Abstract
The catalysis of biomass pyrolysis vapors by metal oxide and zeolite was proposed to produce high-value chemicals. Metal oxides CaO and Al2O3 exhibited strong reactivity with acids and phenols with methoxyl. Acetic acid was reduced by 65.2% and 23.3% under CaO and Al2O3 catalysis, respectively, whereas guaiacols decreased by 40.4% and 27.6%, respectively. The neutralization with acids and the catalytic effect on the oxygenate groups were the main reactions of CaO, while deoxygenation on Lewis sites mainly occurred during the catalyzation by Al2O3. Since the vapors were cracked into low molecular compounds, the combination of CaO and microporous HZSM-5 showed a promotion effect on generating aromatic hydrocarbons, with 56.0% and 44.7% increases of benzene and toluene, respectively. A dual catalyst composed of CaO and mesoporous Al-MCM-41 exhibited a promotion of phenols, furans, etc., due to its large pores, which permitted more vapor to react at acid sites. The enhancement of phenols and furans was 81.2% and 8.78%, respectively. At last, the optimal temperatures for the two dual catalysts, CaO and HZSM-5 and CaO and Al-MCM-41, were 800 °C and 700 °C, respectively.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
In the present study, the dual catalysts of metal oxides and zeolites were employed to enhance the yields of high-value chemical compounds like aromatics and phenols. For different catalyst combination, the reaction mechanism and product distribution were discussed in detail, and the optimal operating conditions were obtained. During the catalytic fast pyrolysis of biomass, the formation of coke would cause block and deactivation of the catalyst, and the generation of target products were suppressed as well. With the catalysis of macroporous metal oxide, the pyrolytic vapors could be cracked into lower molecular compounds firstly, and then passed through the pores of zeolite molecular sieves to crack into aromatics, phenols, etc., so that the yields of target products were enhanced. Due to the low cost and accessibility of the dual catalyst like CaO and HZSM-5, the proposed catalyst could be a promising technique for industry application.
Introduction
For the promotion of renewable energy, scholars all over the world have conducted extensive and in-depth research on topics such as solar energy [1] and wind energy [2]. However, the accumulation of waste biomass remains a major obstacle to reducing the ecological footprint of many industries. Agriculture and forestry play a pivotal role in China’s economic development, and the annual waste biomass has reached as high as 487 million tons. Common treatments for biomass include direct combustion [3], compression molding [4], biogas fermentation [5], gasification [6], pyrolysis [7], and biodiesel [8]. In these technologies, pyrolysis is a promising method that can produce liquid, gas and solid products simultaneously, especially the high-grade alternative liquid fuels and valuable chemical raw materials. Many scholars have investigated the pyrolytic kinetic mechanism and product distribution of waste such as end-of-life tires [9, 10], metal-contaminated oil waste [11], printed circuit boards [12], and polymeric compounds [13, 14]. Recently, new pyrolysis ways like microwave-activation pyrolysis were also proposed [15]. One study focused on different feedstocks, like plant acidified oil degraded from soap stock [16], and reported the different characteristics of the raw materials and the bio-oil components. With this approach, the obtained bio-oil can be stored and transported appropriately, with the heat value reaching about half that of gasoline. It was found that bio-oil can be further refined to generate high-value chemicals, like phenols and aromatic hydrocarbons, which are important raw materials for the chemical industry.
