Abstract
The global impetus to produce alternatives for the petroleum-based fuels and value-added chemicals in order to reduce greenhouse gases is currently emphasizing stringent need on the industries to diversify and valorize byproducts. This further aims at the valorization of agroindustrial by-product into furfural. A thorough investigation of research advances particularly, the pretreatment of biomass, a pertinent reaction mechanism in furfural production, separation of furfural and the various used catalysts were explored in the current review. The biomass, which contains fiber, lignin, pentosans, and pith, can be converted into furfural by the application of suitable chemical, biochemical and microbial methods. Dilute acid, alkali and hydrothermal pretreatment methods for hemicellulose separation from the biomass matrix were discussed in detail. Studies on the development of an effective and stable catalyst to overcome the limitation of the existing commercial processes were also reviewed. The strategies including the steam stripping, nitrogen stripping, supercritical carbon dioxide extraction, mono- and biphasic solvent extractions were investigated in this study, as a way forward towards the removal of furfural from the reaction medium, thereby assisting in the avoidance of the product degradation.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
The purpose of this review is to demonstrate the gaps in producing the furfural from biomass efficiently. It provides insights and understanding of lignocellulosic biomass conversion over various catalysts in view of the production of furfural. It is intended to give a clue for the readers on the reaction mechanisms, pretreatment methods to enhance furfural yield and the various catalysts which can be applied for biomass valorization processes. This review is important as it also elucidates furfural isolation mechanisms to help readers understand the effect of the method on the yield.
Introduction
Conversion of biomass into sustainable energy and various chemicals is recently becoming an attractive alternative to resolve the problems of mankind [1]. Lignocellulosic biomass contains cellulosic, hemicellulosic and lignin components which can be transformed into various platform chemicals such as furfural. Furfural can be produced from xylan, a five-carbon saccharide, undergoing hydrolysis reaction followed by the dehydration upon the release of the three water molecules. Most organic conversions in chemical process industries are based on the acid-base homogeneous catalysis. Mineral acids are used in industrial furfural productions and sulphuric acid is the most widely used catalyst in the production of furfural [2]. Hydrochloric acid, nitric acid, and phosphoric acid are also among the reported homogeneous catalysts used to catalyze the conversions [3, 4]. These catalysts, however, are corrosive to the reaction system and careful handling of them is required [5, 6]. The use of homogeneous acid catalysts requires extra separation steps after reaction which may lead to additional costs. The production of furfural is one of the chemical processes that involve the use of these catalysts which may be toxic, hazardous and corrosive. Besides, these catalysts are not reusable and recoverable from the reaction mixture, as either they may form a complex molecule with the product or they are highly soluble in the reaction mixture resulting in hazardous waste effluents [7]. This also leads to the formation of hazardous inorganic acids as by-products. Hence, the environment is soaringly affected by these chemical processes. Various attempts have been carried out by the researchers to mitigate the chemical pollution occurring due to the use of acid homogeneous catalysts. Heterogeneous solid acids have advantages over the conventional homogeneous acid catalysts, for instance, the simplicity in handling, decreased reactor problems, plant corrosion problems, and they can be easily recovered and reused several times without any loss of their efficiency [8].
Lignocellulosic Biomass as a Source of Carbohydrate for the Furfural Production
Furfural is produced mainly from the lignocellulosic biomass with high hemicellulose content. Lignocellulosic biomass, the raw materials for furfural production, differ in composition and structure based on the species of the biomass and environmental growth conditions [9]. The most common sources to produce furfural are sugarcane bagasse and corncobs [10] Lignocellulosic biomass contains three main components based on their mass contributions [11]. These are cellulose, hemicellulose, and lignin which are mainly linked together by the hydrogen bond. Cellulose is a polymer composed of six carbon glucose linked to each other with β-glycosidic bonds [12,13,14]. Cellobiose is the repeating unit and hydrogen bonds are responsible for the high water insolubility and flexibility of cellulose crystalline structure [15]. The elementary fibrils formed by the adjacent chains contain amorphous and crystalline regions, the amorphous region is subjected to hydrolysis first [16]. Hemicellulose is a mixed polymer of both five and six-carbon monosaccharide molecules. It consists of polymers of glucose, galactose, mannose, xylose, arabinose and other five and six carbon monosaccharides [17]. Xylan, mannans, and galactans are the main amorphous polymer groups in hemicellulose [18]. The polymer xylan is the major component of sugarcane bagasse hemicellulose which is composed of thousands the monomer xylose linked to each other by the β-(1,4)-glycosidic bonds [19]. Its backbone contains xylose of more than 80% [20].
Lignin, one of the major components of lignocellulosic biomass, is a highly cross-linked hydrophobic polymer which binds the hemicellulose to cellulose [9]. It is an amorphous three-dimensional aromatic biomolecule consisting of tyrosine and phenylalanine as building blocks. The three monomer units, p-hydroxyphenylpropane (H), guaiacylpropane (G) and syringylpropane (S), synthesized from coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol respectively, polymerize by dehydrogenation to form lignin having different structure and relatively high recalcitrant property from cellulosic polysaccharides [21]. The cellulose, hemicellulose, and lignin are linked to each other by the hydrogen bonds. Besides these, the lignin is linked to hemicellulose with the covalent feruloyl ester-ether chemical bonds [22]. The presence of non-hydrolyzable C–O–C and C–C bonds in its structure and heterogeneity of the ether & C–C bonds hamper the degradation of lignin. During the acid hydrolysis for the fractionation of the lignocellulosic biomass into cellulose, hemicellulose and lignin, the formation of the complex products due to the possible formation of acid-soluble lignin-based components in the reaction, may occur [23]. Lignin has the tendency to neutralize the acidity and inhibit the formation of furfural by dehydration of the xylose in an acidic reaction medium [24]. Lamminpää et al. [24] studied the effect of the presence of lignin along with the hemicellulose on the yield of furfural and confirmed that it has a negative effect on both the conversion of xylose and the yield of furfural [24]. Daorattanacha et al. [25] reported a 24% decrease in xylose conversion and a 11% decrease in the yield of furfural upon the addition of 50 wt. % lignin loading [25]. On the other hand, the presence of lignin assists the isomerization of glucose to fructose and the subsequent conversion, provided no low catalyst loading conditions, to hydroxymethylfurfural [25]. Several methods, for example, the alkaline and alkaline peroxide pretreatment, organic solvent extraction, ultrasonication, and twin-screw extrusion treatments, and microwave treatment have been used to remove lignin [26, 27]. In alkaline treatment, the most widely used delignification method [26], a soda solution is used to solubilize and remove the lignin after the biomass is exposed to mechanical and hot water pretreatment at around 100 °C and above for about or more than an hour [28, 29]. In the acid pretreatment, dilute sulfuric acid is used to isolate hemicellulose from lignin and cellulose [30]. The combination of the two methods appears to be promising because their advantages could be coupled and minimize their disadvantages. Acid pretreatment followed by alkaline improves the removal of lignin through solubilization by breaking the ether bonds and deprotonation of ionizable groups [31]. Luo et al. [32] fractionated pubescens into 87 wt % cellulose, 93.6 wt % hemicellulose and 80.2 wt% lignin derivatives in 25% GVL/water system at 160 °C within 4 h [32].
Biomass Pre-treatment for Isolation of Hemicellulose
Quaker Oats Technology is the oldest commercial way of producing furfural, conceived in 1921 [13]. Quaker Oats Technology or its modified versions are used to produce furfural. In the Quaker Oats Technology, biomass is treated with sulfuric acid at 153 °C for 5 h to hydrolyze the pentosan content and generate pentoses. In this process, the raw 100 kg biomass is mixed with 2.246 kg sulfuric acid without any pretreatment step and the fed to the reactor. Steam stripping is applied to recover furfural after it is formed by the dehydration of the pentoses in the subsequent stage. Low yield around 50%, high steam requirement, disposal problems due to extremely acidic waste discharge and high operating cost are the major challenges in this process [33]. Various studies have been undertaken to improve furfural production technology to achieve higher yield and lower cost and to make environmentally friendly. In this regard, the scholars have been searching for low-cost catalyst, better product separation technology and a suitable solvent. In order to facilitate, the heat and mass transfer, the biomass should undergo size reduction and then the isolation of the components is carried out [34]. Seaparation of the hemicelluloses component, in order to reduce the recalcitrant nature of the biomass or the biomass pretreatment is an important step. Pretreatment has an impact on the furfural yield by reducing the amount of lignin, which polymerizes with other compounds in acidic conditions [35]. It may also reduce operating cost if the recycling of catalyst is considered [36].
Isolation of hemicellulose can be through several pretreatment ways using chemical, biochemical, physical or physicochemical methods. This step can be performed by steam explosion, organic solvents, acids such as dilute sulfuric acid or phosphoric acid, alkali treatment using alkaline agents such as sodium hydroxide, ammonia or lime, and autohydrolysis [37]. Figure 1 illustrates the various pretreatment methods along with the possible intermediate and final products obtained from the biomass. Applications and purpose of the extraction products, mainly containing the hemicellulose, are the main considerations that should be incorporated in determining the extraction method. The degree of polymerization, reactivity, purity, and solubility are among the important characteristics of the extracted hemicellulose which can be affected by the method of isolation used [38]. Dilute acid pretreatment of bagasse produces monosaccharide and oligosaccharides, while alkaline pretreatment results in the polysaccharides [30, 38].
