Abstract
Coffee, one of the world’s most consumed beverages, is recognized for its peculiar aroma and flavor. Depending on the type of coffee processing, different co-products are generated as lignocellulosic materials that comprise husks, skin, pulp, mucilage, parchment, coffee silverskin and spent coffee grounds. These co-products could be converted into a highly attractive substrate for bioconversion processes, and they can be used due to their high added value. According to the type of coffee co-product and its chemical composition, different enzymatic technologies can be used like enzyme production, enzymatic hydrolysis, enzyme-assisted extraction, and others. The application of enzymatic processes for biomass reuse has been increasingly desired for being an environmentally friendly alternative improving the management of the vast quantity of waste generated by the coffee industry.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Statement of Novelty
Coffee co-products, rich in lignocellulose and valuable bioactives can be used as raw material in fermentation processes and can be exploited for the production of different value-added products. The difficulty of using lignocellulosic materials, like coffee co-products, comes up against two main obstacles: the cellulose-hemicellulose-lignin association, a natural barrier to enzymatic and microbial degradation and the crystalline structure of cellulose, resistant to hydrolysis. Despite the availability of several commercial formulations, there is scope for the development of intelligent enzyme formulations to guarantee an efficient and economical hydrolysis of coffee wastes. Further studies to obtain more effective pretreatments are necessary to achieve viable hydrolysis yields. This review aims to show an overview of types of enzymatic technology for coffee co-products utilization.
Introduction
Researches on the use of enzymes in coffee processing has been increasing, since coffee is a beverage with a characteristic aroma and flavor of great popularity in terms of world consumption [1] and is classified as commodities only losing to petroleum in terms of world trade marketed currency [2]. After water, coffee and tea are the two most commonly consumed beverages worldwide.
There are three different coffee processing routes: the dry, the wet, and the semi-wet route. Depending on the process used, there will be differences in the quality of the final product and the quantity and types of solid and liquid waste. Regardless of the process used, the production is estimated to generate 15 million tonnes per year of waste worldwide, of which 9.4 million tonnes are made up of coffee husks [3]. Therefore, in order to improve the production of the second most popular beverage in the world, enzymes have been used during the processing of the grain and also in the processing of its residues.
During the coffee processing, enzymes can be used to improve the final product, such as the case of pectinases that helps as an aid in the removal of coffee mucilage [4]. In this article, the natural fermentation (also used to demucilize coffee allowing the growth of natural microbe that produces enzymes necessary to depolymerize and hydrolyze the pectin in the mucilage) during the wet route will not be addressed. Beyond this, the enzymes asparaginase and acrylamidase can be used to decrease the content of acrylamide [5,6,7]. Peroxidases can be used to reduce the concentration of phenolic compounds in the wastewater of coffee processing in order to adapt it to the standards required by regulation from Brazilian National Environmental Council (CONAMA Number 430 of 05/13/2011) [8].
Coffee co-products are lignocellulosic materials that comprise husks, skin, pulp, coffee mucilage, coffee parchment, coffee silverskin, and spent coffee grounds. These co-products could be converted into highly attractive substrate for bioconversion processes. The structure of lignocellulosic material is based on three main components: cellulose, hemicellulose, and lignin. These three components offer biotechnological potential to be used as substrate in bioconversion processes and can be effectively exploited for the production of bulk chemicals and value-added products. Some significant products include enzymes (cellulases, hemicellulases, pectinases, amylases, proteases, galactosidases, tannase, and others), organic acids, pharmaceutics, chemicals, fuels and food [9]. Currently coffee co-product applications include biofuel, mushroom, and fertilizer production, and the extraction of dietary fiber, and bioactive compounds [10].
Coffee wastes are rich in valuable bioactives, that can be exploited as a nutraceutical or used in a range of food products. The breakdown of coffee lignocellulose can help the bioactives compounds extraction and generates metabolizable sugars that can be used for the production of various high-value products such as biofuels, amino acids and enzymes [11].
Enzymes are potential catalysts responsible for thousands of biochemical reactions involved in the biological processes of living systems [12]. When compared to chemical catalysts, the enzymes are characterized by their substrate specificity, ensuring the high conversion rates of a product without the formation of co-products [13]. Concerning enzyme applications in the food industry, the use of enzymatic technology is already an accomplished fact worldwide, with employment in baking, breweries, juices, wines, dairy products, and others.
This review aims to show an overview of what has been studied about the application of different types of enzymatic technology for utilization of coffee co-products in order to identify some knowledge gaps and trends on this subject.
Coffee Processing and Wastes
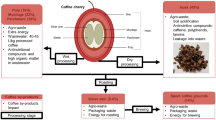
Processing is a significant activity in coffee industry that converts raw coffee fruit into liquid coffee. After harvesting, the fruits can pass through two basic methods of coffee processing, the dry and the wet route, which differ in complexity and the quality of the resultant raw coffee and the liquor [14]. An additional mode is a process known as the semi-wet route, a process variable by the wet route. For a better understanding of each step, it is necessary to observe the differences between the processes and the main stages of generating solid and liquid wastes (Fig. 1). Besides, the type of processes influence the content of the chemical constituents [15, 16], such as carbohydrates, lipids, proteins, minerals, and secondary metabolites present in the raw grains. These are the leading groups of coffee grain constituents, which affect the quality of the beverage characterized by the intense flavor and aroma after the roasting process [17, 18].
The dry route is usually used in regions with higher solar incidence, where the fruits are dried in their full form, immediately after harvesting, without grain separation, in an open yard or a mechanical dryer. In this route, the coffee beans are not selected and have different degrees of maturation, with extremely mature beans till beans that have been totally dried [14]. In a dry way, the coffee, after harvesting, is subjected as soon as possible to the processes of separation of the impurities before the preparation itself. The separation of the coarser impurities can be done by manual screening and shaking, with pre-cleaning machines, or with machines driven by the power take-off of a small tractor [19].
In the wet route, except manual picking, where only mature coffee beans (called cherry) are harvested, coffee fruits are usually harvested at various stages of ripening from green to ripe. As the green fruit grains are not physiologically well developed and smaller than the ripe ones, they can be separated according to their stage of ripening in the wet or semi-wet route during washing, allowing to obtain the mature (cherries), immature and excessively ripe coffees (fermented/float). Immature coffees and fermented coffees can go directly to drying similar to dry route [20].
After this separation, the coffee cherry fruits undergo mechanical removal of the husk still in direct contact with water and through disintegration equipment. Until this stage, there is the process known as semi-wet, where the husked grains, but still containing the mesocarp (mucilage) are subjected to drying in mechanical dryers or open yard. Following the removal of the husk from the wet route, the mesocarp will be removed mechanically or by controlled fermentation using the natural biota. The wet route requires a large volume of water to purge and remove mucilage consuming approximately 6000 L/h using traditional equipment [14, 17].
The dry route results in coffee called natural coffees or coconut, based on the drying of the fruits with husk, pulp, mucilage, parchment, and seeds. Already by wet route originates the pulped, shelled and demucilated coffees [21].
During the coffee fruiting process, however, a large amount of waste is generated in the form of pulp, mucilage, husks, and water waste. Different liquid and solid wastes are generated during coffee processing, both in wet and dry routes. These wastes can be a raw material source for the food, pharmaceutical, and cosmetic industry, and also can be used in power generation in mechanical dryer boilers and as fertilizers [22].
The agro-food wastes, like coffee wastes, are mainly composed of cellulose, hemicellulose, and lignin [23]. The lignocellulosic biomass is an abundant source of renewable organic material that can be converted into biofuels and other value-added products with high economic value.
Coffee wastes can be a source of the most interesting and valuable products, among which metals, oils and fats, lignin, cellulose and hemicelluloses, tannins, antioxidants, caffeine, polyphenols, pigments, flavonoids, through recycling, compound recovery or energy valorization, besides contributing to more sustainable and circular economies [24].
An enormous quantity of waste is generated at various stages of the processing of coffee beans. These residues (skin, pulp, husk, parchment, silverskin, and spent grounds) are substrates rich in carbohydrates (57%), proteins (24%), and minerals with potential for utilization in bioprocesses [25].
Second only to oil, coffee is the world's second most traded commodity, with about half a trillion cups consumed per year. Since more than 50% of the coffee fruit is not used for the production of the commercialized green coffee and, therefore, is discarded during processing, it should be interesting to find applications for these co-products. Like it was said before, depending on the processing, the production of coffee generate several co-products. The main by-product of the dry processing is composed of the skin, pulp, mucilage, and parchment, all together in a single fraction (coffee husks). In wet process, in contrast, potentially allows recovery of the skin and pulp in one fraction (43.2% w/w from the whole fruit), mucilage and soluble sugars in a second fraction when fermentation is not used (11.8% w/w) and, finally, the parchment (6.1% w/w). Husks, skin and pulp comprise nearly 45% (w/w) of the waste produced from coffee cherries, representing the primary residues of the coffee industry [22].
In wet processing, the parchment is removed after drying and hulling separate steps that permit the collection and use of parchment separately from other co-products [26]. The production of 60 kg bag of coffee grains approximately generates 11 kg of coffee parchment. However, parchment is one of the less-studied coffee co-products [22].
Concerning coffee wastes composition, coffee husks are rich in carbohydrates (35%), proteins (5.2%), fibers (30.8%), and minerals (10.7%) [27]. Coffee skin and pulp have a similar composition to that of the husks with carbohydrates (21–32%), protein (7.5–15.0%) and fat (2.0–7.0%) [28]. The mucilage is composed of water (84.2%), protein (8.9%), sugar (4.1%), pectic substances (0.91%) and ash (0.7%) [26]. Coffee parchment is composed of cellulose (40–49%), hemicellulose (25–32%), lignin (33–35%) and ash (0.5–1%) [29].
The primary coffee industry residues are coffee silverskin (CSS) and spent coffee grounds (SCG). Coffee silverskin is an agro-food waste obtained from roasting phase of green coffee beans. At the end of the coffee processing, a portion of coffee silverskin remains with the green coffee beans. Usually, producing countries export the green coffee beans with attached coffee silverskin to consuming countries, and here the beans are roasted by the suppliers [30]. Coffee silverskin represents about 4.2% (w/w) of coffee beans [10]. Considering that the global production of coffee beans is about 159 million of 60-kg bags (IOC, 2017/18), coffee silverskin is collected in a large amount (about 0.4 Mt per year) from large-scale roasting factories, making this residue easy to obtain and reuse for biorefinery applications. However, only a few studies focused on the use of CSS as biorefinery feedstock [30,31,32,33].