However, there are a number of oxygenates in bio-oil, including acids, aldehydes, and ketones. Because of the high oxygen content, the heat value of bio-oil is only half that of gasoline. The reactions between aldehydes and ketones will cause aging to shorten the service life [17]. Many scholars have investigated different ways to refine bio-oil, including catalytic hydrogenation [18] and catalytic cracking [19, 20]. Noble metals, like Pt and Pd, are often used as catalysts for catalytic hydroprocessing. The main drawbacks of this technology are the need for H2 and the high operating pressure, indicating potential safety issues for industrialization. Recently, Hoda et al. [21] proposed an improved hydrogenation method employing 2-proponal as the hydrogen source, which reduced the cost and safety risks. However, a relatively high pressure of N2 was still indispensable for the inert environment. Catalytic cracking mainly employs porous catalysts, which are inexpensive and readily available. The operating condition, though, is an inert environment at constant pressure, indicating a higher security requirement for industrial applications. Zeolites have been investigated extensively due to their great deoxidization capacity. HZSM-5 is a microporous material, and its deoxygenation effect has been demonstrated by many researchers. The catalytic pyrolysis of poplar with HZSM-5 revealed that the selectivity of toluene and xylene was increased from 0.6 and 0.54% to 38.15 and 28.46%, respectively [22]. For a higher yield of aromatics and better anti-coking ability, more attention was focused on the modification of HZSM-5. The catalyst modified by transition metals, such as Ni [23, 24], Zn [25], and Fe [26], was found to generate more aromatic hydrocarbons due to the load of strong Brønsted sites. However, the limited pores prevented large-molecular oxygenates from entering, which affected the catalytic efficiency to some extent. The large molecular compounds also concentrated outside the catalyst and formed coke, which shortened the catalyst’s life. Mesoporous catalysts, like MCM-41 [27] and SBA-15 [28], have also been employed to generate aromatics and phenols. The large pores made it easier for the molecules to get through, so less coke was formed in the catalyst. However, the deoxidization capability of MCM-41 was worse than HZSM-5 since the decarboxylation of long-chain compounds in the pores formed more ketones, aldehydes, etc., which increased the oxygen content [29].
Metal oxides, which are common and inexpensive catalysts, were also evaluated extensively for their capability of reducing oxygen. Zou et al. [30] employed Al2O3, MgO, Ga2O3, etc., to reform the pyrolysis steam from cellulose and found that decarboxylation and decarbonization were promoted, which removed the acids and aldehydes and reduced the oxygen content. Another alkali metal catalyst, CaO, could exhibit different characteristics under different conditions. According to Chen et al. [31], when the mass ratio of CaO to reactant was lower than 0.2, decarboxylation dominated the reaction, which released more CO2 and calcium carboxylate ((RCOO)2Ca), and further decomposed to ketones. By increasing the amount of catalysts, more esters would be catalyzed to generate hydrocarbons. The fluidized-bed pyrolysis conducted by Yuan et al. [32] concluded that, with a 30 wt% addition of CaO, the pH of bio-oil could be raised from 2.4 to 4.4, and most acids and ketones could be removed. Wang et al. [33] also compared the mesoporous catalyst MCM-41 with CaO in terms of their deoxygenation effects. It was found that decarbonization mainly occurred on MCM-41, and decarboxylation mainly occurred on CaO. The carbonyl compounds and acids were reduced by 10.2% and 75.88%, respectively. However, the reaction between CaO and acids to form (RCOO)2Ca was exothermic [31], and a high temperature would affect the reaction negatively.
To reduce the oxygen content, selectively produce high-value chemicals, and prolong the service life of the catalyst, a metal oxide combined with a zeolite catalyst was also proposed. Fe2O3/ZSM-5 prepared by metal–organic chemical vapor deposition showed an excellent deoxygenation ability to generate phenolic and aromatic hydrocarbons [34], although a mechanical combination could exhibit similar effects and lower the cost. Che [35] mechanically combined HZSM-5 with CaO and Al2O3 in turn and found that the selectivity of xylene and toluene was increased by 15.9% and 18.3%, respectively. Although the combination of metal oxides and microporous catalysts has been studied, research on the combination of metal oxides with mesoporous catalysts to form dual catalysts is much more limited. Hence, the reaction mechanisms and product distributions of such catalysts are also needed for a comprehensive evaluation. In the present study, Pyrolysis-gas chromatography/mass spectrometry (Py-GC/MS) was employed for catalytic pyrolysis experiments, and both single catalysts and dual catalysts of metal oxides and zeolite were used for comparison. The generation of specific chemicals, such as phenols and aromatics, was discussed in detail.