Dilute Acid Pretreatment
Acid pretreatment selectively extracts hemicellulose from the cellulose, hemicellulose and lignin complex because hemicellulose is more readily separated under acidic conditions due to its amorphous nature. It is often done using dilute acids to reduce the problems associated with the use of concentrated acids like the corrosion of the reactors, other equipment and energy requirement for acid recovery [38, 39]. H2SO4, HCl and H3PO4 are commonly used to cleave the glucosidic linkage thereby solubilizing the hemicellulose component predominantly to pentose and at some extent to insoluble cellulose and lignin fractions. In this isolation method, furfural is also formed in a small amount [38]. The concentration of 0.5 to 1.5% of sulfuric acid at the temperature above 121–160 °C was reported for the hydrolysis of hemicellulose [39]. About 75 to 95% recovery of sugar can be achieved using the dilute acid treatment.
Hydrothermal Pretreatment
Hydrothermal pretreatment is used to preferentially extract the hemicellulose from the cellulose, hemicellulose and lignin complex. Hemicellulose can be extracted in hot water at a lower temperature than the cellulose and lignin [40, 41]. Hot, compressed water depolymerize and solubilize the hemicellulose into pentoses and di- and poly-saccharides of pentoses. Autohydrolysis and steam explosion are the hydrothermal pretreatment methods.
Autohydrolysis
Autohydrolysis uses hot, compressed water usually at the temperature between 150–230 °C. Like dilute acid pretreatment, this isolation method uses hydronium ion to catalyze the hemicellulose extraction process [42]. O-glycosidic linkages and acetyl groups break, resulting in partial depolymerization when hydronium ion is released because of an increase in temperature. The acetate group cleaves to some extent and the pH decreases resulting in the formation of additional acetic acid. Autohydrolysis is a green process as it uses only aqueous media without the addition of chemicals [37]. The liquor resulting from the hot water pretreatment includes oligosaccharides and polysaccharides [43]. 55 to 84% of hemicellulose was obtained through the autohydrolysis [39]. The solids remaining after the pretreatment contain, mainly cellulose and lignin which can be further converted to useful chemicals [44].
Steam Explosion
Steam explosion is another physical pretreatment method which helps to separate the hemicellulose from the lignocellulosic material by solubilizing the soluble components. In this method, the chipped biomass is exposed to saturated steam under high pressure and then the pressure is suddenly reduced to help the biomass undergo explosive decomposition [45]. The sudden compression and expansion depolymerize the fiber and the soluble products of the pretreatment should be removed from the reactor to reduce destruction reactions. Full recovery of hemicellulose can be achieved in the presence of sulfuric acid or sulfur dioxide [45]. Steam explosion is less expensive and less energy consuming process when compared to acid pretreatment because it uses no external catalyst and requires less amount of water (around 3 to 7 times less) to pretreat the same amount of biomass [46]. Even though it is an inexpensive pretreatment method, the steam explosion has limitations like the destruction of some portion of the pentosan.
Alkaline Pretreatment
Organic solvents and alkaline agents remove lignin together with hemicellulose as they do not selectively remove hemicellulose from the complex matrix [39, 47]. Alkaline pretreatment can be categorized into two, i.e. alkaline/alkaline earth metals and ammonia based, depending on the type of catalyst involved in fractionating the lignocellulosic components [39]. Using Na2CO3, up to 82% xylan extraction from sugarcane bagasse was achieved and low formation of furan aldehyde observed. Alkaline pretreatment under microwave radiation with improved recovery was also studied [48]. Biomass pretreatment should achieve optimized isolation of the hemicellulose component with lower recalcitrance to facilitate the successive conversion to value-added chemicals such as furfural. To mitigate issues related to disposal, the environmentally benign chemicals are required to be used during the pretreatment. Suitable, energy efficient and inexpensive pretreatment methods are also required [49].
Microwave-Assisted Pretreatment
Electromagnetic waves with frequencies ranging from 0.3–300 GHz are useful to heat up dielectric substances with high potential to absorb microwaves [50]. Aqueous systems are very suitable for the application of microwaves because water is a highly polar substance [50]. Furthermore, microwave heating has advantages like the energy efficiency, faster reaction time and lower reaction temperature when compared to the conventional heating [51]. It directly provides energy to substances by molecular interaction with electromagnetic fields. It also saves costs by reducing the amount of chemical/solvents used to fractionate the biomass components, minimizing the by-product formation and increasing the yield. The extent of biomass fractionation depends on the microwave intensity and irradiation time. Several studies have been carried out on the extraction of the hemicellulosic components from various lignocellulosic biomass like spruce wood [52], tobacco biomass [53], aspen wood and sugarcane trash [54]. Up to 66.1 and 5.3%, xylan and xylose were obtained respectively from aspen wood at 195 °C in 20 min and up to 50% (w/w) xylan and 6% (w/w) xylose from sugarcane trash at temperatures above 170 also reported up to about 70 wt% hemicellulose from sugarcane bagasse at 185 °C in 20 min and 77% from softwood sawmill shavings at 190 °C in 10 min [55].
Furfural
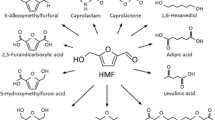
Furfural is a promising chemical platform aldehyde with empirical formula C5H4O2. It is a colorless liquid with a characteristic ‘almond-benzaldehyde’ odor [56]. It was first isolated in 1821 by a German scientist, Dobereiner [57]. These days, furfural is considered as one of the top value-added chemicals with a huge potential to be produced from the lignocellulosic biomass [58]. Furfural can be produced from the agro-industrial residues, including the corncob, wood wastes and sugarcane bagasse. Sugarcane bagasse and corncob are the main raw materials used for the commercial productions [45]. It can also be produced from the fossil-based raw materials like 1,3-dienes, but this is not economical as compared to the lignocellulosic biomass-based sources [59]. As shown in Fig. 2, furfural can be subjected to other different reactions to produce various other useful chemicals [47].
Applications of Furfural
Furfural is a platform chemical having various industrial applications. It has an excellent property for dissolving the components selectively. It can be used to extract unsaturated compounds from oil which can be used to make drying oils and remove aromatic compounds from diesel fuel and lubrication oil to improve their properties and hence produce high quality motor oil. It can also be used as very effective fungicide with better effect and faster action as compared to the formaldehyde, even if applied at low concentrations. It kills wheat smut in 3 h while formaldehyde does in 6 h at 0.5% concentration dose. The advantage of using furfural as fungicide is, its effect on the seed germination power is insignificant whereas the formaldehyde completely damages this power at concentration of 0.5% if wheat seed is soaked for 6 h [33].
Furfural can be converted to other useful compounds such as furfuryl alcohol, methyltetrahydrofuran, furoic acid, tetrahydrofurfuryl alcohol, α-Methylfurfuryl alcohol, levulinic acid, pyrrole, furfurylamine, tetrahydrofurfurylamine, piperidine furoic esters, and tetrahydrofuroic acid. Furfuryl alcohol (FA) is used to make furan resins, cement, composites and corrosion resistant fiber reinforced plastics. FA acts as viscosity reducing agent and reactive solvent in production of epoxy resins and phenolic resins respectively. Tetrahydrofuryl alcohol is specialty solvent and chemical intermediate produced through further conversion of FA (Table 1).
Chemistry of Furfural Formation
The conversion of hemicellulose to furfural takes place through two steps [60]. The first step is the hydrolysis of polysaccharide into simple sugar. In this step, xylan is broken into the monomer xylose. Xylose is then converted to furfural in the second step through dehydration by removal of three molecules of water [57].
Mechanism for the Hydrolysis of Pentosan
Pentosan consists predominantly of rings linked by oxygen bridges (ether bridges). As shown in Fig. 3, protonation of oxygen link is the first step of hydrolysis of pentosan and carbon-oxygen bond cleavage follows resulting in the formation of the hydroxyl group on one side oxygen bridge and carbonium ion is formed during the reaction on the other side. The addition of water molecule onto carbocation forms H2O+ group and then H2O+ group liberates hydrogen ion leaving a hydroxyl group behind. These steps of hydrolysis continue until the all oxygen bridge disappears.
The Mechanism for the Dehydration of Pentose
Various mechanisms for the transformation of xylose to furfural have been proposed but, most studies focus on the cyclodehydration mechanism removing three molecules of water using heterogeneous catalysts. Figure 3 shows the mechanism for removal of three molecules of water from xylose to form furfural. First, protonation of the free electron pair on hydroxyl group results in trivalent positively charged oxygen and the positive charge moves to the adjacent carbon atom to arrange itself to more stable conformation before C–O bond breaks and a water molecule is released. Then, the two electrons from the neighboring C–O bond are attracted to the C–C bond. This causes the formation of the double bond and the C–O bond breaks liberate another hydrogen ion. This hydrogen ion then attacks electron pair on the oxygen of another hydroxyl group to liberate another water molecule. The attacked site splits to another carbocation and water molecule. The resulting hydrogen ion migrates and attacks another non-bonded pair of electrons on another hydroxyl groups and other two water molecules are released upon dissociation. In the end, ring formation takes place by 1,4-elimination [45, 61]. Other reaction mechanisms such as xylose dehydration via enolization and β-elimination were also proposed as shown in Fig. 4.
Xylose dehydration to furfural through lyxose and xylulose formation by functional group rearrangement or by reconfiguration around C1 and C2 carbon atoms were also reported as it can be seen in Fig. 4. With Brønsted acids, furfural can be formed directly from xylose while Lewis acids lead to furfural through the intermediate xylulose. Choudhary et al. [62] reported that the zeolite produce xylulose from xylose and the intermediated xylulose can be quickly transferred into furfural in the presence of Brønsted-acid catalysts such as HCl and Amberlyst-15 [62]. Binder and coworkers also proposed isomerization by enolization and hydride shift mechanisms for the transformation of xylose to furfural using the chromium as a catalyst [63]. First, xylose isomerizes to xylulose by 1,2-hydride shift and the resulting xylulose then dehydrates over chromium catalyst to form furfural. Xylose also isomerizes to xylulose by enolization over chromium and enediol is formed.
