). https://doi.org/10.1007/s40093-019-00313-8
Panwar, N.: Studies on physicochemical characteristics and fertility of soil by addition of banana peels—waste management. Int. J. Sci. Res. Dev. 3, 121–125 (2015)
Cao, Q., Huang, Z., Liu, S., Wu, Y.: Potential of Punica granatum biochar to adsorb Cu(II) in soil. Sci Rep (2019). https://doi.org/10.1038/s41598-019-46983-2
Sial, T.A., Khan, M.N., Lan, Z., Kumbhar, F., Ying, Z., Zhang, J., Sun, D., Li, X.: Contrasting effects of banana peels waste and its biochar on greenhouse gas emissions and soil biochemical properties. Process Saf. Environ. Prot. 122, 366–377 (2019). https://doi.org/10.1016/j.psep.2018.10.030
Maji, D., Misra, P., Singh, S., Kalra, A.: Humic acid-rich vermicompost promotes plant growth by improving microbial community structure of soil as well as root nodulation and mycorrhizal colonization in the roots of Pisum sativum. Appl. Soil Ecol. 110, 97–108 (2017). https://doi.org/10.1016/j.apsoil.2016.10.008
Wu, J., Brookes, P.C., Jenkinson, D.S.: Formation and destruction of microbial biomass during the decomposition of glucose and ryegrass in soil. Soil Biol. Biochem. 25, 1435–1441 (1993). https://doi.org/10.1016/0038-0717(93)90058-J
Frink, C.R., Waggoner, P.E., Ausubel, J.H.: Nitrogen fertilizer: retrospect and prospect. Proc. Natl. Acad. Sci. U.S.A. 96, 1175–1180 (1999). https://doi.org/10.1073/pnas.96.4.1175
Okeleye, K.A., Alofe, C.O.: Effect of nitrogen fertilizer source on dry matter accumulation and grain yields of open pollinatinated maize (Zea mays L.). Samaru J. Agric. Res. 12, 87–98 (1995)
Chinthapalli, B., Dibar, D.T., Chitra, D.S.V., Leta, M.B.: A comparative study on the effect of organic and inorganic fertilizers on agronomic performance of Faba Bean (Vicia faba L.) and Pea (Pisum sativum L.). Agric. For. Fish. 4, 263–268 (2015)
Khan, A., Ishaq, F., Sciences, N., Science, E.: Chemical nutrient analysis of different composts (Vermicompost and Pitcompost ) and their effect on the growth of a vegetative crop Pisum sativum. Asian J. Plant Sci. Res. 1, 116–130 (2011)
Eid, E.M., El-bebany, A.F., Taher, M.A., Alrumman, S.A., Galal, T.M., Shaltout, K.H., Sewelam, N.A., Ahmed, M.T.: Heavy metal bioaccumulation, growth characteristics, and yield of Pisum sativum L. grown in agricultural soil-sewage sludge mixtures. Plants 9, 1–15 (2020). https://doi.org/10.3390/plants9101300
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AEB: Conceptualization, Methodology, Formal analysis, Investigation, Visualization, Writing—original draft. FZM: Investigation, Methodology. HS: Review & editing, Formal analysis. BZ: Methodology. AIIH: Review & editing, Supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El Barnossi, A., Moussaid, F.Z., Saghrouchni, H. et al. Tangerine, Pomegranate, and Banana Peels: A Promising Environmentally Friendly Bioorganic Fertilizers for Seed Germination and Cultivation of Pisum sativum L.. Waste Biomass Valor 13, 3611–3627 (2022). https://doi.org/10.1007/s12649-022-01743-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-022-01743-8