Material and Methods
Material
The biomass sample for catalytic pyrolysis was cedarwood provided by a local furniture factory. After being sieved with a 200-mesh sieve (< 0.95 μm), the feedstock was dried up at 80 °C for 24 h. The ultimate analysis was conducted on the varioELcube element analyzer, where Duma’s combustion dynamic method was employed. The proximate analysis was performed in a muffle furnace according to GB/T28731-2012. The heat value was determined with a SDACM3100 Bomb Calorimeter (HunanSundy Science and Technology Co., Ltd.). The results are listed in Table 1. Metal oxide catalysts CaO and Al2O3 were purchased from Zhiyuan Co., Ltd., and Antai Co., Ltd., respectively. Microporous HZSM-5 (Si/Al ratio = 25) and mesoporous Al-MCM-41 (Si/Al ratio = 30) were produced by Nankai Catalyst Co., Ltd. The properties of these catalysts are listed in Table 2. All the catalysts and biomass samples were dried overnight.
Py-GC/MS Experiment
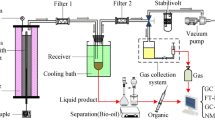
All pyrolysis experiments were conducted in the Py-GC/MS, which included a CDS5200 pyrolyzer and an Agilent GC/MS (GC: 7890B, MS: 5977B). For single metal oxide catalytic pyrolysis, the sample was separated from the catalyst with quartz wool, like a conventional placement. In contrast, for the dual catalyst composed of metal oxide and zeolite, the sample was set up as shown in Fig. 1 to prevent the alkaline metal oxides from destroying acid sites on the zeolite [35]. In each run of the experiment, 0.3 ± 0.02 mg of cedarwood, 0.3 ± 0.02 mg of metal oxide, and 1.5 ± 0.05 mg of zeolite were used.
In the pyrolyzer, the sample was heated at a rate of 20 °C∙ms−1 to the target temperature with a hold time of 20 s. The generated pyrolytic vapors were then directly carried into GC/MS through a transfer line by Helium (99.999%). To avoid the condensation of condensable vapors, the transfer line was kept at 300 °C. In the GC oven, different products were separated by a HP-5 ms capillary column (30 m × 250 μm × 0.25 μm) with a split ratio of 1:60. The GC oven was kept at 40 °C for 2 min, and then the temperature rose to 280 °C in 24 min and stayed at that temperature for 1 min. The mass spectra were operated in electron ionization (EI) mode at 70 eV and obtained from 45 to 550 m/z. Since the response values of different products in GC/MS are inconsistent, a semi-quantitative method was adopted in this paper, that is to say, the absolute peak area of the same substance was compared to determine the yield. To assure the accuracy and reliability of the results, each run was repeated three times.
Results and Discussion
Catalytic Pyrolysis with Metal Oxides
The pyrolysis of cedarwood catalyzed with metal oxide CaO and Al2O3 was conducted by Py-GC/MS under 600 °C. According to the total ion chromatography (Fig. 2), the products detected are listed in Table 3. The production of chemical compounds with oxygen-containing groups, like acids, aldehydes, and phenols with methoxyls, was suppressed. Compared to the non-catalyzed experiment, the removal of acetic acid as a result of catalysis by CaO and Al2O3 was 65.2% and 23.3%, respectively, while for furfural, the removal was 24.7% and 5.2%, respectively. In general, alkali metal oxide CaO removed more acids and aldehydes than Al2O3. CaO is widely used in biomass gasification for its excellent capacity for the crack of tar and the absorption of CO2 [36]. During pyrolysis, the generated acids might react with the CaO through neutralization [31,32,33], in which the carboxyl on the acids would be converted to calcium carboxylate ((RCOO)2Ca) and further decomposed into ketones and calcium carbonate (CaCO3), as shown in Eqs. (1) and (2). However, it is also possible for CaO to act as a catalyst during the decarboxylation of acids, which would generate more hydrocarbons as shown in Eq. (3) [35, 37]. Due to the basic center on the surface of CaO, the phenolic hydroxyl of lignin would lose hydrogen ions, which would accelerate the degradation of the ether bond in the lignin structure [38]. The phenolic hydroxyl, methoxyl, and other unsaturated groups on the single phenol would further be removed with the addition of CaO [37], so the yields of guaiacols and eugenols were decreased as shown in Table 3. After catalyzation, the oxygen content was decreased while the (H/C)eff could be increased effectively. Some researchers [39] also pointed out that the decarbonylation of phenols could be catalyzed by CaO with the oxygen removed in the form of CO. The mild acidity of Al2O3 was attributed to the Lewis sites covering it, which could accelerate the deoxygenation, although not as well as CaO [30]:
Catalytic Pyrolysis with Metal Oxides and Zeolites
Effects of Different Dual Catalysts