Catalysts Used to Produce Furfural from Pentoses
Different studies have been made by many researchers to increase furfural yield. Pentosan content of the feed, catalysts loading, time and temperature of hydrolysis and residence time of furfural in the reaction medium are among the determinant factors to that should be considered to optimize the yield in the production of furfural [64].
Homogeneous Catalysts
In last decades, extensive studies on the conversion of reagent grade xylose and xylan into furfural have been performed instead of raw biomass for simplification of analysis. Homogeneous catalysts, mineral acids [65] and metal chlorides [66, 67] have been investigated for furfural production. Among the mineral acids, sulfuric acid and hydrochloric acid are the most widely used catalysts for the commercial production of furfural [68]. Using sulfuric acid as a catalyst, Quaker Oats produce furfural with around 50% yield at 150 °C in 5 h and similar yields have been reported for most industrial processes [69]. Undesired polymerization reactions are the causes for the formation of solid humins which are in turn responsible for the moderate yield of furfural [70]. Yemis and Mazza [65] studied and compared the catalytic activities of sulfuric acid, nitric acid, phosphoric acid, hydrochloric acid, acetic acid and formic acid targeting at the yield of furfural. The highest yield of furfural from xylose using hydrochloric acid was about 65% at 170 °C [44]. AlCl3 gave 84.8% furfural yield from xylan at 170 °C in reaction time of 10 s [51].
Marcotullio and De Jong have shown the effect of Cl- ions on the dehydration rate of xylose to furfural in aqueous acidic solutions. The presence of Cl- ions have shown improvement in the yield of furfural and selectivity using conventional heating [71]. On the other hand, using microwave heating [72] investigated the effect of sodium chloride on the dehydration rate of xylose to furfural and achieved 76% furfural yield at 200 °C in less than 8 min. However, mineral acids are extremely corrosive to the equipment, recovery of the catalysts from the reaction mixtures is difficult, and we cannot reuse the catalysts [73, 74]. Various attempts have been carried by researchers to mitigate the chemical pollution occuring because of the use of acid homogeneous catalysts.
Heterogeneous Catalysts
Solid acid catalysts could be regenerated and reused in catalytic processes with comparable efficiencies [17, 75,76,77]. Sulfonic acid functionalized mesoporous SBA-15 materials [77], modified zeolites such as HUSY, H-Beta zeolite, H-mordenite [78], and MCM-41 [79]) are among heterogeneous solid catalysts that were developed for furfural production to solve the problems associated with the use of homogeneous catalysts. Arenesulfonic SBA-15 catalysts were prepared via co-condensation for the dehydration for d-xylose to furfural [77]. These selective and hydrothermally stable catalysts incorporate high arenesulfonic-site and posse hexagonal pore arrangement. The activities of sulfonic acid functionalized mesoporous SBA-15 materials were studied with emphasis on the stability of the catalyst. At 160 °C with 99% selectivity, they obtained 82% furfural yield from d-xylose in water/toluene. Even though the solvent (toluene) used in the study for extraction of furfural from water medium is not a green chemical, arenesulfonic functionalized SBA-15 supports aged at higher temperatures show higher stability and recyclability [77]. The most studied solid catalysts are zeolites, which are thermally and chemically stable, have tunable acidities and shape [80]. Furfural yield of mostly lower than 40% was achieved on studies using a variety of H-zeolites (e.g., HY-faujasite, H-mordenite, and H-ferrierite) in water, water/methyl isobutyl ketone (MIBK), water/toluene and dimethyl sulfoxide (DMSO) in a batch reactor at 140–170 °C [81].
A sulfated tin ion-exchanged montmorillonite (SO42−/Sn-MMT) in 2-Methyltetrahydrofuran (2-MTHF)/NaCl-water showed high activity and reusability with furfural yield of 79.64% from xylose at 160 °C [70]. The presence of NaCl showed a decrease in the yield of furfural from xylan. Silico-aluminophosphate (SAPO) based catalysts have shown up to 65% yield of furfural from hemicellulose in bi-phasic systems at 170 °C in 8 h [82]. Bhaumik and Dhepe [83] showed in their work that the SAPO-44 can be washed with water and reused for eight times without loss of efficiency. This catalyst contains both the Lewis and Bronsted acid cites where isomerization and dehydration reactions take place. Bhaumik and Dhepe [84] have also shown in other work that hydrothermally stable SAPO-44 can achieve up to 93% furfural yield from the raw lignocellulosic material such as bagasse, rich husk and wheat straw [85].
Sulfonated metal oxides and ion-exchange resins were also used for dehydration of xylose to produce furfural. 40-70% yield was achieved by using ion-exchange resins [80]. Metal oxides, sulfonated with H2SO4, such as TiO2, ZrO2, SnO2, and Al2O3 were also studied and proved that SnO2 gives the highest xylose conversion and furfural yield [86]. SO42−/ZrO2–TiO2 gave a higher yield than the selected zeolite catalysts [87]. TiO2 coupled nanocellulose was proved to enhance the conversion of cellulose to glucose [88]. Neill et al. [89] studied the conversion of furfural over ZSM-5 zeolite in H+ form at 140–220 °Cin a batch reactor and proposed a kinetic model for the reaction as the first order with activation energy of 32.1 kcal/mol. The catalyst achieved a yield of 46% furfural from xylose at the optimum temperature of 200 °C. The effects of parameters like the catalyst pore size, time and temperature were investigated and found to be highly influential in product distribution [89].
Carbon-based solid acids have suitable catalytic properties to produce furfural by dehydration of xylose. The accessibility to xylose is good as the specific surface area and porosity of these solid acids is high. Besides, these types of catalysts have excellent electron conductivity and have relatively high chemical and thermal stability [90,91,92]. Carbon materials can be prepared from cheap and abundant biomass residue to be used as catalyst and catalyst support for various biomass transformation [93, 94]. Carbon black and activated carbon are prepared via different techniques. Pyrolysis of polymers or hydrocarbon at about 1500 °C can produce an amorphous carbon black with randomly cross-linked structure and roughly planar layers of sp2 hybridization carbons [90]. Activated carbon is amorphous solid with high porosity and surface area. It is prepared by carbonization of carbon materials in the absence of oxygen if physical activation is required. Steam or oxygen is used as physical activation at 600–1200 °C [91]. The carbon material is carbonized after impregnated with an acid, a strong base or a salt in chemical activation [95].
Carbon materials exhibit acid-base character with different oxygen functionality. Biomass-based amorphous carbon has been studied for biomass conversion by bearing SO3H, COOH, and OH groups [14, 68]. The catalyst can be easily prepared from biomass resources by incomplete carbonization and subsequent sulfonation at a high temperature greater than 160 °C with a large amount of fuming sulfuric acid (15 wt% SO3) or concentrated sulfuric acid (98%) for the introduction of SO3H groups into the amorphous carbon surface. Lignin can be used to produce activated carbon and acidic sulfonated carbon for hydrolysis/dehydration of polysaccharides and polyols [96].
Porous carbon materials are becoming very attractive in recent years for their versatile use as catalysts, catalyst supports, sorbent for air and water purification, supercapacitors and fuel cells. For catalytic processes, solid acid catalysts based on the carbon sources are getting attention due to their recyclability, low cost, unique structure, environment-friendly properties, and easy preparation methods. They can be obtained from carbon precursors through carbonization and sulfonation for various applications like dehydration of xylose to furfural. Hydrothermal, ionothermal and molten salt carbonization are among the various techniques adopted to synthesize porous structured carbon materials reported in some literature. Thermochemical conversions take place in water for the case of hydrothermal carbonization and in ionic liquids and molten salt for ionothermal and molten salt carbonization respectively. Hydrothermal carbonization is an inexpensive, mild and green process involving thermal decomposition of carbon precursor in aqueous solution in the presence of heat [97].
Lam et al. [98] reported that the water-tolerant carbon catalyst sulfonated graphene oxide (SGO) with 2% loading converted xylose to furfural with selectivity of about 75% and yield of 62% in 35 min at 200 °C. At these same conditions, i.e. catalyst loading, time of reaction and temperature; graphene, graphene oxide and, sulfonated graphene achieved relatively lower yield of 51,53 and 55% as compared SGO. SGO exhibit large surface area (680 m2/g), contains aryl sulfonic acid group which are active sites responsible for the dehydration process and remains active after repeated reactions.
Properties of carbon-based solid catalysts that affect the catalytic performance include acidic property, specific surface area and pore size [99]. The acidity of the catalyst is among the properties of catalyst that can significantly affect the performance of a catalyst. The strength and distribution of acid sites on the solid surfaces in addition to their nature are determinant catalytic properties of a solid acid catalyst that affect the conversion and selectivity of a reaction. A Brønsted acid donates proton while Lewis acids accept electrons. Lewis acid sites promote xylose-xylulose-furfural route instead of xylose to furfural direct conversion and Brønsted acid sites catalyze the conversion of xylulose to furfural [4]. Agirrezabal-Telleria et al. [77] used partially hydroxylated MgF2 catalysts by varying the Lewis/Brønsted acid ratio to produce furfural in water/toluene [100]. Lewis acidity is due to Mg2+ ions and hydroxyl groups are responsible for Brønsted acid property of the catalyst. Combination of Sn-beta zeolite as a Lewis acid and Amberlyst-15 as a Brønsted acid can be used to convert xylose to xylulose and then to furfural in an aqueous medium [101]. The Lewis/Brønsted acid site ratio is, therefore, one of the factors that affect the rate of dehydration reaction and furfural yield. The optimum Lewis/Brønsted loading is required to achieve the optimized conversion and yield [100]. Carbonaceous by-products will be formed at higher L/B ratio and lower L/B ratio leads to undesired polymerization reaction. The rate of reaction increases with an increase in acid content and strength, but too strong acidity accelerates undesired reactions. The variation of acidity of zeolite catalysts is mainly due to the preparation conditions and Si/Al ratio [4]. Carbon-based catalysts possess Brønsted acid sites like -SO3H and –COOH. Phenolic –OH group are also present in these types of catalysts [102].