Spent coffee ground (SCG) is the leading coffee industry waste. SCGs are the insoluble residue that remains after coffee beans are dehydrated, milled and brewed, and there are two sources: those generated by the soluble coffee industry, which utilizes 50% of the global coffee harvest each year, and those generated by cafés and the public, accounting for the remaining 50%. Previously, soluble or “instant” coffee producers dumped large quantities of SCGs in landfills, a practice that might alter the local ecology because of the high oxygen demand during decomposition and the potential release of contaminants (e.g. caffeine, tannins, polyphenols, and others) [34]. Incorporating SCGs into animal feed is also limited due to the anti-nutritional activity of tannins. SCG contains large amounts of organic components (fatty acids, cellulose, etc.) making it suitable for different applications [35].
Soluble coffee production is responsible for the generation of approximately six million tons of SCG worldwide annually [36]. SCG contains a high proportion of polysaccharides (approximately 50% of dry weight), mainly cellulose (47.5%) and mannan (39.3%). The application of innovative methods such as enzymatic hydrolysis seems to be a promising strategy to reduce the amount of waste generated [37].
Life Cycle Assessment of Coffee
The importance of preserving the environment and control the emissions to the atmosphere in a more responsible manner is well known. The coffee activity covers several stages, including cultivation, transportation, roasting, packaging, beverage manufacture and disposal. In all the stages mentioned, there are potential risks to the environment [38, 39].
The high consumption of water and energy, the generation of solid waste and waste water with high levels of organic compounds, the release of gases, as well as deforestation and loss of biodiversity are some examples of environmental impacts generated with the cultivation, processing and use of coffee [40, 41].
In the meantime, life cycle assessment (LCA) is an effective tool used to assist in decision making to improve environmental performance by assessing the potential environmental impacts caused by products or activity systems since the beginning of the process until the end of using the product, from a “cradle to grave” perspective [39, 42].
However, depending on the evaluator objective, the LCA can be functional in a limited perspective “from process to process” if the focus is on an area of specific interest, enabling a careful evaluation of a part of the entire life cycle [39].
When it comes to coffee, studies using LCA have elucidated questions about environmental impacts and helped in decision making. The cultivation and manufacture of the beverage are significant from the point of view of environmental impacts [38, 43]. The cultivation of coffee is a major contributor to the damage caused to the ecosystem and the use of fertilizers is largely linked to this effect, since it requires a high quantity of fertilizers, whether chemical or organic, which are the main responsible for the emission of greenhouse gases [44,45,46].
Depending on the way in which the drink is made, this stage can be considered to be of great energy consumption. Coffee made by moka pot, for example, represented a large proportion of the energy consumption of the coffee life cycle, when compared to the manufacturing process by manual drip with filter and electric coffee maker, which generates environmental problems related to energy impacts [38, 43].
The stages of cultivation, treatment, processing, packaging and distribution carried out by coffee producers and suppliers are responsible for approximately 50% of the total environmental impacts and the other half is generated by consumers in the stages of use and disposal [38, 47]. Therefore, organic cultivation can reduce environmental impacts and also, consumers, who are responsible for the use stage, can act to reduce the environmental impacts of the coffee life cycle, choosing a more environmentally correct method of preparation or with devices with high energy efficiency [38].
Coffee Composting
It is known that population growth leads to an increase in the volume of solid waste that is generated and the lack of proper management of this waste brings unfavorable consequences to the environment, seriously reflecting on public health. Rural regions are of particular concern because they are often neglected in several aspects, including the lack of waste collection and this condition is no different in coffee plantation properties [48].
The coffee agribusiness produces a large amount of solid waste, such as coffee husk and pulp, being responsible for causing environmental damage and even the appearance of pathogenic insects by mistakenly discarding them in arable land and surface water, a responsible practice the main source of river pollution in Ethiopia and northern Latin America, for example [49,50,51]. The solid residue from coffee processing represents 50% of the coffee harvested, since for 1 kg of coffee produced 1 kg of husk is generated in dry processing, therefore it is necessary to find an alternative uses for these residues [52, 53].
In this context, composting can be an auxiliary practice in sustainable coffee waste management, since it is a spontaneous biological decomposition process, which has a fundamental help from the enzymatic arsenal produced by the local biota, that absorbs organic materials in a predominantly anaerobic manner. In this way, composting not only reduces harmful impacts on the environment, but also generates a source of nutrients for the soil [54]. The coffee husk and pulp, that is, the two main coffee by-products can be composted alone or co-composted with other solid urban waste or with animal manure, which is more common, generating a mature and stable compost ideal for agriculture because they contain in addition to iron and potassium, other micronutrients necessary for plant growth [55, 56]. The fact that composting allows complete conservation of residual energy stored in organic material was another advantage reported by Preethu et al. [49]. In view of this scenario, the importance of composting in the care of the environment is evident, aiming at the sustainability of coffee production and processing using its by-products and adding value to them.
Enzymatic Applications for Coffee Wastes Uses
Regarding the enzymatic application for the utilization of solid co-products generated in the various stages of coffee processing (pulp, husk, parchment, silverskin and spent ground), it can be divided into four major groups (Fig. 2). The first is the use of co-products (husk, pulp, CSS and SCG) as substrate for the production of several enzymes. The second is enzymatic hydrolysis of the co-products pulp, CSS and SCG aiming to obtain sugar, oligosaccharides, and other products derived from the hydrolysate as bioethanol, butanol, isopropanol, acetone, chemicals and others. The third group uses mainly the pulp and SCG for recover phenolic compounds using enzyme-assisted extraction and the last uses enzymatic technology for biodiesel production from SCG. Parchment is one of the less studied coffee co-products. The scarce information available reports that parchment is rich in phenolic compounds and dietary fiber [57].
The liquid co-product generated on wet and semi-wet route can be treated with peroxidases. Peroxidases are capable of reacting with aqueous phenolic compounds to form non-soluble substances, which could easily be removed from the aqueous solution by filtration, sedimentation or centrifugation. During semi-wet and wet processing, large amounts of wastewater are generated (from 20 to 45 kg per kg of coffee beans) [58].
Enzyme Production
As mentioned before, there is a massive amount of co-products generated during the coffee process steps. In this contest, new ways of harnessing these abundant residues need to be developed. As their composition is rich mainly of carbohydrates, proteins, and phenolic compounds, they can be used as substrates especially for solid-state fermentation processes to produce various enzymes, as shown in Table 1. The selection of an ideal agro-biotech waste for enzyme production by solid-state fermentation process depends upon several factors, mainly related to the cost and availability of the raw material.
In the literature, some studies have shown the growth of various microorganisms in different co-products, including coffee pulp and husk [59,60,61], coffee beans and coffee spent grounds [37] and coffee compost waste [62].
Putri et al. isolated 10 actinomycetes strains with ability to grow and degrade the cellulose and hemicellulose of coffee pulp using the congo red plate method [59]. Screening of fungi strains isolated from coffee husk waste collected in Dak Lak province was evaluated for pectinase production. It was found that 17 different fungi strains were isolated and only 9 strains could hydrolyze pectin. Rhizopus oryzae, Aspergillus oryzae, and Hypocrea pseudokoningii were those giving the largest halo zones. Hypocrea pseudokoningii presented the best pectinase activity and was chosen for enzyme production [60]. In another work, from the 20 fungi strains isolated from coffee wastes (coffee pulp and coffee husk), only seven were found to be capable of producing at least one esterase of interest and were identified by morphological characteristics into genera Aspergillus and Rhizopus [61].
Different samples of coffee beans and spent coffee ground were used as a substrate for the isolation of microorganisms with appropriate hydrolytic capacity. Wild fungi were isolated and screened for mannanase, endoglucanase, exoglucanase, xylanase, and pectinase production. Out of the 51 screened microorganisms, 2 belonging to Aspergillus ssp. and Penicillium ssp., respectively, showed potential for mannanase production when using spent coffee ground as substrate. The enzymes produced from the selected wild fungi and recombinant fungi were then evaluated for enzymatic hydrolysis of spent coffee ground, in comparison to commercial enzyme preparations. The application of mannanase enzymes of different origin (from isolated and recombinant microorganisms and commercial preparations) resulted in higher soluble yields when compared with other enzyme activities such as cellulases and pectinases [37].
Another study focused on the evaluation of cellulolytic and hemicellulolytic fungi isolated from coffee residue compost (CRC). For the enzyme production, isolates were inoculated onto wheat bran agar plates, and enzymes were extracted and tested for cellulase, xylanase, β-glucanase, mannanase, and protease activities. In total, 29 isolates from CRC were identified and evaluated. Five genera (Aspergillus, Coniochaeta, Fusarium, Penicillium, and Trichoderma/Hypocrea) were dominant in CRC. Penicillium sp., Trichoderma sp., and Aspergillus sp. displayed high cellulolytic and hemicellulolytic activities. The enzyme analyses revealed that Penicillium, Aspergillus, and Trichoderma isolates had a dominant role in the degradation of CRC [62].
A screening of 248 strains, isolated in Mexico's coffee-growing areas, permitted to select a wild strain of Aspergillus niger, which in 72 h attains a peak production of pectinase measured by viscosimetry. It was possible to isolate pectinase hyperproductive mutant strains using a selective culture medium [63].
The enzyme production using coffee wastes can use only one strain or a mixed culture of different microorganisms. Three cellulolytic enzymes (cellulase, xylanase and arabinofuranosidase) was produced by Aspergillus sp. using fresh coffee pulp waste by solid-state fermentation (SSF) [64]. Another work studied the cellulase and xylanase production by actinobacteria using coffee pulp from Coffea canephora. The coffee pulp was dried, milled and characterized (18.19% cellulose, 5.46% hemicellulose and 17.56% lignin) before the fermentation process [59].
A mixture of coffee husks and wood chips mixed in a proportion 9:1 (w:w) was used as a substrate for cellulase production by solid-state fermentation using a mixed culture. The wood chips were used as bulking agent to provide adequate porosity to the final mixture. It was used a compost provided by the waste biological treatment plant (Granollers, Barcelona, Spain) to increase the initial microbial population [65]. In another work, it was used a specialized consortium as inoculum for cellulase production by SSF using coffee husk (25.7% cellulose, 14.6% hemicellulose and 17.6% lignin) as a sole substrate. The predominant cellulolytic microorganisms present were bacteria (Pseudoxanthomonas taiwanensis and Sphingobacterium composti) and some yeasts (Cyberlindnera jardinii and Barnettozyma californica) [66].
Spent coffee grounds were used to produce lignocellulolytic enzymes (cellulase, xylanase, pectinase, and peroxidase) by Bacillus sp. R2. Untreated spent coffee grounds presented the highest content of polyphenols and were the best inducers for cellulase and pectinase production. However, xylanase and peroxidase correlated moderately with polyphenols content, and their highest activities were registered with spent coffee grounds treated with boiling water and 1% EDTA. The obtained results indicated that polyphenols content of the pretreated substrates influences the production of lignocellulolytic enzymes by Bacillus sp. R2 [67].