During the dual-catalyst experiment, the pyrolysis vapors passed through the metal oxides and zeolites in turn, and the two reforming processes exhibited great effects on the final yields of products, as shown in Table 4. Both the microporous HZSM-5 and mesoporous Al-MCM-41 showed positive effects on the promotion of aromatics, while the yields of aromatics for HZSM-5 were higher than those for Al-MCM-41. HZSM-5 had excellent deoxidation capability and higher selectivity to the aromatics than Al-MCM-41. Among one of the three main components, lignin was composed of three kinds of phenylpropane units, which were connected through a variety of C–O and C–C bonds. The spatial structure and aromaticity of lignin made it able to be converted into aromatics to enhance the chemical value [40]. Lignin was first decomposed into phenols under heating, and the secondary reactions took place when the products entered the pores of catalysts. The strong Brønsted sites located in the channel of HZSM-5 would promote the dehydroxylation, demethylation, etc., to convert the phenols into aromatic hydrocarbons [41, 42]. For holocellulose, the decomposed product monosaccharide would be converted into furan and other low molecular oxygenates like aldehydes and ketones, as Wang et al. [43] proposed. HZSM-5 would accelerate the decarbonization of furans to aliphatic hydrocarbons and finally convert these hydrocarbons into aromatics during aromatization. The weak aromatization and hydrodeoxygenation capability of the mesoporous catalyst resulted in the lower yield than HZSM-5. However, due to the limited pore size of HZSM-5, the yields of phenols and furans were lower than those of Al-MCM-41. The large pore size of the mesoporous catalyst would let more long-chain compounds arrive at the acid sites,thus, decarbonization, dehydration, etc., would happen on these C=O and C–C bonds to generate more phenols, olefins, and furans. Specifically, the phenol and methyl phenol were not detected during the catalytic pyrolysis when HZSM-5 was employed, which could be attributed to the limited amounts of guaiacols getting into the pores and the strong hydrodeoxygenation ability to convert these into aromatics.
When cedarwood was catalyzed by the dual catalyst, due to the difference between the two zeolite catalysts, the continuous reforming process exhibited different effects. According to Fig. 3, the aromatics’ yields were dramatically increased when the metal oxide and HZSM-5 were employed. Compared with the single HZSM-5, the yields of benzene, toluene, and xylene with Al2O3 and HZSM-5 were increased by 56.0%, 41.0%, and 26.9%, respectively, and the increased yields with CaO and HZSM-5 were 56.0%, 44.7%, and 42.8%, respectively. As a result, the obtained bio-oil exhibited a high yield for the aromatic hydrocarbons. In detail, CaO and HZSM-5 showed a better promotion effect on the final aromatics’ yields. When Al-MCM-41 was employed, the yields of the aromatics were far less than those of HZSM-5. As discussed in section “Catalytic Pyrolysis with Metal Oxides”, the advance catalyzation by the metal oxide effectively reduced the length of the compounds, which would make more small molecules be catalyzed on the strong Brønsted sites. Meanwhile, the generated olefins and ketones would also experience aromatization in the channel of HZSM-5 [44, 45]. However, the production of polycyclic aromatics was also promoted, which could be attributed to the reactions between monocyclic aromatics and oxygenates on the outer acid sites of the catalyst. Regardless of the aromatics’ yield, with the mesoporous catalyst, more phenols and furans were produced, as shown in Fig. 4. The deoxidation of the oxygen-containing groups of guaiacols was greatly accelerated when the metal oxide and Al-MCM-41 functioned as a dual catalyst, especially for CaO; thus, the phenol and methyl phenol’s yields exhibited increases of 81.2% and 19.3%, respectively, compared with the single Al-MCM-41 catalyzation. During the experiment, the wide pores of CaO allowed more glucosan and other polysaccharides to be exposed to the reaction site on Al-MCM-41. With Al substituting for Si in the structure, sites with negative charges form and attract protons; thus, reactions between vapors and catalysts take place [46]. As He et al. [47] pointed out, MCM-41 showed a positive effect on the hydrolysis of cellulose, which would also explain the 8.78% increase of furan in this work. Lewis sites located on Al2O3 might cause further reaction of furan, and the yield could be affected by these further reactions, which was consistent with the result in Table 4. When it comes to acetic acid, the low yield made it difficult to be detected, and undoubtedly the decarboxylation capability of the dual catalyst was verified.