IUPAC defines pore size less than 2 nm as micropores, 2–50 nm as mesopores and greater than 50 nm as macropores. The pore size of the catalyst should match the molecular size of xylose and furfural to facilitate the diffusion of reactants into the catalyst and removal of products in furfural production processes. Small pore size than xylose and furfural results in diffusional resistance problem by inhibiting the diffusion rate and larger sizes may be suitable for furfural rearrangement into large molecules because of longer residence time product inside the porous structure [4]. Catalyst screening by Iglesias et al. [103] showed that H-ZSM5 containing narrow pore size were unable to promote the desired transformation and probably transformations may occur on the active sites located at the outer surface of the catalyst [103]. The size of xylose and furfural were approximate to be 0.68 and 0.57 nm, respectively based on literature. Neill et al. [89] concluded in their work that zeolite with a pore size (0.8 nm) close to the size of xylose and furfural would be effective for xylose conversion to furfural [89].
The specific surface area of the catalysts is another factor that affects the catalytic performance. Higher specific surface area promotes the performance of a catalyst by increasing the accessibility of the active sites to xylose [4]. Lima et al. [104] experimented delaminated Nu-6 zeolite with the greater surface area in about seven folds than of standard Nu-6 zeolite and increased rate of reaction in two folds with furfural yield of about 50% at 453 K [82]. Table 2 shows the summary of the reaction conditions and the yield of furfural with various catalysts.
Conversion of Hexoses to Furfural
Most of the investigations towards furfural focus on the conversion of hemicellulose component of biomass, but there are also several published works that confirm the possibility of converting cellulose component into furfural. Jin et al. [105] illustrated two possible mechanisms of furfural formation from hexoses. The first pathway is the conversion of hexose to HMF and then to furfural, while the second is hexose to pentose and then to furfural [105]. In the conversion of glucose to HMF, glucose isomerizes in the rate-limiting step to fructose and fructose then dehydrates to form HMF [25]. The formed HMF then loses –CH2O by decarboxylation to form furfural [8]. The formation of pentose via retro-aldol reaction from ketose is the other plausible pathway to produce furfural from C6 sugars [106]. Aida et al. [107] investigated the effect of temperature, residence time and pressure on the yield of furfural from d-glucose in water and the yield was 12.2% at 400 °C [107]. Some literature reported the higher yield of furfural from raw biomass when compared to the pure xylose, where the possible reason could be the co-conversion of five- and six-carbon sugar in the polymer matrix. Looking for simultaneous utilization of cellulose and hemicellulose component derived sugars, 51.1 and 40.9% yield of furfural on molar basis were achieved from corncob and sugarcane bagasse respectively in γ-valerolactone at 185 °C within 85 min [108]. A maximum yield of 22.3% furfural on the molar basis was obtained from cellulose at 175 °C in 100 min using Fe-beta zeolite as a catalyst γ-valerolactone [108]. In one pot processing of corn stalk, a furfural yield of 83.8% (at 170 °C, 100 min), 84.5% (at 180 °C, 80 min) and 86.3% (at optimal conditions of 190 °C, 40 min) were obtained while a maximum yield of 80.4% was obtained from xylose (at 170 °C, 10 min) using PTSA-POM catalyst in GVL/water system [109].
Reduction of Yield Loss Through Isolation of Furfural from the Reaction Mixture
Since furfural is very reactive, in the acidic medium it undergoes through various reactions such as polymerization which lead to lower furfural yield. Resinification and condensation reactions are the causes for the decrease in the yield of furfural [33]. These reactions occur when the furfural formed during the reaction stays dissolved in the liquid phase. Resinification reactions basically take place when furfural reacts with another furfural molecule and condensation reactions take place when furfural combines with an intermediate.

Difurfural xylose is also formed as a degradation product if additional furfural molecule reacts with an intermediate [33]. These furfural degradation reactions are possible only when furfural is present as dissolved product in the liquid phase, whereas impossible in the vapor phase where the catalytically active components are absent [33]. Table 3 shows the summary of various furfural separation approaches.

In industrial processing of biomass for the production of furfural, steam is injected into the reaction chamber to supply heat for the reaction to take place and also to preferentially remove furfural from the liquid phase. This selective removal of furfural from the liquid phase into vapor phase help the process to achieve higher furfural yield by preventing the product loss due to resinification and condensation reactions. In the analytical method the amount of furfural produced can be escalated to 100% yield because the presence of HCl saturated with NaCl raises the boiling point and result in superheating of vapor [33].
Continuous removal of furfural product from the acidic medium is required to stop the polymerization and condensation reactions which reduce yields. This can be done by steam stripping, reactive distillation, water/organic biphasic systems, use of supercritical fluids (CO2) and gaseous acid catalysis (using superheated steam and HCl vapor) [110].
Steam Stripping
Steam stripping is a separation process which uses steam to preferentially extract components from a liquid stream. Commercially furfural is produced from biomass using H2SO4 and recovered from the aqueous solution by steam stripping to stop the further transformation of furfural to larger molecules and purification is done by double distillation [59]. The energy consumption by this method of product recovery has been reported to be very high as it uses 25 to 35 tons of steam to produce a ton of furfural [71]. In Rosenlew process 30 to 1 ratio of steam to furfural is used to achieve 59.5% yield of furufal at 180 °C. Gandarias and Arias [73] suggested in their work that high amount of utilities are required when fresh steam is used and cooling of vapors are required. Further purification also requires high energy input if the concentration of furfural in the steam is too low [73]. Volume of steam used for stripping can affect yield of furfural. Too low amount of steam is not sufficient for fast isolation of the furfural formed during the reaction. The furfural remaining in the acidic medium will be lost due to resinification and condensation reactions. Too high steam input hinders the catalystic activity towards the formation of furfural by reducing the acidity of the medium [33]. Therefore, optimum amount of steam is required to produce higher amount of furfural as both too much and too low volume of steam reduce the product output . Costs of steam stripping appear to be high because of high steam usage to extract the furfural from the reaction medium and the high cooling requirement for stripping vapor and high energy requirement for boilers in the distillation process for the dilute furfural-water stream separation [33].
Nitrogen-Stripping
Nitrogen is another stripping agent for producing furfural from biomass at higher yields. It is inert, easily separable and recyclable gas. Agirrezabal-Telleria et al. [111] achieved 67%, a higher yield of furfural as compared to that obtained using steam stripping [112]. Agirrezabal-Telleria et al. [77] studied partially hydroxylated MgF2 catalysts by varying the Lewis/Brønsted acid ratio and simultaneous stripping with nitrogen achieved 87% furfural selectivity at 160 °C in water/toluene [100]. Introduction of the nitrogen stripping current commercial process reduces the energy requirements and allows the recycling of the stripping agent and reduces the cost up to 60%. But, high recompression is required to recycle if the reduction of the use of fresh nitrogen is to be applied [73]. High product purity, high furfural yield, lower product dilution, easy separation of catalyst and stripping agent recycling are among the advantages of nitrogen stripping [80]. Nitrogen stripping showed better industrial feasibility as compared to biphasic water/organic solvent systems. 39% furfural yield was reported in water toluene system using ion-exchange sulfonic resin (A70) as a catalyst, which is less than that obtained using nitrogen as an extracting agent at the same temperature of 175 °C [41, 80, 111]. Nitrogen stripping has also the advantage of reducing the cost of final product separation challenging the commercial production processes of furfural [95]. Considering the stripping agent recycling and product dilution, nitrogen stripping has a cost advantage over the steam stripping [111].
Water/Organic Biphasic Systems
Furfural can be produced in biphasic systems consisting of water and organic mixture [73]. In this system, furfural produced in the aqueous phase will be immediately transferred to the organic phase. The spontaneous transfer of furfural into organic phase protects it from undergoing further transformation to a larger molecule. The purpose of a biphasic reaction is to increase furfural yield by reducing the side reactions and to simplify the separation process by avoiding the azeotropic point of the furfural-water mixture. Organic phase includes solvents like toluene, butanol, methyl isobutyl ketone (MIBK), MIBK-2-butanol mixture, tetrahydrofuran (THF), or dichloromethane (DCM), which have a great affinity for absorbing furfural. Furfural resinification reactions appear to be negligible in the organic phase of toluene/water system. The yield of furfural was 70, 66 and 35 in water/toluene byphasic system and 52, 54 and 13 in water only in the presence of H-MMC-22(24), ITQ-2(24) and HY-ferrierite(20) respectively [113, 114]. Deng et al. [115] reported higher yield of furfural in water/dichloromethane than in water only [115]. The addition of NaCl into the water/dichloromethane further improved the yield of furfural.