Coffee pulp wastes (CPW) generated during the industrial processing of coffee cherries by wet and also by dry process was used as substrate for protease production using Bacillus sp., a local bacteria isolated from an agro-waste dumping site. The maximum yield of protease production was achieved after 60 h of incubation using 3.0 g/L of CPW and 2.0 g/L of corn cob [68].
The coffee pulp and mucilage have the chemical composition to support the growth of microorganisms and the production of a value‐added product. The coffee pulp was evaluated as carbon and inductor source for β‐glucosidase by Bacillus subtilis CCMA0087 strain. The response surface methodology based on a central composite rotatable design was employed for this optimization. The highest β‐glucosidase production was reached in 24 h of culturing at coffee pulp concentration of 36.8 g/L, temperature of 36.6 °C and pH of 3.64 [58].
Another enzyme, the β-fructofuranosidases (FFase—EC.3.2.1.26), was produced by Aspergillus japonicus using coffee silverskin as solid matrix for SSF and nutrient source [33]. The enzyme FFase exhibits transferase activity, so can be exploited for synthesis of fructooligosaccharides (FOS) using sucrose as substrate. FOS are oligosaccharides that can be used as artificial sweeteners and are considered small dietary fibers with low caloric value. Additionally, FOS has important functional properties due to its capacity to serve as substrate for microflora in the large intestine, increasing the gastrointestinal tract health. Therefore, and due to the increased use of this ingredient in food and pharmaceutical products, the development of a suitable and economically viable process that allows obtaining FOS on industrial scale with higher yields and productivities has been actively encouraged. Later, the same research group evaluated and compared three different fermentation processes for the production of fructooligosaccharides (FOS) in terms of economic aspects and environmental impact. The processes included: submerged fermentation of sucrose solution by Aspergillus japonicus using free cells or using the cells immobilized in corn cobs, and SSF using coffee silverskin as support material and nutrient source. SSF was the most attractive process in both economic and environmental aspects since it is able to generate FOS with higher annual productivity (232.6 tonnes) and purity (98.6%) compared with the others processes [69].
An enzymatic extract produced by Rhizomucor pusillus strain 23aIV by SSF using olive oil or coffee pulp as an inducer for the feruloyl esterase production were evaluated. The feruloyl esterase activity obtained in the fermentation using coffee pulp as an inducer was 31.8% higher in comparison with that obtained in the fermentation using olive oil as the inducer [70].
Different coffee wastes can be compare for enzyme production. The coffee co-products such as coffee pulp, coffee cherry husk, silverskin, and spent coffee were evaluated for their efficacy as a sole carbon source for the production of xylanase by Penicillium sp. CFR 303 by SSF. Among the residues, coffee cherry husk was observed to produce maximum xylanase activity. The enzyme production was further improved when coffee husk was pretreated with steam [71]. In other work, coffee pulp, coffee cherry husk, coffee silverskin, spent ground coffee and a mixture of these coffee wastes (MC) were evaluated for their efficacy as a sole carbon source for the synthesis of α‐amylase in SSF using a fungal strain of Neurospora crassa CFR 308. The coffee pulp and the mixture MC were the best carbon sources for α‐amylase production. The enzyme production was further improved when both substrates were subjected to pre‐treatment by steaming. Neurospora crassa CFR 308 was reported for the first time in an application of α‐amylase production on coffee co‐products using SSF [72].
Coffee pulp and yeast extract were the best substrate and nitrogen source respectively for laccase production by Streptomyces psammoticus MTCC 7334 strain in submerged fermentation at 32 °C and 175 rpm [73].
A novel thermophilic fungi Mycotypha sp. strain AKM1801 was used to evaluate its efficiency for endo-pectinase production using coffee pulp as substrate. The culture was cultivated on coffee pulp through submerged fermentation in aerated and stationary conditions. The pectin content was reduced up to 85% in both conditions [74].
The mixture of coffee husk with wheat bran was used for tannase production by a lineage of Paecilomyces variotii. The variables were optimized using surface response methodology. The best conditions for tannase production were: temperature (29–34 °C); tannic acid (8.5–14%); % residue (coffee husk:wheat bran 50:50) and incubation time of 5 days. After the optimization process, the tannase activity increased 8.6-fold [75].
Summarizing, as coffee wastes compositions are rich mainly of carbohydrates, proteins, and minerals, they can be used as raw material especially for SSF processes to produce various enzymes, like cellulases, xylanases, glucanases, mannanases, pectinases, esterases, proteases, peroxidases, amylases, beta-glucosidases, beta-fructofuranosidases, laccases, tannases and others. As mention, many studies have shown the screening of various microorganisms in different coffee co-products. The use of alternative culture media, such as coffee residues, can help in the search for new species. When finding new species it is possible to find new enzymes with different functions. Therefore, the largest possible number of microorganisms and raw materials should be examined, through a powerful screening system. The selected microorganism must be GRAS, and preferably have a high capacity for synthesis and excretion of enzymes.
However, wild strains of microorganisms that produce industrially important enzymes show low yield and cannot thrive on artificial substrates. The application of recombinant DNA technology and metabolic engineering has enabled researchers to develop superior strains that can not only withstand harsh environmental conditions during the fermentation process but also ensure timely delivery of optimal results [76].
Thus, new enzymes may have their genes cloned and produced in host microorganisms that are easy to grow on a large scale. Therefore, the search for new species, with new enzymatic characteristics, must be prioritized so that we have new opportunities to obtain enzymes with important characteristics for biotechnological applications.
In parallel, the cost and availability of the raw material will influence on the selection of an ideal coffee waste for enzyme production. Up to 30% of the total production, cost of enzymes is attributed to the raw materials costs. The food industry expels copious amounts of processing waste annually, which is mostly lignocellulosic in nature. Upon proper treatment, lignocellulose can replace conventional carbon sources in media preparations for industrial microbial processes, such as enzyme production [76].
In order to reduce the cost of enzyme production, there is a growing interest in the use of agroindustrial and forestry lignocellulosic residues as raw materials, as coffee wastes, for industrial microbial processes. The use in bioprocesses thus represents a way of adding value to abundant waste, providing a solution to its accumulation, which represents a serious environmental problem. As most coffee residues are solid, solid-state fermentation will preferably be chosen. This fermentation process has already established advantages such as simplicity of the culture medium; reduced energy consumption due to the absence of sophisticated machinery and equipment requirements; low humidity, reducing contamination problems; growth conditions similar to those found in the natural environment. However, further studies are needed to help with some limitations such as less accessibility to the substrate, problems with mass and heat transfer, difficulty in controlling variables (pH, temperature, oxygen) and increasing scale.
Enzymatic Hydrolysis
Plant cell walls are the source of lignocellulosic materials, also known as biomass, whose structure is represented by the physicochemical interaction of cellulose (a linear glucose polymer), with hemicellulose (a highly branched heteropolymer of xylose, mannose, galactose, arabinose, glucose, as well as different sorts of uronic acids) and lignin (a very high molecular weight and cross-linked aromatic macromolecule). The biomass polysaccharides can be hydrolyzed by enzymatic hydrolysis within an advantage over the chemical conversion as it is environmentally friendly, has a higher conversion efficiency, is substrate-specific with absence of substrate loss due to chemical modifications, produces fewer degradation co-products and requires mild reaction using moderate and non-corrosive physical–chemical operating conditions. Enzymes like cellulases, hemicellulases and auxiliary enzymes, such feruloyl esterases, arabinofuranosidase, mannanases and others are necessary to break up the biomass lignocellulosic structure and to hydrolyze its polysaccharides such that its sugars, derived from glucan and xylan, can be used as feedstock for biotechnological processes including the production of biofuels and others products [77]. It is mentioned that additional research on the pretreatment of feedstock is required to improve the components yield and the cellulose digestibility to the level, which would make usage of such residues economically viable [9].
Besides, the use of biomass as a sustainable renewable resource represents a promising way to shift from a fossil-based to a bio-based society [78]. In the case of biofuels, there are three generations. The first-generation biofuels use different feedstocks, in competition with human/animal food. The second-generation biofuels show advantages over the first-generation in terms of land-use efficiency and environmental performance, since biofuels can be produced from vegetal wastes (biomass). The third-generation of biofuels are produced from algal biomass [30]. Enzymes like cellulases and xylanases are essential for the biodegradation of lignocellulosic material, with great interest in bioethanol production and other second-generation fuels. However, cellulase purchase has been reported as the most expensive point in the entire chain of bioethanol production, accounting up to 40% of the total cost [79].
Coffee wastes are rich in lignocellulose, a covalently bonded network of lignin, cellulose, and hemicellulosic polysaccharides that gives structural stability to the plant cell wall. Consequently, the biomass pretreatment is required to overcome the covalent linkages between the lignin and hemicellulose. Different approaches have been employed to convert hemicellulose into mono sugars, among which dilute acid hydrolysis (DAH) is one of the most efficient methods to selectively solubilize the hemicellulose of the biomass and to increase the enzymatic digestibility of cellulose. Disrupting lignocellulose improves hydrolysis of hemicellulose and cellulose into monosaccharides. Other kinds of pretreatment have also been used, as alkaline, steam, and others. In Table 2, are shown results of enzymatic hydrolysis using different enzymes on diverse coffee co-products.
The crude enzyme extract from Aspergillus sp. VTM5, with xylanase, arabinofuranosidase and cellulase activities, were used for hydrolyzed the coffee pulp pretreated with alkali. The main effect of this type of pretreatment is the removal of lignin from biomass, thus improving polysaccharide reactivity. The results showed that the consortium of these three enzymes from Aspergillus could efficiently hydrolyze the coffee pulp into pure sugar with a degree hydrolysis 79% [64].
Niglio et al. evaluated the enzymatic hydrolysis of coffee silverskin alkali-pretreated using cellulase aiming at maximizing the sugar release. Increasing cellulase load resulted in increasing hydrolysis rate and sugar final concentration. The hydrolyzate obtained from the coffee silverskin was used to produce butanol biofuel using Clostridium acetobutylicum as a fermentation agent and succinic acid using Actinobacillus succinogenes as a fermentation agent. The results highlight the possibility of using coffee silverskin to produce fermentable sugars, solvents and biochemicals of industrial interest [30].
Although different coffee beans wastes have been widely studied, the application of coffee flower (CF) has not been much investigated. The use of CF was evaluated for the production of bio-sugars through the green process. The bio-sugar was obtained at a 92.8% conversion rate using cellulase and pectinase as hydrolytic enzymes [80].