Effects of the Temperature
Temperature would affect the catalyzation reaction greatly, and the yields of products by CaO and HZSM-5 and CaO and Al-MCM-41 catalysis are exhibited in Fig. 5. According to section “Effects of Different Dual Catalysts”, benzene, toluene, xylene, naphthalene, and 2-methyl-naphthalene were selected as the characteristic products with the CaO and HZSM-5 catalyst, whereas butene, furans, and methyl phenols were chosen for the CaO and Al-MCM-41 catalyst. From Fig. 5a, when the temperature increased from 600 to 800 °C, all aromatics experienced an increase, and the highest yield was achieved at 800 °C. When the temperature kept rising to 900 °C, the yield of all products decreased, although toluene and p-xylene were reduced more seriously. A high temperature might cause further decomposition of the products, and the catalyst’s pore structure would also be destroyed under such a harsh environment. However, the optimal temperature for another dual catalyst was 700 °C, as shown in Fig. 5b. Compared with the microporous HZSM-5, the hydrothermal stability of Al-MCM-41 was worse, so a high temperature was not appropriate for the reaction in the mesoporous catalyst [48]. Moreover, the generation of furans and phenols might need lower temperatures than aromatics since the production of aromatics was a further reaction. To conclude, the optimal reaction temperature for producing the main characteristic products with CaO and HZSM-5 and CaO and Al-MCM-41 catalysts was 800 °C and 700 °C, respectively.
Conclusion
In order to selectively produce high value-added chemicals or high-quality oxygenated liquid fuels, and prolong the service life of the catalyst, the duel catalysts of metal oxides and zeolites, viz. CaO and HZSM-5, CaO and Al-MCM-41, Al2O3 and HZSM-5 and Al2O3 and Al-MCM-41 were studied in comparison with single zeolite catalyst. Pyrolysis-Gas Chromatography/Mass Spectrometry (Py-GC/MS) was employed for catalytic pyrolysis experiments. The generation of specific chemicals, such as phenols and aromatics, were discussed in detail.
The dual catalysts, CaO and HZSM-5 and CaO and Al-MCM-41, exhibited different effects on the promotion of high-value chemicals during catalytic fast pyrolysis, and are better than the other two dual catalysts with Al2O3. CaO and HZSM-5 increased benzene production by 56.0% and toluene by 44.7%, and the optimal temperature was 800 °C. CaO and Al-MCM-41 increased the yield of phenol and furan by 81.2% and 8.78%, respectively, and the optimal temperature was 700 °C. It was proved that the two dual catalysts have the potential to convert biomass feedstock into significant industrial chemicals and relieve the pressure on fossil fuels in the chemical industry.
In further study, experiments will be carried out on fluidized bed devices, and the scale-up of the device and the comprehensive use of gas, liquid and solid products will be considered to make it more economical. For a scaled-up application, it will be necessary to conduct a comprehensive assessment on the service life of the catalysts, and an economic feasibility analysis on the system will be carried out.