With the aim to produce furfural using green process and develop an appropriate kinetic model for conversion of xylose to furfural, Hua et al. [116] achieved improved furfural yield under optimal conditions [116]. They used high-temperature water as a catalyst to dehydrate xylose to furfural and ethyl butyrate, butyl acetate, n-butanol, n-butyl ether, and toluene solvents to extract the product. Ethyl butyrate showed the highest extraction efficiency. Selection of an appropriate solvent along with a proper catalyst can give high furfural yields. Zeolites, sulfated and tungstated zirconia catalysts, and sulfonic acid materials, which have different textural, surface, and acid properties in methanol, ethanol, and 2-propanol were tested. β-zeolite achieved best results as it exhibits best Brønsted and Lewis acid site combinations among the tested catalysts and 2-propanol was found to be the best medium to hinder side reactions that may occur in aqueous media and to give higher furfural yield than methanol and ethanol [103]. 68.3% furfural yield was achieved in H2O/toluene over SBA-15-SO3H [117]. The MCM-41 resulted in a furfural yield of about 44% in DMSO [81] and less than 40% in H2O/1-butanol [79], while 51% in water/MIBK and 76% yield achieved in water/toluene by MCM-41-SO3H [81, 117]. With H3PW12O40 and Cs3PW12O40 supported on MCM-41, the yields in H2O/toluene were 48% and 33%, respectively, and increased to 52% and 45% in DMSO, respectively [4].
Presence of biomass particles in water also reduce the furfural yield loss by preventing movement of furfural molecules and catalytically active components towards each other [80]. Environmental issues, solvent recovery, and process complexity are the major problems associated with biphasic systems [118]. For large scale processing, separation of the product furfural from the organic solvents consumes a substantial amount of energy and vigorous mixing of water and solvent for efficient extraction of furfural is a problem. Use of solvents with a low boiling point is another challenge in biphasic systems as heat treatment develops high pressure in the reaction vessel which in turn lead to additional capital cost [119]. In water/organic biphasic systems the use of organic solvents in commercial processes could add economic burdens because of the consumption of solvents, costs extra separation steps and waste management to protect the invironment from pollution [111]. Cleaning the water effluents from these systems are difficult as high energy is required to remove the solvents and recovery of solvents is also a must to make the process economical [120].
Monophasic systems
Gürbüz et al. [106] reported the use of a γ-valerolactone solvent as a monophasic system for conversion of xylose into furfural using a solid acid catalyst. γ-valerolactone has the advantage that it is green solvent can be produced from biomass. But, multi-step processing for γ-valerolactone production, its high boiling point, and miscibility with water makes it feasible only for laboratory [119]. Use of γ-valerolactone as a solvent has advantages like the promotion of xylose conversion rate and inhibition of the furfural degradation rate and the separation of furfural from the solvent is simple due to the difference in their volatility. There is no further step needed for liquid-liquid separation as in the case of biphasic systems to recover organic solvents from aqueous/organic phases.
Supercritical Carbon Dioxide
Super critical carbon dioxide was also reported for the extraction of furfural from reaction solution. In this extraction type liquified CO2 is introduced into a reactor containing the solution and transferred to container with cooling system. Supercritical CO2 is easily recoverable, green and also has tendency to acidify the reaction system. Hydrothermal conversion of sugar under the pressure up to 12 MPa achieved 68% furfural yield [121]. Sako et al. [122] evaluated the conversion of different concentrations of xylose to furfural to envestigate the effect of supercritical CO2 on the selectivity and the yield of furfural at 150 °C. From the experimental data they reported, it is clear that the yield obtained without the use of supercritical CO2 is less than the yield obtained in the presence of supercritical CO2 for the same xylose conversion and they obtained an improved maximum yield of 70% [122]. Sangarunlert et al. [123] reported a maximum yield of furfural aproching 90% from rice husk using supercritical CO2 for extraction of the product [123]. Diffusivity, surface tension and solublity are highly desirable properties for supercritical CO2 extraction. High diffusivity enhances the mass transfer, reduced surface tension is required for easy penetration and wetting of pore of biomass materials and solublity is important for selective extraction [124]. The compression of CO2 takes the major part of energy requirement for supercritical extraction [125]. This method of extraction is at experimental level due to the high capital and operating costs [13].
Challenge in Furfural Production
Solvent recycling, catalyst reuse, and product purification are very crucial factors to be considered for the economical industrial conversion of the various feedstocks to furfural. Currently, azeotropic distillation is in use for purification of furfural as the product streams contain up to 6% furfural and over 90% water in pentoses conversion processes [126]. But, it requires a large investment and high energy [127]. In producing furfural, various technologies have been developed at the experimental level. For instance, the supercritical CO2 extraction technology is one of the methods applied to separate furfural from the reaction mixture in view of getting a higher yield by suppressing reactions leading to furfural loss. But, this technology is being applied only at the laboratory stage due to its high operating and material cost requirements to be applied in industrial scale [127].
To reuse a catalyst for the next catalytic runs, separating it from reaction mixture and regeneration may be required. But, catalyst reuse is still a challenge for industrial applications due to reduced activity and deactivation of catalysts upon regeneration. In catalytic valorization of biomass, difficulty in the separation of solid catalysts from the solid residue remaining in the reaction mixture is often observed [8, 128]. Washing the used catalyst with water and organic solvent to separate it from corn stover residue and the drying is a difficult task [8] and it incurs additional costs of solvents for washing and energy for drying. Up to 5% furfural yield reduction was observed in the first run after regeneration and calcination deactivated the Al-beta [108]. In the MSPFR catalyzed conversion of xylose to furfural, the yield was 77.3% when the catalyst was used for the first time, but on the 5th run, 62.5% was obtained [8]. Besides the loss of active sites due to leaching, accumulation of by-products and corn stover residue were the main reasons for the reduced performance of the catalyst. CrPO4 catalyst was tasted for furfural production by washing and drying after each use and achieved a yield of 47% from xylose on the 4th run with a significant difference of 41% from the first run and this reduced activity can be due to the partial dissolution of the catalyst [44]. The activity of H-SAPO-34 zeolite in producing furfural from eucalyptus sawdust was about 88%, but it reduced to 57.24% in the 5th run and it couldn’t be recovered by calcination [129].
Solvent recycling is one of the important processes in chemical industries for economical operations because it reduces the costs associated with waste stream management and chemical consumption [130]. Environmental pollution is a problem of high priority these days. Solvent released in waste streams from industries needs to be reduced to protect the environment. And, also to keep consistent operating conditions throughout the process in furfural production, the solvent used should be fully recovered and reused or makeup solvent is required. Generally, to reduce the amount of solvent required and to minimize the negative environmental impact associated with the disposal of solvents in waste streams, recovery and reuse of solvents is required. Recovery processes might be possible at a laboratory scale, but it would be though for commercialization [118].
Conclusions
Significant studies have been carried out in the last several decades on the catalytic transformation of lignocellulosic biomass to furfural. This review provided an overview of the catalytic valorization process of biomass to valuable platform product, the furfural. Various catalysts employed by the researchers to achieve high catalytic activity and selectivity in converting the carbohydrate fractions of various biomass, but economical scale-up is still the challenge due to the difficulties in solvent recycling, catalyst reuse, and product purification. Isolation of hemicellulose is required to reduce the recalcitrant nature of biomass and to allow use of the other lignocellulosic fractions, like cellulose, to obtain diversified products through integration of processes with furfural production. Extracting hemicellulose with a desired characteristic such as the degree of polymerization, reactivity, purity, and solubility remains still a challenge. Researchers have also pioneered the furfural stripping methods from the reaction medium for suppressing product degradation. Resinification and condensation reactions, which cause the degradation of furfural, could be minimized by removing the furfural formed in the liquid phase and transferring the same into the vapor phase, which is devoid of the catalytically active components.