Another study proved that coffee silverskin is a good candidate as renewable feedstock for isopropanol-butanol-ethanol production. Coffee silverskin was pretreated with alkaline hydrolysis and after the pretreated biomass was treated by enzymatic hydrolysis using the commercial cellulases cocktail Cellic CTec2 aiming to obtain sugars which were used as carbon source to produce butanol and isopropanol by Clostridium beijerinckii [31].
Hijosa-Valsero et al. evaluated coffee silverskin as a feedstock for biobutanol production by acetone–butanol–ethanol fermentation. The biomass contained approximately 30% total carbohydrates and 30% lignin. Coffee silverskin was subjected to autohydrolysis at 170 °C for 20 min, with a biomass-to-solvent ratio of 20%. This physicochemical pretreatment by autohydrolysis is technically easy and environmentally friendly because it does not require any reagent but water. After, the enzymatic hydrolysis was made using a commercial enzyme (Cellic CTec2 from Novozymes) in order to release simple sugars. The fermentability of the hydrolysate was assessed with four solventogenic strains from the genus Clostridium [81].
The potential bioconversion of spent coffee grounds (SCG) into lactic acid was investigated. SCG was hydrolyzed by a combination of dilute acid treatment and subsequent application of cellulase. The pretreatment of lignocellulosic biomass with dilute acid promotes hydrolysis and consequent removal of the hemicellulosic fraction, thus significantly improving the enzymatic hydrolysis of cellulose. For enzymatic hydrolysis, Celluclast (Novozymes, Denmark), β‐glucosidase (Novozymes), and Viscozyme (Novozymes) were used. The percentage of the released sugars after hydrolysis is 74.5% of glucose, 53.2% of galactose + mannose and 70.8% of arabinose. The SCG hydrolysate contained a considerable amount of reducing sugars (glucose 9 g/L; galactose + manose 26 g/L and arabinose 2 g/L), and it was used as a substrate for culturing several lactic acid bacteria (LAB). Among the screened microorganisms, Lactobacillus rhamnosus CCM 1825 was identified as the most promising producer of LA on an SCG hydrolysate. Therefore, it could be demonstrated that SCG is a promising raw material for the production of lactic acid and could serve as a feedstock for the sustainable lactic acid large-scale production [82].
Pleissner et al. also studied coffee pulp as carbon source in fermentative lactic acid production using Bacillus coagulans. After thermo-chemical pretreatment at 121 °C for 30 min (in presence of 0.18 mol/L H2SO4) and following an enzymatic digestion using Accellerase 1500, carbon-rich hydrolysates were obtained. Fermentations were carried out at laboratory (2 L) and pilot (50 L) scales. At pilot scale carbon utilization and lactic acid yield per gram of sugar consumed were 94.65% and 0.78 g/g, respectively [83].
The conversion of the lignocellulosic biomasses is challenged by the presence of lignin that prevents the hydrolysis of polysaccharides, hence demanding a pretreatment step. The effectiveness of Pleurotus ostreatus laccases to lignin remotion was assessed, improving the subsequent saccharification. The commercial cellulolytic enzyme cocktail Cellic® CTec2 (Novozyme); an endo-1,4-β-xylanase from Trichoderma viride (Megazyme) and an α-amylase from Bacillus licheniformis (Megazyme) was used for enzymatic hydrolysis. As regard coffee silverskin, a sugar yield of 73% was obtained using laccases as a pretreatment step, while with the untreated coffee silverskin, a sugar yield of 27% was obtained. The herein developed sequential protocol, raising soluble sugars and reducing the amount of wastewater, can improve the overall process for obtaining chemicals or fuels from agro-food wastes [32].
Kim et al. studied bio-sugar and bioethanol production from spent coffee ground pretreated by chloride acid. They mentioned that coffee residue after the roasting process is one of the most useful resources for biofuel and biomaterial production. The acid pretreatment before enzymatic hydrolysis can efficiently improve the bioconversion of bio-sugar and bioethanol by removing the phenolic and brown compounds. The commercial enzymes used for hydrolysis and simultaneous saccharification and fermentation (SSF) process were Cellulase (Celluclast 1.5 L), pectinase (Pectinex SP-L) and xylanase (Sigma-Aldrich). The yeast used in the SSF process was Saccharomyces cerevisiae. The yields of bio-sugar conversion and bioethanol production were 78.0 and 73.8%, respectively [84].
A different pretreatment for spent coffee waste (SCW) has been proposed which utilises the superior oxidising capacity of alkaline KMnO4 assisted by ultra-sonication and resulted in 98% cellulose recovery and a maximum lignin removal of 46%. 1.7 fold increase in reducing sugar yield was obtained after enzymatic hydrolysis of KMnO4 pretreated SCW as compared to raw. Ultrasound-assisted potassium permanganate oxidation was found to be an effective pretreatment for SCW, and can be a used as a potential feedstock pretreatment strategy for bioethanol production [85]. The same group proposed another pretreatment which combines two techniques, atmospheric air plasma and FeCl3, to create a superior pretreatment that involves Fenton chemistry. The pretreated SCW after enzymatic hydrolysis yielded 0.496 g of reducing sugar/g of SCW. The hydrolysate was subjected to fermentation by S. cerevisiae and led to the production of 18.642 g/L of ethanol with a fermentation efficiency of 74%, which was a two fold increase in yield compared to the control [86]. In the same year, Ravindran et al. also studied eight different pretreatments of varying nature (physical, chemical and physico-chemical) followed by a sequential, combinatorial pretreatment strategy was applied to spent coffee waste to attain maximum sugar yield. Pretreated samples were analysed for total reducing sugar, individual sugars and generation of inhibitory compounds such as furfural and hydroxymethyl furfural (HMF) which can hinder microbial growth and enzyme activity. Results showed that sequential pretreatment yielded 0.350 g of reducing sugar/g of substrate [87].
Another work investigated the optimal condition of dilute acid pretreatment of SCG at high solids and mild temperature conditions to release the reducing sugars with enzymatic hydrolysis. Under the optimal condition, the mean yield of reducing sugars from enzymatic saccharification using the cellulase enzyme complex Accellerase® 1500 (DuPont™ Genencor® Science) was 0.563 g/g (81.5% conversion of SCG total carbohydrate). The SCG hydrolysate was then successfully applied to culture Lipomyces starkeyi for microbial oil fermentation without showing any inhibition. The results suggested that dilute acid hydrolysis followed by enzymatic saccharification has the great potential to convert SCG carbohydrates to reducing sugars [88].
The spent coffee ground may represent a potential feedstock for mannooligosaccharides (MOS) production by enzymatic hydrolysis of the mannan polysaccharides found in the structure of spent coffee ground. The cellulose and galactomannans are polysaccharides that remain unextractable during the process for instant coffee production. These polysaccharides can be hydrolyzed to oligosaccharide molecules, such as MOS, which have potential applications as prebiotic products in human and animal feed. MOS can be used in nutraceutical products for humans/animals or added to instant coffee, increasing process yield and improving product health properties [36].
Like already mentioned, spent coffee grounds (SCG) or coffee residue wastes (CRW) provide excellent raw material to produce valuable biosugars, including oligosaccharides (OSs), mannooligosaccharides (MOSs), mannose, and bioethanol. SCG were subjected to delignification and defatting, producing SCG-derived polysaccharides. Two-stage enzymatic hydrolysis (short- and long-term) was performed to produce short-chain mannooligosaccharides (MOSs) and monosaccharides (MSs), respectively. From 100 g dry weight (DW) amounts of SCG, approximately 77 g delignified SCG and 61 g SCG-derived polysaccharides, amounts of 15.9 g of first biosugars (mostly MOSs), 25.6 g of second biosugars (mostly MSs), and 3.1 g of bioethanol, were recovered [89]. The same group evaluated a integrated process for economical high-yield production of d-mannose and ethanol from coffee residue waste (CRW). The process involves pretreatment, enzymatic hydrolysis, fermentation, color removal, and pervaporation, which can be performed using environmentally friendly technologies. The CRW was pretreated with ethanol at high temperature and then hydrolyzed with enzymes produced in-house to yield sugars. The key points of the process are: manipulations of the fermentation step that allowing bioethanol-producing yeasts to use almost glucose and galactose to produce ethanol, while retaining large amounts of d-mannose in the fermented broth; removal of colored compounds and other components from the fermented broth; and separation of ethanol and d-mannose through pervaporation. Under optimized conditions, approximately 15.7 g dry weight (DW) of d-mannose (approximately 46% of the mannose) and approximately 11.3 g DW of ethanol from 150 g DW of ethanol-pretreated CRW, were recovered [90].
Another work studied the SCG for MOS production by steam pretreatment and enzymatic hydrolysis with a recombinant mannanase and a commercial cellulase cocktail (Acremonium, Bioshigen Co. Ltd., Japan). The mannanase was produced using a recombinant strain of Yarrowia lipolytica, used to produce and secrete endo-1,4-β,d-mannanase from Aspergillus aculeatus in bioreactor cultures. The steam pretreatment was effective in generating a substrate amenable to enzyme action and allowed the reduction of the mannanase and cellulase cocktail loading while keeping the same yield obtained with higher enzyme loadings for hydrolysis of untreated SCG. Combined enzymatic hydrolysis of untreated or steam-pretreated SCG with mannanase and cellulase cocktail resulted in 36–57% (based on mannan content) of MOS production with a degree of polymerization of up to 6 [36].
The application of an enzyme cocktail (mannanase, endoglucanase, exoglucanase, xylanase and pectinase) that acts synergistically on enzymatic hydrolysis of SCG with each other is regarded as a promising strategy to solubilise/hydrolyse remaining solids, either to increase the soluble solids yield of instant coffee or for use as raw material in the production of bioethanol and food additives (mannitol). Out of the enzymes evaluated, the application of mannanase gave better yields than when only cellulase or xylanase was used for hydrolysis. The combination of mannanase with other enzyme activities revealed an additive effect on the hydrolysis yield, but not synergistic interaction, suggesting that the highest soluble solid yields were mainly due to the hydrolysis action of mannanase [37].
The use of SCG for bioethanol production was evaluated. The carbohydrate content of SCG was analyzed for fermentable sugars such as glucose, galactose, and mannose, which can be fermented by Saccharomyces cerevisiae. Pretreatment at a pressure of 1.47 MPa for 10 min with popping pretreatment was required to increase enzymatic hydrolysis. The enzymatic conversion rate of SCG to fermentable sugars was 85.6%. Ethanol concentration and yield (based on sugar content) following enzymatic hydrolysis after simultaneous saccharification and fermentation were 15.3 g/L and 87.2%, respectively [91].