References
Kabir, E., Kumar, P., Kumar, S., Adelodun, A.A., Kim, L.: Potential and future prospects. Renew. Sustain. Energy Rev. 82, 894–900 (2018)
Sahu, B.K.: Wind energy developments and policies in China: a short review. Renew. Sustain Energy Rev. 81, 1393–1405 (2018)
Khan, A.A., Jong, W.D., Jansens, P.J., Spliethoff, H.: Biomass combustion in fluidized bed boilers: Potential problems and remedies. Fuel Process Technol. 90, 21–50 (2009)
Uranga, J., Etxabide, A., Guerrero, P., Caba, K.D.L.: Development of active fish gelatin films with anthocyanins by compression molding. Food Hydrocoll. 84, 313–320 (2018)
Blume, F., Bergmann, I., Nettmann, E., Schelle, H., Rehde, H., Mundt, K., Klocke, M.: Methanogenic population dynamics during semi-continuous biogas fermentation and acidification by overloading. J. Appl. Microbiol. 109, 441–450 (2010)
Sansaniwal, S.K., Pal, K., Rosen, M.A., Tyagi, S.K.: Recent advances in the development of biomass gasification technology: a comprehensive review. Renew. Sustain. Energy Rev. 72, 363–384 (2017)
Anca-Couce, A., Scharler, R.: Modelling heat of reaction in biomass pyrolysis with detailed reaction schemes. Fuel 206, 572–579 (2017)
Shahzad, K., Nizami, A.S., Sagir, M., Rehan, M., Maier, S., Khan, M.Z., Ouda, O.K.M., Ismail, I.M.I., BaFail, A.O.: Biodiesel production potential from fat fraction of municipal waste in Makkah. PLoS ONE 12, e0171297 (2017)
Al-Salem, S.M.: 5—feedstock and optimal operation for plastics to fuel conversion in pyrolysis. In: Al-Salem, S.M. (ed.) Plastics to Energy, pp. 117–146. William Andrew Publishing, Norwich (2019)
Al-Salem, S.M., Lettieri, P., Baeyens, J.: Kinetics and product distribution of end of life tyres (ELTs) pyrolysis: a novel approach in polyisoprene and SBR thermal cracking. J. Hazard. Mater. 172, 1690–1694 (2009)
Tian, Y., Li, J., Yan, X., Whitcombe, T., Thring, R.: Co-pyrolysis of metal contaminated oily waste for oil recovery and heavy metal immobilization. J. Hazard. Mater. 373, 1–10 (2019)
Quan, C., Li, A., Gao, N.: Synthesis of carbon nanotubes and porous carbons from printed circuit board waste pyrolysis oil. J. Hazard. Mater. 179, 911–917 (2010)
Al-Salem, S.M., Khan, A.R.: On the degradation kinetics of poly(ethylene terephthalate) (PET)/poly (methyl methacrylate) (PMMA) blends in dynamic thermogravimetry. Polym. Degrad. Stab. 104, 28–32 (2014)
Al-Salem, S.M., Lettieri, P.: Kinetic study of high density polyethylene (HDPE) pyrolysis. Chem. Eng. Res. Des. 88, 1599–1606 (2010)
Liew, R.K., Azwar, E., Yek, P.N.Y., Lim, X.Y., Cheng, C.K., Ng, J., Jusoh, A., Lam, W.H., Ibrahim, M.D., Ma, N.L., Lam, S.S.: Microwave pyrolysis with KOH/NaOH mixture activation. A new approach to produce micro-mesoporous activated carbon for textile dye adsorption. Biores. Technol. 266, 1–10 (2018)
Xu, L., Cheng, J.H., Liu, P., Wang, Q., Xu, Z.X., Liu, Q., Shen, J.Y., Wang, L.J.: Production of bio-fuel oil from pyrolysis of plant acidified oil. Renew. Energy 130, 910–919 (2019)
Lu, Q., Li, W., Zhu, X.: Overview of fuel properties of biomass fast pyrolysis oils. Energy Convers. Manag. 50, 1376–1383 (2009)