References
Afreen, G., Patra, T., Upadhyayula, S.: Thermodynamic insights into valorization of biomass-derived oxygenates and reconciliation with experimental study. J. Chem. Eng. Data 63, 2197–2210 (2018). https://doi.org/10.1021/acs.jced.8b00171
Moghaddam, L., Rencoret, J., Maliger, V.R., Rackemann, D.W., Harrison, M.D., Gutiérrez, A., Del Río, J.C., Doherty, W.O.S.: structural characteristics of bagasse furfural residue and its lignin component: an NMR, Py-GC/MS, and FTIR Study. ACS Sustain. Chem. Eng. 5, 4846–4855 (2017). https://doi.org/10.1021/acssuschemeng.7b00274
Bamufleh, H.S., Alhamed, Y.A., Daous, M.A.: Furfural from midribs of date-palm trees by sulfuric acid hydrolysis. Ind. Crops Prod. 42, 421–428 (2013). https://doi.org/10.1016/j.indcrop.2012.06.008
Li, X., Jia, P., Wang, T.: Furfural: a promising platform compound for sustainable production of C4 and C5 chemicals. ACS Catal. 6, 7621–7640 (2016). https://doi.org/10.1021/acscatal.6b01838
Vekariya, R.H., Patel, H.D.: Cellulose sulfuric acid (CSA) and starch sulfuric acid (SSA) as solid and heterogeneous catalysts in green organic synthesis: recent advances. Arkivoc 1, 136–159 (2015). https://doi.org/10.3998/ark.5550190.p008.975
Delbecq, F., Wang, Y., Len, C.: Conversion of xylose, xylan and rice husk into furfural via betaine and formic acid mixture as novel homogeneous catalyst in biphasic system by microwave-assisted dehydration. J. Mol. Catal. A 423, 520–525 (2016). https://doi.org/10.1016/j.molcata.2016.07.003
Corma, A., García, H.: Lewis acids: from conventional homogeneous to green homogeneous and heterogeneous catalysis. Chem. Rev. 103, 4307–4365 (2003). https://doi.org/10.1021/cr030680z
Zhang, T., Li, W., An, S., Huang, F., Li, X., Liu, J., Pei, G., Liu, Q.: Efficient transformation of corn stover to furfural using p-hydroxybenzenesulfonic acid-formaldehyde resin solid acid. Bioresour. Technol. 264, 261–267 (2018). https://doi.org/10.1016/j.biortech.2018.05.081
Foston, M., Ragauskas, A.J.: Biomass characterization: recent progress in understanding biomass recalcitrance. Ind. Biotechnol. 8, 191–208 (2012). https://doi.org/10.1089/ind.2012.0015
Uppal, S.K., Kaur, R.: Hemicellulosic furfural production from sugarcane bagasse using different acids. Sugar Tech. 13, 166–169 (2011). https://doi.org/10.1007/s12355-011-0081-5
Jabasingh, S.A., Ki, Z.: Expanding sustenance in Ethiopia based on renewable energy resources: a comprehensive review. Renew. Sustain. Energy Rev. (2016). https://doi.org/10.1016/j.rser.2016.11.082
Hara, M., Yoshida, T., Takagaki, A., Takata, T., Kondo, J.N., Hayashi, S., Domen, K.: A carbon material as a strong protonic acid. Angew. Chemie Int. Ed. 43, 2955–2958 (2004). https://doi.org/10.1002/anie.200453947
Zhang, Y., Xu, B., Zhou, W.: On a novel mechanistic model for simultaneous enzymatic hydrolysis of cellulose and hemicellulose considering morphology. Biotechnol. Bioeng. 111, 1767–1781 (2014). https://doi.org/10.1002/bit.25244
Hu, L., Zhao, G., Tang, X., Wu, Z., Xu, J., Lin, L., Liu, S.: Catalytic conversion of carbohydrates into 5-hydroxymethylfurfural over cellulose-derived carbonaceous catalyst in ionic liquid. Bioresour. Technol. 148, 501–507 (2013). https://doi.org/10.1016/j.biortech.2013.09.016
Bobleter, O.: Hydrothermal degradation of polymers derived from plants. Prog. Polym. Sci. 19, 797–841 (1994). https://doi.org/10.1016/0079-6700(94)90033-7
Ramos, L.P.: The chemistry involved in the steam treatment of lignocellulosic materials. Quim. Nova. 26, 863–871 (2003). https://doi.org/10.1590/S0100-40422003000600015
Carà, P.D., Pagliaro, M., Elmekawy, A., Brown, D.R., Verschuren, P., Shiju, N.R., Rothenberg, G.: Hemicellulose hydrolysis catalysed by solid acids. Catal. Sci. Technol. 3, 2057–2061 (2013). https://doi.org/10.1039/c3cy20838a
Fan, L.T., Gharpuray, M.M., Lee, Y.H.: Cellulose Hydrolysis. Springer, Berlin (1987)
Yang, T., Zhou, Y.H., Zhu, S.Z., Pan, H., Huang, Y.B.: insight into aluminum sulfate-catalyzed xylan conversion into furfural in a Γ-valerolactone/water biphasic solvent under microwave conditions. ChemSusChem 10, 4066–4079 (2017). https://doi.org/10.1002/cssc.201701290
de Moraes Rocha, G.J., Nascimento, V.M., Gonçalves, A.R., Silva, V.F.N., Martín, C.: Influence of mixed sugarcane bagasse samples evaluated by elemental and physical-chemical composition. Ind. Crops Prod. 64, 52–58 (2015). https://doi.org/10.1016/j.indcrop.2014.11.003
Chen, H.: Chemical composition and structure of natural lignocellulose. In: Chutani, P., Sharma, K.K. (eds.) Biotechnology of Lignocellulose, pp. 25–71. Springer, Dordrecht (2014). https://doi.org/10.1007/978-94-007-6898-7
Luo, A.Y., Li, Z., Li, X., Liu, X., Fan, J., Clark, J.H., Hu, C.: The production of furfural directly from hemicellulose in lignocellulosic biomass. Catal. Today 319, 14–24 (2019). https://doi.org/10.1016/j.cattod.2018.06.042
Girisuta, B., Janssen, L.P.B.M., Heeres, H.J.: Green chemicals: a kinetic study on the conversion of glucose to levulinic acid. Chem. Eng. Res. Des. 84, 339–349 (2006). https://doi.org/10.1205/cherd05038
Lamminpää, K., Ahola, J., Tanskanen, J.: Acid-catalysed xylose dehydration into furfural in the presence of kraft lignin. Bioresour. Technol. 177, 94–101 (2015). https://doi.org/10.1016/j.biortech.2014.11.074
Daorattanachai, P., Viriya-empikul, N., Laosiripojana, N., Faungnawakij, K.: Effects of Kraft lignin on hydrolysis/dehydration of sugars, cellulosic and lignocellulosic biomass under hot compressed water. Bioresour. Technol. 144, 504–512 (2013). https://doi.org/10.1016/j.biortech.2013.06.124
Bian, J., Peng, F., Peng, X., Xu, F., Sun, R., Kennedy, J.F.: Isolation of hemicelluloses from sugarcane bagasse at different temperatures : structure and properties. Carbohydr. Polym. 88, 638–645 (2012). https://doi.org/10.1016/j.carbpol.2012.01.010
De Freitas, C., Carmona, E., Brienzo, M.: Xylooligosaccharides production process from lignocellulosic biomass and bioactive effects. Bioact. Carbohydr. Diet. Fibre. (2019). https://doi.org/10.1016/j.bcdf.2019.100184
Al Arni, S.: Industrial Crops & Products Extraction and isolation methods for lignin separation from sugarcane bagasse : a review. Ind. Crop. Prod. 115, 330–339 (2018). https://doi.org/10.1016/j.indcrop.2018.02.012
Moubarik, A., Grimi, N., Boussetta, N., Pizzi, A.: Isolation and characterization of lignin from Moroccan sugar cane bagasse : production of lignin–phenol-formaldehyde wood adhesive. Ind. Crop. Prod. 45, 296–302 (2013). https://doi.org/10.1016/j.indcrop.2012.12.040
Farhat, W., Venditti, R.A., Hubbe, M., Taha, M., Becquart, F., Ayoub, A.: A review of water-resistant hemicellulose-based materials: processing and applications. ChemSusChem 10, 305–323 (2017). https://doi.org/10.1002/cssc.201601047
Camargos, C.H.M., Silva, R.A.P., Csordas, Y., Silva, L.L., Rezende, C.A.: Industrial crops & products experimentally designed corn biomass fractionation to obtain lignin nanoparticles and fermentable sugars. Ind. Crop. Prod. 140, 111649 (2019). https://doi.org/10.1016/j.indcrop.2019.111649
Luo, Y., Li, Z., Zuo, Y., Su, Z., Hu, C.: A simple two-step method for the selective conversion of hemicellulose in pubescens to furfural. ACS Sustain. Chem. Eng. 5, 8137–8147 (2017). https://doi.org/10.1021/acssuschemeng.7b01766
Zeitsch, K.J.: The Chemistry and Technology of Furfural and Its Many By-Products. Elsevier, Amsterdam (2001)
Saydut, A., Tonbul, Y., Baysal, A., Duz, M.Z., Hamamci, C.: Froth flotation pretreatment for enhancing desulfurization of coal with sodium hydroxide. J. Sci. Ind. Res. (India) 66, 72–74 (2007). https://doi.org/10.1098/rsta.1987.0029
Zhang, Y., Yu, G., Li, B., Mu, X., Peng, H., Wang, H.: Hemicellulose isolation, characterization, and the production of xylo-oligosaccharides from the wastewater of a viscose fiber mill. Carbohydr. Polym. 141, 238–243 (2016). https://doi.org/10.1016/j.carbpol.2016.01.022
Rackemann, D.W.: Production of levulinic acid and other chemicals from sugarcane fibre, In: Ph.D. Thesis, Queensland University of Technology, Brisbane (2014)
Senila, L., Miclean, M., Senila, M., Roman, M., Roman, C.: New analysis method of furfural obtained from wood applying an autohydrolysis pretreatment. Rom. Biotechnol. Lett. 18, 7947–7955 (2013)
Brienzo, M., Carvalho, A.F., Figueiredo, F.C.A., Oliva Neto, P.D.: Sugarcane bagasse hemicellulose properties, extraction technologies and xylooligosaccharides production. Food Waste 21, 155–188 (2016)
Gírio, F.M., Fonseca, C., Carvalheiro, F., Duarte, L.C., Marques, S., Bogel-Łukasik, R.: Hemicelluloses for fuel ethanol: a review. Bioresour. Technol. 101, 4775–4800 (2010). https://doi.org/10.1016/j.biortech.2010.01.088
Yajima, M., Yokotsuka, K.: Volatile compound formation in white wines fermented using immobilized and free yeast. Am. J. Enol. Vitic. 52, 210–218 (2001). https://doi.org/10.1002/jctb
Weiqi, W., Shubin, W., Liguo, L.: Combination of liquid hot water pretreatment and wet disk milling to improve the efficiency of the enzymatic hydrolysis of eucalyptus. Bioresour. Technol. 128, 725–730 (2013). https://doi.org/10.1016/j.biortech.2012.08.130
Carvalheiro, F., Duarte, L.C., Gírio, F., Moniz, P.: Hydrothermal/liquid hot water pretreatment (Autohydrolysis): a multipurpose process for biomass upgrading. Technol. Lignocellul. Feed. Based Biorefinery, Biomass Fract (2016). https://doi.org/10.1016/B978-0-12-802323-5.00014-1
Rivas, S., Vila, C., Santos, V., Parajó, J.C.: Furfural production from birch hemicelluloses by two-step processing: a potential technology for biorefineries. Holzforschung 70, 901–910 (2016). https://doi.org/10.1515/hf-2015-0255
Liu, L., Chang, H.M., Jameel, H., Park, S.: Furfural production from biomass pretreatment hydrolysate using vapor-releasing reactor system. Bioresour. Technol. 252, 165–171 (2018). https://doi.org/10.1016/j.biortech.2018.01.006
Axelsson, L., Franzén, M., Ostwald, M., Berndes, G., Lakshmi, G., Ravindranath, N.H.: Perspective: jatropha cultivation in southern India: assessing farmers’ experiences. Biofuels Bioprod. Biorefining 6, 246–256 (2012). https://doi.org/10.1002/bbb
Steinbach, D., Kruse, A., Sauer, J.: Pretreatment technologies of lignocellulosic biomass in water in view of furfural and 5-hydroxymethylfurfural production: a review. Biomass Convers. Biorefinery. 7, 247–274 (2017). https://doi.org/10.1007/s13399-017-0243-0
Al Arni, S.: Extraction and isolation methods for lignin separation from sugarcane bagasse: a review. Ind. Crops Prod. 115, 330–339 (2018). https://doi.org/10.1016/j.indcrop.2018.02.012
Wang, L.J., Liu, X.L., Weng, M.L., Wu, F.S., Li, Z.J., Wang, S.F.: Extraction of hemicellulose from sugarcane bagasse under microwave radiation. Adv. Mater. Res. 634–638, 975–980 (2013). https://doi.org/10.7326/P15-9029
Shrotri, A., Kobayashi, H., Fukuoka, A.: Catalytic conversion of structural carbohydrates and lignin to chemicals. Adv. Catal. 60, 1–57 (2017). https://doi.org/10.1016/bs.acat.2017.09.002
Tsubaki, S., Azuma, J.I., Yoshimura, T., Maitani, M.M., Suzuki, E., Fujii, S., Wada, Y.: Microwave-induced biomass fractionation. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery, pp. 103–126. Elsevier (2016). https://doi.org/10.1016/B978-0-12-802323-5.00005-0.