In conclusion, the difficulty of effectively using lignocellulosic materials in biotechnological processes comes up against two main obstacles: the cellulose-hemicellulose-lignin association, which is a natural barrier to enzymatic and microbial degradation of these materials and the crystalline structure of cellulose, resistant to hydrolysis. The acid hydrolysis of cellulose, a fast process, has the drawback of requiring high temperatures and pressure, leading to the destruction of part of the carbohydrates and the obtaining of toxic degradation products to microorganisms. Enzymatic hydrolysis, in turn, requires the use of physical pretreatments (grinding, heating, irradiation) or chemicals (sulfuric acid, phosphoric acid, alkalis), to achieve viable yields. As seen in this review, several studies have evaluated different types of pretreatment.
There is still a need for further studies to obtain more effective pretreatments, which do not generate toxic substances that can hinder alcoholic fermentation. It is also important not alter the structure of lignin in order to prevent its use as a solid fuel or as an input for industry chemistry.
Another important question is about the availability of several commercial formulations for hydrolysis. Depending of biomass recalcitrance, different enzymes must be present for warranty the complete hydrolysis. So, there is scope for the development of intelligent enzyme formulations (stable above 50 °C) to guarantee an efficient and economical hydrolysis of lignocellulosic materials.
Phenolics Compounds Extraction
Traditional methods for extraction of bioactive compounds are traditionally based on solid–liquid extraction or liquid–liquid extraction. However, these procedures are not selective for specific bioactive compounds, use high temperatures, have low efficiency, and may degrade thermolabile compounds. For this reason, advanced extraction techniques such as microwave-assisted extraction, ultrasound-assisted extraction, pressurized liquid extraction, supercritical fluid extraction and enzyme-assisted extraction (EAE) have emerged during the last decades for improved green extraction of bioactive compounds [92]. EAE is a potential alternative to conventional solvent-based extraction methods, which benefits from the high selectivity, specificity, and ability of enzymes to degrade cell walls and membranes [93].
According to Croteau et al., the bioactive compounds are divided into three main categories: phenolic, terpenoids and alkaloids [94]. Phenolic compounds are ubiquitous constituents of higher plants, including fruits, vegetables, cereals, and legumes, and in beverages of plant origin, such as wine, tea, and coffee. These compounds are secondary metabolites of plants generally involved in defense against ultraviolet radiation or aggression by pathogens. Pulp and skin of coffee beans are a great source of different phenolic compounds such as chlorogenic acids, ferulic acid, and tannins, among others. Most of these compounds and its derivatives have received considerable attention as potential protective factors against human chronic degenerative diseases such as diabetes mellitus, cancer, AIDS, and cardiovascular disease. Therefore, enzymes that allow the release or transformation of these aromatic compounds offer particular interest. Feruloyl esterase, chlorogenic acid esterase, p-coumaroyl esterase, and tannases are enzymes useful in phenolic modifications of pharmaceutical relevance [61].
Feruloyl esterases (E.C. 3.1.1.73) are used as a tool for the release of phenolic compounds from agro-industrial co-products or as a critical enzyme allowing better hydrolysis of lignocellulosic substrates by polysaccharide hydrolases [95]. Chlorogenic acid esterase (E.C. 3.1.1.42) can be used for the production of caffeic acid from natural substrates rich in chlorogenic acid like coffee pulp [96]. Only a few studies have focused on p-coumaroyl esterase (EC 3.1.1.B10). In Table 3 is shown different enzymes used for enzyme-assisted extraction. The co-products used for extraction normally are coffee pulp and spent coffee ground (wet wastes).
The extracellular esterase activity was detected in submerged cultures of Rhizoctonia solani. The putative type B feruloyl esterase coding sequences found in the genome data of the basidiomycete were heterologously expressed in Pichia pastoris. This recombinant enzyme production exceeded the productivity of the wild type strain by a factor of 800. Based on substrate specificity profiling, the purified recombinant enzyme (Rs pCAE) was classified as a p-coumaroyl esterase (pCAE) with a pronounced chlorogenic acid esterase side activity. The Rs pCAE was also active on methyl cinnamate, caffeate, and ferulate and feruloylated saccharides. Hydroxycinnamic acids were released from coffee pulp. Overnight incubation of coffee pulp with the Rs pCAE resulted in the efficient release of p-coumaric (100%), caffeic (100%) and ferulic acid (85%) indicating possible applications for the valorization of food processing wastes and the enhanced degradation of lignified biomass [97].
The feruloyl esterases from Schizophyllum commune were functionally expressed in Pichia pastoris. The recombinant enzymes, ScFaeD1 and ScFaeD2, were used for hydroxycinnamic acid extraction of coffee pulp, and it released caffeic (> 60%), ferulic (> 80%) and p-coumaric acid (100%) indicating applicability for the valorization of food processing wastes and enhanced biomass degradation. ScFaeD1 and ScFaeD2 preferably hydrolyzed feruloylated saccharides with ferulic acid esterified to the O-5 position of arabinose residues and showed an unprecedented ability to hydrolyze benzoic acid esters [98].
The response surface methodology was used to optimize the enzymatic saccharification of lignocellulose in SCGs following hydrothermal pretreatment. A maximum reducing sugar yield was obtained at the following optimized hydrolysis conditions: 4.97 g of pretreated SCGs, 120 h reaction time, and 1246 and 250 µL of cellulase and hemicellulase, respectively. Industrially relevant sugars (glucose, galactose, and mannose) were identified as the main hydrolysis products under the studied conditions. Total flavonoids, total polyphenols, and DPPH free-radical scavenging activity increased significantly after enzymatic saccharification. A 14-fold increase in caffeine levels was also observed. This study provides insight into SCGs as a promising source of essential sugars and polyphenols [11].
Ferulic, caffeic, p-coumaric, and chlorogenic acids are classified as hydroxycinnamic acids, presenting anticarcinogenic, anti-inflammatory and antioxidant properties. The total content of ferulic, caffeic, p-coumaric and chlorogenic acids was 5276 mg per kg of coffee pulp. Distribution was as follows (in %): chlorogenic acid 58.7, caffeic acid 37.6, ferulic acid 2.1 and p-coumaric acid 1.5. Most of the hydroxycinnamic acids were covalently bound to the cell wall (in %): p-coumaric acid 97.2, caffeic acid 94.4, chlorogenic acid 76.9 and ferulic acid 73.4. An enzymatic extraction using a commercial pectinase and an enzymatic extract containing feruloyl esterase produced by Rhizomucor pusillus strain 23aIV was evaluated in order to extract high value-added products like hydroxycinnamic acids from coffee pulp. The highest yield of extraction of hydroxycinnamic acids was obtained by mixing the produced enzyme using coffee pulp as an inducer and a commercial pectinase. Extraction yields were as follows (in %): chlorogenic acid 54.4, ferulic acid 19.8, p-coumaric acid 7.2 and caffeic acid 2.3 [70].
Spent coffee grounds were subjected to solid–liquid extractions to study the influence of some critical variables on the phenol content of extracts. Enzymatic extraction using cellulase was compared with hydroalcoholic extraction. After grinding, spent coffee grounds were passed through several sieve sizes (125, 250, 500, and 1000 μm) and classified into four different particle size groups. The highest yields of total phenols were consistently obtained from the smallest particles. HPLC analysis confirmed chlorogenic acid as the major phenolic acid presented on spent coffee grounds. Chromatograms of extracts obtained after the enzyme treatment showed that cellulases catalyzed the transformation of chlorogenic acid, resulting in a derivative with similar spectrum, but shorter retention time. Results confirmed the feasibility of upgrading spent coffee grounds as a promising source of chlorogenic acid, which may be used in biofunctional dietary supplements [99].
Agro-industrial co-products, as coffee pulp, are a potential source of added-value phenolic acids with promising applications in the food and pharmaceutical industries. Two purified feruloyl esterases from Aspergillus niger, FAEA and FAEB, were tested for their ability to release phenolic acids such as caffeic acid, p-coumaric acid and ferulic acid from coffee pulp. Only FAEB was active in the release of caffeic (100%), p-coumaric (73%), and ferulic (50%) acids [100].
In relation to the extraction of phenolic compounds, it can be observed that most studies have used the residues of coffee pulp and SCG, which are moist and easier residues for the enzymatic extraction process. Further studies are needed to recover the phenolics from dry residues (such as husk, parchment and silverskin), where a previous stage of pretreatment of biomass will probably be necessary.
Biodiesel Production
The biodiesel is a potential alternative to fossil fuels. The main obstacle biodiesel faces are the long-term commercial viability because of sustainability issues and high cost of feedstocks. Feedstock alone is responsible for 70–80% of the final biodiesel costs. Waste materials, like non-food crops with high oil content and non-edible oils, can be used as viable alternatives for the production of biodiesel [101]. Spent coffee grounds could be a feedstock that qualifies for both as a non-food crop and a waste product. The spent coffee grounds generated worldwide can produce ~ 0.9 million tons of biodiesel, assuming 16% of oil can be extracted from it. The oil from spent coffee ground is usually extracted using organic solvent [102]. Due to high antioxidant presence, biodiesel obtained from spent coffee grounds oil has excellent oxidative stability.
Biodiesel can be produced via acid-, alkali-, and enzyme-catalyzed reactions. Various disadvantages of acid- and alkali-catalyzed reactions are as follows: high energy requirements, difficult recovery of catalysts and glycerol, and the toxic and corrosive nature of catalysts. As an alternative, lipase can be used as a catalyst for biodiesel production instead of chemical catalysts. Lipases are extensively used under moderate conditions, easy to recover, and moisture and free fatty acid-tolerant [103]. Lipases (EC 3.1.1.3) catalyze the hydrolysis of long-chain triacylglycerols. Contrary to many other enzymes, they show remarkable levels of activity and stability in non-aqueous environments that facilitate the catalysis of several unnatural reactions such as esterification and transesterification [61].
Different lipases are evaluated for biodiesel production via lipase catalysis. Lipases, namely Mucor miehei, Pseudomonas cepacia, Rhizopus delemar, Geotrichum candidum, Candida rugosa, Porcine pancreas-II, Pseudomonas fluorescence and Candida antarctica lipase-B (Novozyme-435) were employed for biodiesel synthesis from spent coffee oil. Around 96% oil-to-biodiesel conversion was obtained using lipase Novozyme-435 as a catalyst at 1:5 oil-to-methanol molar ratio [103].
The immobilized lipase from Thermomyces lanuginosus was evaluated for the biodiesel production from spent coffee ground oil in a solvent-free system. The immobilized lipase retains, after 60 days, more than 90% of its initial activity. Biodiesel yield of 51.7% after 3 h of synthesis was measured, and it increased up to ∼ 100% after 24 h indicating an enzymatic fast kinetic. No significant decrease during the first three cycles of use of the lipase activity occured [104].