Pstrowska, K., Walendziewski, J., Luzny, R., Stolarski, M.: Hydroprocessing of rapeseed pyrolysis bio-oil over NiMo/Al2O3 catalyst. Catal. Today 223, 54–65 (2014)
Wan, S., Pham, T., Zhang, S., Lobban, L., Resasco, D., Mallinson, R.: Direct catalytic upgrading of biomass pyrolysis vapors by a dual function Ru/TiO2 catalyst. AIChE J. 59, 2275–2285 (2013)
Wang, K., Johnson, P.A., Brown, R.C.: Comparison of in-situ and ex-situ catalytic pyrolysis in a micro-reactor system. Biores. Technol. 173, 124–131 (2014)
Shafaghat, H., Lee, I.G., Jae, J., Jung, S.C., Park, Y.K.: Pd/C catalyzed transfer hydrogenation of pyrolysis oil using 2-propanol as hydrogen source. Chem. Eng. J. 377, 119986 (2019)
Zhao, Y., Liu, Y.: Production of aromatic hydrocarbons through catalytic pyrolysis of biomass used HZSM-5 as catalyst. New Chem. Mater. 45, 145–147 (2017)
Lei, X., Bi, Y., Zhou, W., Chen, H., Hu, J.: Catalytic fast pyrolysis of cellulose by integrating dispersed nickel catalyst with HZSM-5 zeolite. IOP Conf. Ser. 108, 22017 (2018)
Yan, L., Kong, X., Bai, Y., Xie, K., Li, F.: Catalytic upgrading of gaseous tar from coal pyrolysis over Mo and Ni modified HZSM. J. Fuel Chem. Technol. 44, 30–36 (2016)
Ramos, R., Garcia, A., Botas, J.A., Serrano, D.P.: Enhanced production of aromatic hydrocarbons by rapeseed oil conversion over Ga and Zn modified ZSM-5 catalysts. Ind. Eng. Chem. Res. 55, 12723–12732 (2016)
Sun, L., Zhang, X., Chen, L., Zhao, B., Yang, S., Xie, X.: Comparision of catalytic fast pyrolysis of biomass to aromatic hydrocarbons over ZSM-5 and Fe/ZSM-5 catalysts. J. Anal. Appl. Pyrol. 121, 342–346 (2016)
Chen, Y., Zheng, Y., Li, M., Zhu, X.: Arene production by W2C/MCM-41-catalyzed upgrading of vapors from fast pyrolysis of lignin. Fuel Process Technol. 134, 46–51 (2015)
Bao, W., Xue, X., Cao, Q., Lu, J., Lv, Y.: Study on biomass pyrolytic liquid products with MCM-41/SBA-15 as catalyst. J. Fuel Chem. Technol. 06, 675–679 (2006)
Dong, L., Li, X., Zhang, J., Zhang, X., Hu, C., Cai, Y., Shao, S.: Effects of HZSM-5 and MCM-41 mixing ratios on rape straw catalytic pyrolysis. Trans. Chin. Soc. Agric. Eng. 33, 241–247 (2017)
Zou, Q., Dai, G., Lin, H., Wang, S.: Catalytic pyrolysis of microcrystalline cellulose with metal oxides. Chem. Ind. Eng. Prog. 37, 1837 (2018)
Chen, X., Chen, Y., Yang, H., Chen, W., Wang, X., Chen, H.: Fast pyrolysis of cotton stalk biomass using calcium oxide. Biores. Technol. 233, 15–20 (2017)
Yuan, L., Liu, Y., Wang, D., Zang, Y.: Effects of catalyst CaO on fast pyrolysis of pine for the production of bio-oils. Biomass Chem. Eng. 47, 1–6 (2013)
Wang, D., Xiao, R., Zhang, H., He, G.: Comparison of catalytic pyrolysis of biomass with MCM-41 and CaO catalysts by using TGA-FTIR analysis. J. Anal. Appl. Pyrol. 89, 171–177 (2010)
David, E., Kopač, J.: Upgrading the characteristics of the bio-oil obtained from rapeseed oil cake pyrolysis through the catalytic treatment of its vapors. J. Anal. Appl. Pyrol. 141, 104638 (2019)