Zhang, L., Yu, H., Wang, P., Dong, H., Peng, X.: Conversion of xylan, d-xylose and lignocellulosic biomass into furfural using AlCl3 as catalyst in ionic liquid. Bioresour. Technol. 130, 110–116 (2013). https://doi.org/10.1016/j.biortech.2012.12.018
Chadni, M., Bals, O., Ziegler-Devin, I., Brosse, N., Grimi, N.: Microwave-assisted extraction of high-molecular-weight hemicelluloses from spruce wood. Comptes. Rendus. Chimie. 22(8), 574–584 (2019).
Yuan, Y., Zou, P., Zhou, J., Geng, Y., Fan, J., Clark, J., Li, Y., Zhang, C.: Microwave-assisted hydrothermal extraction of non-structural carbohydrates and hemicelluloses from tobacco biomass. Carbohyd. Polym. 223, 1–8 (2019). https://doi.org/10.1016/j.carbpol.2019.115043.
Mihiretu, G.T., Brodin, M., Chimphango, A.F., Øyaas, K., Hoff, B.H., Görgens, J.F.: Single-step microwave-assisted hot water extraction of hemicelluloses from selected lignocellulosic materials – A biorefinery approach. Bioresour. Technol. 241, 669–680 (2017). https://doi.org/10.1016/j.biortech.2017.05.159.
Gulbrandsen, T.A., Johnsen, I.A., Opedal, M.T., Toven, K., Øyaas, K., Pranovich, A., Mikkola, J.P., Hoff, B.H.: Extracting hemicelluloses from softwood and bagasse as oligosaccharides using pure water and microwave heating. Cellulose Chem. Technol. 49(2), 117–126 (2014)
Sing, S., Solomon, S.: Sugarcane diversification. Recent developments and future prospects in sugarcane. In: Singh, G.B., Solomon, S. (eds.) Agro-industrial alternatives. Oxford IBH, New Delhi (1995)
Shafeeq, A., Muhammad, A., Sarfaraz, S., Akram, Z., Saeed, H.M.U., Farooq, U.: Effect of acid concentration on the extraction of furfural from corn cobs. Int. J. Chem. Eng. Appl. 6, 381–384 (2015). https://doi.org/10.7763/IJCEA.2015.V6.514
Werpy, T.A., Holladay, J.E., White, J.F., Peterson, G., Aden, A., Bozell, J., Holladay, J.E., White, J.F., Manheim, A., Elliot, D., Lasure, L., Jones, S.: Top value added chemicals from biomass: I. results of screening for potential candidates from sugars and synthesis gas, in university of pennsylvania law review. Tech. Inf, Sci (2004). https://doi.org/10.2172/926125
Mariscal, R., Maireles-Torres, P., Ojeda, M., Sádaba, I., López Granados, M.: Furfural: a renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 9, 1144–1189 (2016). https://doi.org/10.1039/c5ee02666k
Ruiz, H.A., Rodríguez-Jasso, R.M., Fernandes, B.D., Vicente, A.A., Teixeira, J.A.: Hydrothermal processing, as an alternative for upgrading agriculture residues and marine biomass according to the biorefinery concept: a review. Renew. Sustain. Energy Rev. 21, 35–51 (2013). https://doi.org/10.1016/j.rser.2012.11.069
Shittu, A.A.: Catalytic conversion of hemicellulosic sugars into furfural in ionic liquid media, Diss. Univ. Toledo. 75 (2010)
Choudhary, V., Pinar, A.B., Sandler, S.I., Vlachos, D.G., Lobo, R.F.: Xylose isomerization to xylulose and its dehydration to furfural in aqueous media. ACS Catal. 1, 1724–1728 (2011). https://doi.org/10.1021/cs200461t
Binder, J.B., Blank, J.J., Cefali, A.V., Raines, R.T.: Synthesis of furfural from xylose and xylan. ChemSusChem 3, 1268–1272 (2010). https://doi.org/10.1002/cssc.201000181
Yazdizadeh, M., Jafari Nasr, M.R., Safekordi, A.: A new catalyst for the production of furfural from bagasse. RSC Adv. 6, 55778–55785 (2016)
Vazquez, M.J.: Acid-catalyzed conversion of xylose, xylan and straw into furfural. Bioresour. Technol. 102, 7371 (2011)
Yang, W., Li, P., Bo, D., Chang, H., Wang, X. and Zhu, T.: Optimization of furfural production from d-xylose with formic acid as catalyst in a reactive extraction system. Bioresour. Technol. 133, 361–369 (2013)
Branca, C., Di Blasi, C., Galgano, A.: Pyrolysis of corncobs catalyzed by zinc chloride for furfural production. Ind. Eng. Chem. Res. 49, 9743–9752 (2010). https://doi.org/10.1021/ie101067v
Yan, K., Wu, G., Lafleur, T., Jarvis, C.: Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew. Sustain. Energy Rev. 38, 663–676 (2014)
Cai, C.M., Zhang, T., Kumar, R., Wyman, C.E.: Integrated furfural production as a renewable fuel and chemical platform from lignocellulosic biomass. J. Chem. Technol. Biotechnol. 89, 2–10 (2014). https://doi.org/10.1002/jctb.4168
Lin, Q., Li, H., Wang, X., Jian, L., Ren, J., Liu, C., Sun, R.: SO42−/Sn-MMT solid acid catalyst for xylose and xylan conversion into furfural in the biphasic system. Catalysts. 7, 118 (2017). https://doi.org/10.3390/catal7040118
Danon, B., Marcotullio, G., De Jong, W.: Mechanistic and kinetic aspects of pentose dehydration towards furfural in aqueous media employing homogeneous catalysis. Green Chem. 16, 39–54 (2014). https://doi.org/10.1039/c3gc41351a
Xiouras, C., Radacsi, N., Sturm, G., Stefanidis, G.D.: Furfural synthesis from d-xylose in the presence of sodium chloride: microwave versus conventional heating. ChemSusChem 9, 2159–2166 (2016). https://doi.org/10.1002/cssc.201600446
Agirrezabal-Telleria, I., Gandarias, I., Arias, P.L.: Production of furfural from pentosan-rich biomass: analysis of process parameters during simultaneous furfural stripping. Bioresour. Technol. 143, 258–264 (2013). https://doi.org/10.1016/j.biortech.2013.05.082
Zhang, T., Li, W., Xu, Z., Liu, Q., Ma, Q., Jameel, H., Chang, H.M., Ma, L.: Catalytic conversion of xylose and corn stalk into furfural over carbon solid acid catalyst in γ-valerolactone. Bioresour. Technol. 209, 108–114 (2016). https://doi.org/10.1016/j.biortech.2016.02.108
Shi, X., Wu, Y., Li, P., Yi, H., Yang, M., Wang, G.: Catalytic conversion of xylose to furfural over the solid acid SO 42-/ZrO2-Al2O3/SBA-15 catalysts. Carbohydr. Res. 346, 480–487 (2011). https://doi.org/10.1016/j.carres.2011.01.001
Zou, J., Cao, D., Tao, W., Zhang, S., Cui, L., Zeng, F., Cai, W.: Sorbitol dehydration into isosorbide over a cellulose-derived solid acid catalyst. RSC Adv. 6, 49528–49536 (2016). https://doi.org/10.1039/c6ra05214b
Agirrezabal-Telleria, I., Requies, J., Güemez, M.B., Arias, P.L.: Dehydration of d-xylose to furfural using selective and hydrothermally stable arenesulfonic SBA-15 catalysts. Appl. Catal. B 145, 34–42 (2014). https://doi.org/10.1016/j.apcatb.2012.11.010
Sahu, R., Dhepe, P.L.: A one-pot method for the selective conversion of hemicellulose from crop waste into C5 sugars and furfural by using solid acid catalysts. ChemSusChem 5, 751–761 (2012). https://doi.org/10.1002/cssc.201100448
Zhang, J., Zhuang, J., Lin, L., Liu, S., Zhang, Z.: Conversion of d-xylose into furfural with mesoporous molecular sieve MCM-41 as catalyst and butanol as the extraction phase. Biomass Bioenerg. 39, 73–77 (2012). https://doi.org/10.1016/j.biombioe.2010.07.028
Agirrezabal-Telleria, I., Larreategui, A., Requies, J., Güemez, M.B., Arias, P.L.: Furfural production from xylose using sulfonic ion-exchange resins (Amberlyst) and simultaneous stripping with nitrogen. Bioresour. Technol. 102, 7478–7485 (2011). https://doi.org/10.1016/j.biortech.2011.05.015