Another work also studied immobilized lipases to the enzymatic conversion spent coffee ground oil into biodiesel. The enzymatic synthesis of biodiesel was initially carried out on a model substrate (triolein) in order to select the most promising immobilized biocatalysts. The results indicate that oils can be converted quantitatively within hours. The role of the nature of the immobilization support emerged as a critical factor affecting reaction rate, most probably because of partition and mass transfer barriers occurring with hydrophilic solid supports. Finally, oil from spent coffee ground was transformed into biodiesel with yields ranging from 55 to 72%. The synthesis is of particular interest in the perspective of developing sustainable processes for the production of biofuels from food wastes and renewable materials [105].
Different methods for biodiesel production were used: acid-catalyzed esterification followed by alkali-catalyzed transesterification and lipase-catalyzed transesterification. It was found that the high level of free fatty acids in the coffee oil was reduced from 16.3 to 2.64% by acid-catalyzed esterification, and reduced conversion was obtained for the further alkali-catalyzed transesterification. In comparison, 98.5% conversion was achieved by using enzymatic catalysis, demonstrating the feasibility of using this approach to process low-quality coffee oil from spent coffee grounds for biodiesel production [106].
In summary, the production of biodiesel with lipases has been evaluated using predominantly the oil extracted from spent coffee grounds, due to the high concentration of lipids (16%) in this residue in comparison with the other coffee co-products.
Wastewater Treatment
Wastewater from coffee processing is rich in organic matter (cellulose, hemicellulose, pectin, sucrose, monosaccharides, lipids, proteins, polyphenols, and vitamins), which is released during coffee pulping, mucilage removal and fermentation [107]. Due to their varied composition, several enzymes could be used to minimize the organic pollutant load of coffee wastewater, such as cellulases, hemicellulases, pectinases, lipases, proteases, and peroxidases. However, the literature usually mentions studies using pectinase and peroxidase [8, 108].
Chagas et al. studied the application of peroxidase from immobilized soybean hull for the oxidation of phenolic compounds in coffee processing wastewater. The enzyme was stabilized with immobilization to investigate its application on caffeic acid oxidation, one of the most abundant phenolic compounds in coffee processing wastewater. The researchers evaluated the peroxidase enzyme in both coffee processing wastewater and a synthetic solution of phenolic compounds containing caffeic acid, chlorogenic acid, ferulic acid, catechin and epicatechin, the main phenolic compounds present in coffee processing wastewater. The initial concentration of total phenolic compounds in the coffee processing wastewater, as well as the synthetic solution, was 218.21 and 219.54 mg/L, respectively. The study showed a reduction in phenolic compounds for the synthetic solution of 79% and 61% for immobilized and free enzymes, respectively. For wastewater from coffee processing, the reduction was 32% and 19% for immobilized and free enzymes, respectively. The immobilized enzyme had a better result than the free enzyme because some of the phenolic compounds can be absorbed in the chitosan support. The performance of the enzyme in real sample was limited due to the high complexity of coffee processing wastewater. The peroxidase immobilization provided an increase in oxidation, as well as allowing the enzyme recovery from the reaction medium and its reuse [8].
A similar study, by Torres et al., with soybean peroxidase to reduce phenolic compounds in coffee processing wastewater was evaluated. In order to improve the enzyme approach and minimize enzyme deactivation, the researchers used Triton X-100 surfactant and polyethylene glycol as chemical additives. The concentration of total phenols showed a value of 257.84 mg/L. The study showed that there was no influence on the oxidation of phenolic compounds in the coffee processing wastewater sample in the presence of polyethylene glycol and Triton X-100, since the reduction in oxidation of phenolic compounds in coffee processing wastewater in the absence of these additives was 31% and using the additive polyethylene glycol, and Triton X-100, the reduction in oxidation was 28% and 24%, respectively. There was no adsorption of the enzyme to polymeric products through association with reaction products [108].
Like it was said before, due to the complex composition of coffee wastewater, a multienzymatic system is needed to minimize the organic pollutant load. Further studies should be done to help reuse compounds with high added value, such as phenolics. In addition, new enzyme mixtures must be developed for the treatment of coffee processing effluents.
Conclusion
Enzymatic technology is fundamental in coffee processing, not only to remove mucilage but also, to improve sensory quality characteristics of this beverage. Currently the number of enzymes applied either in coffee processing to improve the beverage or in coffee wastes uses aiming at minimize environmental problems due to the large volume generated by the sector is still small. However, as presented in this review, diverse research investments using enzymatic technology have been made in order to increase this number in the coming years. Regarding the waste generated during coffee processing, there are several sustainable ways to take advantage of these co-products. The coffee residues (pulp, husk, silverskin, and spent ground) can be used as substrates for different enzyme production (cellulase, xylanase, pectinase, β‐glucosidase, feruloyl esterase, arabinofuranosidase, β‐fructofuranosidase, α-amylase, protease, peroxidase, and others). The primary coffee industry residues, coffee silverskin and spent coffee grounds, can be as sustainable renewable resources to produce fermentable sugars, solvents, biofuels and biochemicals of industrial interest using enzymatic technology. The coffee co-products are a great source of different phenolic compounds such as chlorogenic acids, ferulic acid, and tannins, among others. These compounds and their derivatives can be extracted by enzyme technology from coffee wastes, promoting the reduction of the environmental passive, making possible an additional gain to the coffee chain through valorizing these agroindustrial residues.
References
Paiva, A., Ranocchia, K., Marques, M., Silva, M.G., Alves, V., Coelhoso, I., Simões, P.: Evaluation of the quality of coffee extracts concentrated by osmotic evaporation. J. Food Eng. 222, 178–184 (2018)
Oliveira, L.S., Franca, A.S.: Chap. 31—An overview of the potential uses for coffee husks A2. In: Preedy, V.R. (ed.) Coffee in Health and Disease Prevention. Academic Press, San Diego (2015)
Dessie, W., Zhu, J., Xin, F., Zhang, W., Jiang, Y., Wu, H., Ma, J., Jiang, M.: Bio-succinic acid production from coffee husk treated with thermochemical and fungal hydrolysis. Res. Paper 41, 1461–1470 (2018)
Oumer, O.J., Abate, E.D.: characterization of pectinase from bacillus subtilis strain Btk 27 and its potential application in removal of mucilage from coffee beans. Hindawi (2017). https://doi.org/10.1155/2017/7686904
Hendriksen, H.V., Kornbrust, B.A., Ostergaard, P.R., Stringer, M.A.: Evaluating the potential for enzymatic acrylamide mitigation in a range of food products using asparaginase from Aspergillus oryzae. J. Agr. Food Chem. 57(10), 4168–4176 (2009)
Hendriksen, H.V., Budolfsen, G., Baumann, M.J.: Asparaginase for acrylamide mitigation in fodd. Asp. Appl. Biol. 116, 41–50 (2013)
Bedade, D., Sutar, Y.B., Singhal, R.S.: Chitosan coated calcium alginate beads for covalent immobilization of acrylamidase: process parameters and removal of acrylamide from coffee. Food Chem. 275, 95–104 (2019)
Chagas, P.M.B., Torres, J.A., Silva, M.C., Corrêa, A.D.: Immobilized soybean hull peroxidase for the oxidation of phenolic compounds in coffee processing wastewater. Int. J. Biol. Macromol. 81, 568–575 (2015)
Pandey, A., Soccol, C.R.: Economic utilization of crop residues for value addition: a futuristic approach. J. Sci. Ind. Res. 59(1), 12–22 (2000)
Ballesteros, L.F., Teixeira, J.A., Mussatto, S.I.: Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioprocess Technol. 7, 3493–3503 (2014)
Scully, D.S., Jaiswal, A.K., Abu-Ghannam, N.: An investigation into spent coffee waste as a renewable source of bioactive compounds and industrially important sugars. Bioengineering 3(4), 1–13 (2016)
Smith, A.D., Datta, S.P., Howard Smith, G., Campbell, P.N., Bentley, R., McKenzie, H.A. (eds.): Oxford Dictionary of Biochemistry and Molecular Biology. Oxford University Press, Oxford (1997)
Divakar, S.: Enzymatic Transformation. Springer, India (2013)
Murthy, P.S., Naidu, M.M.: Sustainable management of coffee industry by-products and value addition—a review. Resour. Conserv. Recycl. 66, 45–58 (2012)
Dias, E.C., Pereira, R.G.F.A., Borém, F.M., Mendes, E., Lima, R.R., Fernandes, J.O., Casal, S.: Biogenic amine profile in unripe Arabica coffee beans processed according to dry and wet methods. J. Agric. Food Chem. 60, 4120–4125 (2012)
Kleinwachter, M., Selmar, D.: Influence of drying on the content of sugars in wet-processed green Arabica coffees. Food Chem. 119, 500–504 (2010)
Borém, F.M.: Handbook of Coffee Post-Harvest Technology, 1st edn. UFLA, Lavras (2014)
Bytof, G., Knopp, S.-E., Schieberle, P., Teutsch, I., Selmar, D.: Influence of processing on the generation of gamma-aminobutyric acid in green coffee beans. Eur. Food Res. Technol. 220, 245–250 (2005)
Silva, J.S., Nogueira, R.M., Roberto, C.D.: Tecnologia de Secagem e Armazenagem para a Agricultura Familiar, 1st edn, pp. 7–14. Suprema Gráfica e Editora, Viçosa, MG (2005)
Durán, C.A.A., Tsukui, A., Santos, F.K.F., Martinez, S.T., Bizzo, H.R., Rezende, C.M.: Coffee: General aspects and its use beyond drink / Café: Aspectos Gerais e seu Aproveitamento para além da Bebida. Rev. Virtual Quim 9(1), 107–134 (2017)
Pereira, R.G.F.A., Lillela, T.C, Andrade, E.T.: Composição química de grão de cafés (Coffea Arabica L.) submetidos a diferentes tipos de pre-processamento. II Simpósio de Pesquisa dos Cafés do Brasil (2001)
Esquivel, P., Jiménez, V.M.: Functional properties of coffee and coffee by-products. Food Res. Int. 46, 488–495 (2012)
Sarkar, N., Ghosh, S.K., Bannerjee, S., Aikat, K.: Bioethanol production from agricultural wastes: an overview. Renew. Energy 37(1), 19–27 (2012)
Mata, T.M., Martins, A.A., Caetano, N.S.: Bio-refinery approach for spent coffee grounds valorization. Bioresour. Technol. 247, 1077–1084 (2018)
Pandey, A., Soccol, C.R., Nigam, P., Brand, D., Mohan, R., Roussos, S.: Biotechnological potential of coffee pulp and coffee husk for bioprocesses. Biochem. Eng. J. 6, 153–162 (2000)
Belitz, H.-D., Grosch, W.: Schieberle P., Food Chemistry. 4th ed., Springer, Heidelberg (2009).