Che, Q., Yang, M., Wang, X., Chen, X., Chen, W., Yang, Q., Yang, H., Chen, H.: Aromatics production with metal oxides and ZSM-5 as catalysts in catalytic pyrolysis of wood sawdust. Fuel Process Technol. 188, 146–152 (2019)
Florin, N.H., Harris, A.T.: Enhanced hydrogen production from biomass with in situ carbon dioxide capture using calcium oxide sorbents. Chem. Eng. Sci. 63, 287–316 (2008)
Zhang, X., Sun, L., Chen, L., Xie, X., Zhao, B., Si, H., Meng, G.: Comparison of catalytic upgrading of biomass fast pyrolysis vapors over CaO and Fe(III)/CaO catalysts. J. Anal. Appl. Pyrol. 108, 35–40 (2014)
Thring, R.W., Chornet, E., Bouchard, J., Pierre, F.V., Palph, P.O.: Characterization of lignin residues derived from the alkaline hydrolysis of glycol lignin. Can. J. Chem. 68, 82–89 (1990)
Jia, Y., Huang, J., Wang, Y.: Effects of calcium oxide on the cracking of coal tar in the freeboard of a fluidized bed. Energy Fuels 18, 1625–1632 (2004)
Zhao, J.: Research on the Production of Aromatic Hydrocarbons from Fast Catalytic Pyrolysis of Lignin. Southeast University, Dhaka (2016)
Yu, N., Cai, Y., Li, X., Fan, Y., Yin, H., Zhang, R.: Catalytic pyrolysis of rape straw for upgraded bio-oil production using HZSM-5 zeolite. Trans. Chin. Soc. Agric. Eng. 30, 264–271 (2014)
Foster, A.J., Jae, J., Cheng, Y., Huber, G.W., Lobo, R.F.: Optimizing the aromatic yield and distribution from catalytic fast pyrolysis of biomass over ZSM-5. Appl. Catal. A 423–424, 154–161 (2012)
Wang, K.G., Kim, K.H., Brown, R.C.: Catalytic pyrolysis of individual components of lignocellulosic biomass. Green Chem. 16, 727–735 (2014)
Lu, Q., Guo, H., Zhou, M., Cui, M., Dong, C., Yang, Y.: Selective preparation of monocyclic aromatic hydrocarbons from catalytic cracking of biomass fast pyrolysis vapors over Mo2N/HZSM-5 catalyst. Fuel Process. Technol. 173, 134–142 (2018)
Lu, Q., Guo, H., Zhou, M., Zhang, Z., Cui, M., Zhang, Y., Yang, Y., Zhang, L.: Monocyclic aromatic hydrocarbons production from catalytic cracking of pine wood-derived pyrolytic vapors over Ce-Mo2N/HZSM-5 catalyst. Sci. Total Environ. 634, 141–149 (2018)
Antonakou, E., Lappas, A., Nilsen, M.H., Bouzga, A., Stocker, M.: Evaluation of various types of Al-MCM-41 materials as catalysts in biomass pyrolysis for the production of bio-fuels and chemicals. Fuel 85, 2202–2212 (2006)
He, D., Bai, C., Jiang, C., Zhou, T.: Synthesis of titanium containing MCM-41 and its application for catalytic hydrolysis of cellulose. Powder Technol. 249, 151–156 (2013)
Chen, G., Dai, Q., Wang, X.: Hydrothermal stability of mesoporous sieves MCM-41. Chem. Bull. 04, 271–276 (2005)
Acknowledgements
This work was supported by the National Natural Science Foundations [Grant Numbers 51676039 and 51861145102].
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, J., Liu, Q., Zhou, J. et al. Production of High-Value Chemicals by Biomass Pyrolysis with Metal Oxides and Zeolites. Waste Biomass Valor 12, 3049–3057 (2021). https://doi.org/10.1007/s12649-020-00962-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-00962-1