Brand, D., Pandey, A., Rodríguez-León, J.A., Roussos, S., Brand, I., Soccol, C.R.: Packed bed column fermenter and kinetic modeling for upgrading the nutritional quality of coffee husk in solid-state fermentation. Biotechnol. Progress 17, 1065–1070 (2001)
Ulloa-Rojas, J.B., Verreth, J.A.J., Amato, S., Huisman, E.A.: Biological treatments affect the chemical composition of coffee pulp. Bioresour. Technol. 89, 267–274 (2003)
Bekalo, S.A., Reinhardt, H.-W.: Fibers of coffee husk and hulls for the production of particleboard. Mater. Struct. 43, 1049–1060 (2010)
Niglio, S., Procentese, A., Russo, M.E., Sannia, G., Marzocchella, A.: Investigation of enzymatic hydrolysis of coffee silverskin aimed at the production of butanol and succinic acid by fermentative processes. BioEnergy Res. 12(2), 312–324 (2019)
Procentese, A., Raganati, F., Navarini, L., Olivieri, G., Russo, M.E., Marzoccchella, A.: Coffee silverskin as a renewable resource to produce butanol and isopropanol. Chem. Eng. Trans. 64, 139–144 (2018)
Giacobbe, S., Pezzella, C., Lettera, V., Sannia, G., Piscitelli, A.: Laccase pretreatment for agro-food wastes valorization. Bioresour. Technol. 265, 59–65 (2018)
Mussatto, S.I., Ballesteros, L.F., Martins, S., Maltos, D.A.F., Aguilar, C.N., Teixeira, J.A.: Maximization of fructooligosaccharides and β-fructofuranosidase production by Aspergillus japonicus under solid-state fermentation conditions. Food Bioprocess Technol. 6, 2128–2134 (2013)
Echeverria, M.C., Nuti, M.: Valorization of the residues of coffee agro-industry: perspective and limitation. Open Waste Manage. J. 10, 13–22 (2017)
Campos-Vega, R., Loarca-Pina, G., Vergara-Castañeda, H.A., Oomah, B.D.: Spent coffee grounds: a review on current research and future prospects. Trends Food Sci. Technol. 45, 24–36 (2015)
Chiyanzu, I., Brienzo, M., García-Aparicio, M.P., Görgens, J.F.: Application of endo-β-1,4, D-mannanase and cellulase for the release of mannooligosaccharides from steam-pretreated spent coffee ground. Appl. Biochem. BioTechnol. 172(7), 3538–3557 (2014)
Jooste, T., García-Aparicio, M.P., Brienzo, M., Van Zyl, W.H., Görgens, J.F.: Enzymatic hydrolysis of spent coffee ground. Appl. Biochem. BioTechnol. 169(8), 2248–2262 (2013)
Phorommarat, B.: Life cycle assessment of ground coffee and comparison of different brewing methods: a case study of organic Arabica coffee in Northern Thailand. Environment and Natural Resources Journal 17(2), 96–108 (2019)
Monte, M., Padoano, E., Pozzetto, D.: Alternative coffee packaging: an analysis from a life cycle point of view. J. Food Eng. 66, 405–411 (2005)
Ambinacudige, S., Choi, J.: Global coffee market influence on land-use and land-cover change in the Western Ghats of India. Land Degrad. Dev. 20, 327–335 (2009)
Goncalves, M., Guerreiro, M.C., Ramos, P.H., de Oliveira, L.C.A., Sapag, K.: Activated carbon prepared from coffee pulp: potential adsorbent of organic contaminants in aqueous solution. Water Sci. Technol. 68(5), 1085–1090 (2013)
Emara, Y., Lehmann, A., Siegert, M.W., Finkbeiner, M.: Modelling pharmaceutical emissions and their toxicity-related impacts in life cycle assessment (LCA)—a review. Integr. Environ. Assess. Manage. 15(1), 6–18 (2018)
Chayer, J.A., Kicak, K.: The Final Report of Study Project: Life Cycle Assessment of Coffee Consumption: Comparison of Single-serve Coffee and Bulk Coffee Brewing. Quantis Press, Montreal (2015)
Hick, A.L.: Environmental implications of consumer convenience coffee as a case study. J. Ind. Ecol. 22(1), 79–91 (2017)
Noponen, M.R.A., Edwards-Jones, G., Haggar, J.P., Soto, G., Attarzadeh, N., Healey, J.R.: Greenhouse gas emissions in coffee grown with differing input levels under conventional and organic management. Agric. Ecosyst. Environ. 151, 6–15 (2012)
Hassard, H.A., Couch, M.H., Techa-Erawan, T., McLellan, B.C.: Product carbon footprint and energy analysis of alternative coffee products in Japan. J. Clean. Prod. 73, 310–321 (2014)
Humbert, S., Loerincik, Y., Rossi, V., Margni, M., Jolliet, O.: Life cycle assessment of spray dried soluble coffee and comparison with alternatives (drip filter and capsule espresso). J. Clean. Prod. 17, 1351–1358 (2009)
Lima, P.M., Paulo, P.L.: Solid-waste management in the rural area of BRAZIL: a case study in Quilombola communities. J. Mater. Cycles Waste Manage. (2017). https://doi.org/10.1007/s10163-018-0722-9
Preethu, D., Bhanu Prakash, B., Srinivasamurthy, C., Vasanthi, B.: Maturity indices as an index to evaluate the quality of compost of cofee waste blended with other organic wastes. In: Proceeding of International Conference on Sustainable Solid Waste Management, Chennai, India, Citeseer, pp. 270–275 (2007).
Beyene, A., Kassahun, Y., Addis, T., Assefa, F., Amsalu, A., Legesse, W., Kloos, H., Triest, L.: The impact of traditional coffee processing on river water quality in Ethiopia and the urgency of adopting sound environmental practices. Environ. Monit. Assess. 184, 7053–7063 (2012)
Rice, R.A.: A place unbecoming: the coffee farm of northern Latin America. Geogr. Rev. 89, 554–579 (1999)
Franca, A.S., Oliveira, L.S.: Coffee processing solid wastes: current uses and future perspectives. In: Ashworth, G.S., Azevedo, P. (eds.) Agricultural Wastes, pp. 155–189. Nova Science Publishers Inc, New York (2009)
Zoca, S.M., Penn, C.J., Rosolem, C.A., Alves, A.R., Neto, L.O., Martins, M.M.: Cofee processing residues as a soil potassium amendment. Int. J. Recycl. Org. Waste Agric. 3, 155–165 (2014)
Bernal, M.P., Alburquerque, J., Moral, R.: Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 100, 5444–5453 (2009)
Dadi, D., Daba, D., Beyene, A., Luis, P., Van der Bruggen, B.: Composting and co-composting of coffee husk and pulp with source-separated municipal solid waste: a breakthrough in valorization of coffee waste. Int. J. Recycl. Org. Waste Agric. 8, 263–277 (2019)
Echeverria, M.C., Pellegrino, E., Nuti, M.: The solid wastes of coffee production and of olive extraction: management perspectives in rural areas. In: Mihai, F.-C. (ed.) Solid Waste Management in Rural Areas, pp 165–189. Mirena Calmic, Croacia (2017)
Benitez, V., Rebollo-Hernanz, M., Hernanz, S., Chantres, S., Aguilera, Y., Martin-Cabrejas, M.A.: Coffee parchment as a new dietary fiber ingredient: functional and physiological characterization. Food Res. Int. 122, 105–113 (2019)
Dias, M., Melo, M.M., Schwan, R.F., Silva, C.F.: A new alternative use for coffee pulp from the semi-dry process to β-glucosidase production by Bacillus subtilis. Lett. Appl. Microbiol. 61(6), 588–595 (2015)
Putri, E., Rukayadi, Y., Sunarti, T.C., Meryandini, A.: Cellulolytic and Xylanolytic Actinomycetes selection to degrade Lignocellulosic biomass of Robusta coffee pulp (Coffea canephora). IOP Conf. Ser.: Earth Environ. Sci. 299(012014), 1–11 (2019)
Ty Na, N.T., Huan, P.T., Bich Tram, N.T.: Screening of fungal strains grown in solid-state culture for production of pectinase from coffee husk. Int. J. Adv. Sci. Eng. Inf. Technol. 6(3), 273–276 (2016)
Ramírez, L., Arrizon, J., Sandoval, G., Cardador, A., Bello-Mendoza, R., Lappe, P., Mateos-Díaz, J.C.: A new microplate screening method for the simultaneous activity quantification of feruloyl esterases, tannases, and chlorogenate esterases. Appl. Biochem. BioTechnol. 151(2–3), 711–723 (2008)
Eida, M.F., Nagaoka, T., Wasaki, J., Kouno, K.: Evaluation of cellulolytic and hemicellulolytic abilities of fungi isolated from coffee residue and sawdust composts. Microbes Environ. 26(3), 220–227 (2011)
Antier, P., Minjares, A., Roussos, S., Raimbault, M., Viniegra-Gonzalez, G.: Pectinase-hyperproducing mutants of Aspergillus niger C28B25 for solid-state fermentation of coffee pulp. Enz. Microb. Technol. 15(3), 254–260 (1993)
Muzakhar, K.: A consortium of three enzymes: xylanase, arabinofuranosidase, and cellulase from Aspergillus sp, which liquefied coffee pulp wastes. IOP Conf. Ser. Mater. Sci. Eng. 546(2), 1–8 (2019)
Marín, M., Artola, A., Sánchez, A.: Optimization of down-stream for cellulases produced under solid-state fermentation of coffee husk. Waste Biomass Valoriz. 10(10), 2761–2772 (2019)
Cerda, A., Gea, T., Vargas-Garcia, M.C., Sánchez, A.: Towards a competitive solid-state fermentation: cellulases production from coffee husk by sequential batch operation and role of microbial diversity. Sci. Total Environ. 589, 56–65 (2017)
Khelil, O., Choubane, S., Cheba, B.A.: Polyphenols content of spent coffee grounds subjected to physicochemical pretreatments influences lignocellulolytic enzymes production by Bacillus sp. R2. Bioresour. Technol. 211, 769–773 (2016)
Kandasamy, S., Muthusamy, G., Balakrishnan, S., Duraisamy, S., Thangasamy, S., Seralathan, K.-K., Chinappan, S.: Optimization of protease production from surface-modified coffee pulp waste and corncobs using Bacillus sp by SSF. 3 Biotech 6(2), 1–11 (2016)
Mussatto, S.I., Aguiar, L.M., Marinha, M.I., Jorge, R.C., Ferreira, E.C.: Economic analysis and environmental impact assessment of three different fermentation processes for fructooligosaccharides production. Bioresour. Technol. 198, 673–681 (2015)
Torres-Mancera, M.T., Cordova-López, J., Rodríguez-Serrano, G., Roussos, S., Ramírez-Coronel, M.A., Favela-Torres, E., Saucedo-Castañeda, G.: Enzymatic extraction of hydroxycinnamic acids from coffee pulp. Food Technol. BioTechnol. 49(3), 369–373 (2011)
Murthy, P.S., Naidu, M.M.: Production and application of xylanase from Penicillium sp. utilizing coffee by-products. Food Bioprocess Technol. 5(2), 657–664 (2012)
Murthy, P.S., Naidu, M.M., Srinivas, P.: Production of α-amylase under solid-state fermentation utilizing coffee waste. J. Chem. Technol. Biotechnol. 84(8), 1246–1249 (2009)
Niladevi, K.N., Prema, P.: Effect of inducers and process parameters on laccase production by Streptomyces psammoticus and its application in dye decolourization. Bioresour. Technol. 99(11), 4583–4589 (2008)
Venugopal, C., Jayachandra, T., Appaiah, K.A.A.: Effect of aeration on the production of endo-pectinase from the coffee pulp by a novel thermophilic fungi Mycotypha sp. strain No. AKM 1801. Biotechnology 6(2), 245–250 (2007)
Battestin, V., Macedo, G.A.: Tannase production by Paecilomyces variotii. Bioresour. Technol. 98(9), 1832–1837 (2007)
Ravindran, R., Jaiswal, A.K.: Microbial enzyme production using lignocellulosic food industry wastes as feedstock: a review. Bioengineering 3(4), 30 (2016)
Gottschalk, L.M.F., Oliveira, R.A., Bon, E.P.S.: Cellulases, xylanases, β-glucosidase and ferulic acid esterase produced by Trichoderma and Aspergillus act synergistically in the hydrolysis of sugarcane bagasse. Biochem. Eng. J. 51(1–2), 72–78 (2010)
Fava, F., Totaro, G., Diels, L., Reis, M., Duarte, J., Carioca, O.B., Poggi-Varaldo, H.M., Ferreira, B.S.: Biowaste biorefinery in Europe: opportunities and research and development needs. New Biotechnol. 32, 100–108 (2015)
Arora, R., Behera, S., Kumar, S.: Bioprospecting thermophilic/thermotolerant microbes for production of lignocellulosic ethanol: a future perspective. Renew. Sustain. Energy Rev. 51, 699–717 (2015)
Nguyen, T.M.T., Cho, E.J., Song, Y., Oh, C.H., Funada, R., Bae, H.-J.: Use of a coffee flower as a novel resource for the production of bioactive compounds, melanoidins, and bio-sugars. Food Chem. 299, 1–8 (2019)
Hijosa-Valsero, M., Garita-Cambronero, J., Paniagua-García, A.I., Díez-Antolínez, R.: Biobutanol production from coffee silverskin. Microb. Cell Fact. 17(1), 1–9 (2018)
Hudeckova, H., Neureiter, M., Obruca, S., Frühauf, S., Marova, I.: Biotechnological conversion of spent coffee grounds into lactic acid. Lett. Appl. Microbiol. 66(4), 306–312 (2018)
Pleissner, D., Neu, A.-K., Mehlmann, K., Schneider, R., Puerta-Quintero, G.I., Venus, J.: Fermentative lactic acid production from coffee pulp hydrolysate using Bacillus coagulans at laboratory and pilot scales. Biores. Technol. 218, 167–173 (2016)
Kim, H.M., Choi, Y.S., Lee, D.S., Kim, Y.H., Bae, H.J.: Production of bio-sugar and bioethanol from the coffee residue (CR) by acid-chlorite pretreatment. Bioresour. Technol. 236, 194–201 (2017)
Ravindran, R., Jaiswal, S., Abu-Ghannam, N., Jaiswal, A.K.: Evaluation of ultrasound assisted potassium permanganate pretreatment of spent coffee waste. Bioresour. Technol. 224, 680–687 (2017)
Ravindran, R., Sarangapani, C., Jaiswal, S., Cullen, P.J., Jaiswal, A.K.: Ferric chloride assisted plasma pretreatment of lignocellulose. Bioresour. Technol. 243, 327–334 (2017)
Ravindran, R., Jaiswal, S., Abu-Ghannam, N., Jaiswal, A.K.: Two-step sequential pretreatment for the enhanced enzymatic hydrolysis of coffee spent waste. Bioresour. Technol. 239, 276–284 (2017)
Wang, H.M.D., Cheng, Y.S., Huang, C.H., Huang, C.W.: Optimization of high solids dilute acid hydrolysis of spent coffee ground at mild temperature for enzymatic saccharification and microbial oil fermentation. Appl. Biochem. BioTechnol. 180(4), 753–765 (2016)
Nguyen, Q.A., Cho, E.J., Lee, D.-S., Bae, H.-J.: Development of an advanced integrative process to create valuable biosugars including manno-oligosaccharides and mannose from spent coffee grounds. Bioresour. Technol. 272, 209–216 (2019)
Nguyen, Q.A., Cho, E., Trinh, L.T.P., Jeong, J.-S., Bae, H.-J.: Development of an integrated process to produce D-mannose and bioethanol from coffee residue waste. Bioresour. Technol. 244, 1039–1048 (2017)
Choi, I.S., Wi, S.G., Kim, S.-B., Bae, H.-J.: Conversion of coffee residue waste into bioethanol with using popping pretreatment. Bioresour. Technol. 125, 132–137 (2012)
Mena-García, A., Ruiz-Matute, A.I., Soria, A.C., Sanz, M.L.: Green techniques for the extraction of bioactive carbohydrates. Trends Anal. Chem. 119(115612), 1–10 (2019)
Nadar, S.S., Rao, P., Rathod, V.K.: Enzyme assisted the extraction of biomolecules as an approach to novel extraction technology: a review. Food Res. Int. 108, 309–330 (2018)
Croteau, R., Kutchan, T.M., Lewis, N.G.: Natural products (secondary metabolites). In: Buchanan, B., Gruissem, W., Jones, R. (eds.) Biochemical and Molecular Biology of Plants, pp. 1250–1318. American Society of Plant Physiologists, Rockville (2000)
Crepin, V.F., Faulds, C.B., Connerton, I.F.: Functional classification of the microbial feruloyl esterases. Appl. Microbiol. BioTechnol. 63(6), 647–652 (2004)
Asther, M., Alvarado, M.I.E., Haon, M., Navarro, D., Asther, M., Lesage-Meessen, L., Record, E.: Purification and characterization of a chlorogenic acid hydrolase from Aspergillus niger catalyzing the hydrolysis of chlorogenic acid. J. BioTechnol. 115(1), 47–56 (2005)
Nieter, A., Kelle, S., Linke, D., Berger, R.G.: A p-coumaroyl esterase from Rhizoctonia solani with a pronounced chlorogenic acid esterase activity. New BioTechnol. 37, 153–161 (2017)
Nieter, A., Kelle, S., Linke, D., Berger, R.G.: Feruloyl esterases from Schizophyllum commune to treat food industry side-streams. Bioresour. Technol. 220, 38–46 (2016)
Pinelo, M., Tress, A.G., Pedersen, M., Arnous, A., Meyer, A.S.: Effect of cellulases, solvent type and particle size distribution on the extraction of chlorogenic acid and other phenols from spent coffee grounds. Am. J. Food Technol. 2(7), 641–651 (2007)
Benoit, I., Navarro, D., Marnet, N., Rakomanomana, N., Lesage-Meessen, L., Sigoillot, J.-C., Asther, M., Asther, M.: Feruloyl esterases as a tool for the release of phenolic compounds from agro-industrial by-products. Carbohydr. Res. 341(11), 1820–1827 (2006)
Yang, X., Choi, H.S., Park, C., Kim, S.W.: Current states and prospects of organic waste utilization for biorefineries. Renew. Sustain. Energy Rev. 49, 335–349 (2015)
Phimsen, S., Kiatkittipong, W., Yamada, H., Tagawa, T., Kiatkittipong, K., Laosiripojana, N., Assabumrungrat, S.: Oil extracted from spent coffee grounds for bio-hydrotreated diesel production. Energy Convers. Manage. 126, 1028–1036 (2016)
Karmee, S.K., Swanepoel, W., Marx, S.: Biofuel production from spent coffee grounds via lipase catalysis. Energy Sources Part A: Recov. Util. Environ. Effects 40(3), 294–300 (2018)
Sarno, M., Iuliano, M.: Active biocatalyst for biodiesel production from spent coffee ground. Bioresour. Technol. 266, 431–438 (2018)
Ferrario, V., Veny, H., De Angelis, E., Navarini, L., Ebert, C., Gardossi, L.: Lipases immobilization for effective synthesis of biodiesel starting from coffee waste oils. Biomolecules 3(3), 514–534 (2013)
Burton, R., Fan, X., Austic, G.: Evaluation of two-step reaction and enzyme catalysis approaches for biodiesel production from spent coffee grounds. Int. J. Green Energy 7(5), 530–536 (2010)
Pires, J.F., Cardoso, L.S., Schwan, R.F., Silva, C.F.: Diversity of microbiota found in coffee processing wastewater treatment plant. World J. Microbiol. Biotechnol. 33(12), 1–12 (2017)
Torres, J.A., Chagas, P.M.B., Silva, M.C., Santos, C.D., Corrêa, A.D.: Evaluation of the protective effect of chemical additives in the oxidation of phenolic compounds catalyzed by peroxidase. Environ. Technol. 37(10), 1288–1296 (2016)
Acknowledgements
The authors are thankful to National Council for Scientific and Technological Development (CNPq), and Research Support in the State of Rio de Janeiro (FAPERJ). Luna, A. also thanks to from UERJ (Pro-Science Program). We are particularly grateful for the assistance given by Luiz Fernando Menezes da Silva, technician of Embrapa Food Agroindustry for the improvement of the illustrations and Figures.
Funding
This study was funded by the program CAPES (Higher Education Improvement Coordination)/EMBRAPA (The Brazilian Agricultural Research Corporation) no 15/2014.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Corrêa, C.L.O., Penha, E.M., Freitas-Silva, O. et al. Enzymatic Technology Application on Coffee Co-products: A Review. Waste Biomass Valor 12, 3521–3540 (2021). https://doi.org/10.1007/s12649-020-01208-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01208-w