Dias, A.S., Pillinger, M., Valente, A.A.: Dehydration of xylose into furfural over micro-mesoporous sulfonic acid catalysts. J. Catal. 229, 414–423 (2005). https://doi.org/10.1016/j.jcat.2004.11.016
Lima, S., Fernandes, A., Antunes, M.M., Pillinger, M., Ribeiro, F., Valente, A.A.: Dehydration of xylose into furfural in the presence of crystalline microporous silicoaluminophosphates. Catal. Letters. 135, 41–47 (2010). https://doi.org/10.1007/s10562-010-0259-6
Zhang, L., He, Y., Zhu, Y., Liu, Y., Wang, X.: Camellia oleifera shell as an alternative feedstock for furfural production using a high surface acidity solid acid catalyst. Bioresour. Technol. 249, 536–541 (2018). https://doi.org/10.1016/j.biortech.2017.10.061
Bhaumik, P., Dhepe, P.L.: Exceptionally high yields of furfural from assorted raw biomass over solid acids. RSC Adv. 4, 26215–26221 (2014). https://doi.org/10.1039/c4ra04119d
Bhaumik, P., Dhepe, P.L.A.: A novel one-pot method for furfural synthesis from crop wastes using stable SAPO-44, 1–4 (2015). https://doi.org/10.13140/RG.2.1.3800.4561
Suzuki, T., Yokoi, T., Otomo, R., Kondo, J.N., Tatsumi, T.: Dehydration of xylose over sulfated tin oxide catalyst: influences of the preparation conditions on the structural properties and catalytic performance. Appl. Catal. A 408, 117–124 (2011). https://doi.org/10.1016/j.apcata.2011.09.009
Zhang, J., Lin, L., Liu, S.: Efficient production of furan derivatives from a sugar mixture by catalytic process. Energy Fuels 26, 4560–4567 (2012). https://doi.org/10.1021/ef300606v
Jabasingh, S.A., Lalith, D., Prabhu, M.A., Yimam, A., Zewdu, T.: Catalytic conversion of sugarcane bagasse to cellulosic ethanol : TiO2 coupled nanocellulose as an effective hydrolysis enhancer. Carbohydr. Polym. 136, 700–709 (2016). https://doi.org/10.1016/j.carbpol.2015.09.098
O’Neil, R., Ahmad, M.N., Vanoye, L., Aiouache, F.: Kinetics of aqueous phase dehydration of xylose into furfural catalyzed by ZSM-5 zeolite. Ind. Eng. Chem. Res. 48, 4300–4306 (2009). https://doi.org/10.1021/ie801599k
Trogadas, P., Fuller, T.F., Strasser, P.: Carbon as catalyst and support for electrochemical energy conversion. Carbon N. Y. 75, 5–42 (2014). https://doi.org/10.1016/j.carbon.2014.04.005
Lam, E., Luong, J.H.T.: Carbon materials as catalyst supports and catalysts in the transformation of biomass to fuels and chemicals. ACS Catal. 4, 3393–3410 (2014). https://doi.org/10.1037/a0037499
Anthonysamy, S.B.I., Afandi, S.B., Khavarian, M., Bin Mohamed, A.R.: A review of carbon-based and non-carbon-based catalyst supports for the selective catalytic reduction of nitric oxide. Beilstein J. Nanotechnol. 9, 740–761 (2018). https://doi.org/10.3762/bjnano.9.68
Zhang, H., Luo, X., Li, X.: Preparation and characterization of a sulfonated carbon- based solid acid microspheric material (SCSAM) and its use for the esterification of oleic acid with methanol. Austin Chem. Eng. 3, 1024–1029 (2016)
Duyckaerts, N., Trotuş, I.T., Nese, V., Swertz, A.C., Auris, S., Wiggers, H., Schüth, F.: Mesoporous sulfonated carbon materials prepared by spray pyrolysis. ChemCatChem. 7, 2891–2896 (2015). https://doi.org/10.1002/cctc.201500483
Falcao, E.H.L.: Carbon allotropes: beyond graphite and diamond. J. Chem. Technol. Biotechnol 82, 524–531 (2007)
Zhao, H., Kwak, J.H., Wang, Y., Franz, J.A., White, J.M., Holladay, J.E.: Effects of crystallinity on dilute acid hydrolysis of cellulose by cellulose ball-milling study. Energy Fuels (ACS Publications) 20, 807–811 (2006)
Liang, X., Zeng, M., Qi, C.: One-step synthesis of carbon functionalized with sulfonic acid groups using hydrothermal carbonization. Carbon N. Y. 48, 1844–1848 (2010). https://doi.org/10.1016/j.carbon.2010.01.030
Lam, E., Chong, J.H., Majid, E., Liu, Y., Hrapovic, S., Leung, A.C.W., Luong, J.H.T.: Carbocatalytic dehydration of xylose to furfural in water. Carbon N. Y. 50, 1033–1043 (2012). https://doi.org/10.1016/j.carbon.2011.10.007
Termvidchakorn, C., Itthibenchapong, V., Songtawee, S., Chamnankid, B., Namuangruk, S., Faungnawakij, K., Charinpanitkul, T., Khunchit, R., Hansupaluk, N., Sano, N., Hinode, H.: Dehydration of d-xylose to furfural using acid-functionalized MWCNTs catalysts. Nat. Sci. Nanosci. Nanotechnol, Adv (2017). https://doi.org/10.1088/2043-6254/aa7234
Agirrezabal-Telleria, I., Guo, Y., Hemmann, F., Arias, P.L., Kemnitz, E.: Dehydration of xylose and glucose to furan derivatives using bifunctional partially hydroxylated MgF2 catalysts and N2-stripping. Catal. Sci. Technol. 4, 1357–1368 (2014). https://doi.org/10.1039/c4cy00129j
Choudhary, V., Sandler, S.I., Vlachos, D.G.: Conversion of xylose to furfural using Lewis and Brønsted acid catalysts in aqueous media. ACS Catal. 2, 2022–2028 (2012). https://doi.org/10.1021/cs300265d
Kang, S., Ye, J., Chang, J.: Recent advances in carbon-based sulfonated catalyst: preparation and application. Int. Rev. Chem. Eng. 5, 133–144 (2013). https://doi.org/10.15866/ireche.v5i2.6912
Iglesias, J., Melero, J.A., Morales, G., Paniagua, M., Hernández, B.: Dehydration of xylose to furfural in alcohol media in the presence of solid acid catalysts. ChemCatChem 8, 2089–2099 (2016). https://doi.org/10.1002/cctc.201600292
Lima, S., Pillinger, M., Valente, A.A.: Dehydration of d-xylose into furfural catalysed by solid acids derived from the layered zeolite Nu-6(1). Catal. Commun. 9, 2144–2148 (2008). https://doi.org/10.1016/j.catcom.2008.04.016
Jin, F., Enomoto, H.: Rapid and highly selective conversion of biomass into value-added products in hydrothermal conditions : chemistry of acid/base-catalysed and oxidation reactions. Energy Environ Sci 4, 382–397 (2011). https://doi.org/10.1039/c004268d
Gürbüz, E.I., Gallo, J.M.R., Alonso, D.M., Wettstein, S.G., Lim, W.Y., Dumesic, J.A.: Conversion of hemicellulose into furfural using solid acid catalysts in γ-valerolactone. Angew. Chemie Int. Ed. 52, 1270–1274 (2013). https://doi.org/10.1002/anie.201207334
Aida, M.T., Sato, Y., Watanabe, M., Tajima, K., Nonaka, T., Hattori, H., Arai, K.: Dehydration of d -glucose in high temperature water at pressures up to 80 MPa. J. Supercrit Fluids 40, 381–388 (2007)
Zhang, L., Xi, G., Yu, K., Yu, H., Wang, X.: Furfural production from biomass; derived carbohydrates and lignocellulosic residues via heterogeneous acid catalysts. Ind. Crop. Prod. 98, 68–75 (2017). https://doi.org/10.1016/j.indcrop.2017.01.014
Xu, Z., Li, W., Du, Z., Wu, H., Jameel, H., MinChang, H., Ma, L.: Conversion of corn stalk into furfural using a novel heterogeneous strong acid catalyst in γ-valerolactone. Bioresour. Technol. 198, 764–771 (2015). https://doi.org/10.1016/j.biortech.2015.09.104
Rackemann, D.W., Doherty, W.O.: A review on the production of levulinic acid and furanics from sugars. Int. Sugar J. 115, 28–34 (2013)
Agirrezabal-Telleria, I., Requies, J., Güemez, M.B., Arias, P.L.: Furfural production from xylose + glucose feedings and simultaneous N2-stripping. Green Chem. 14, 3132–3140 (2012). https://doi.org/10.1039/c2gc36092f
Gorrindo, T., Goldfarb, E., Birnbaum, R.J., Chevalier, L., Meller, B., Alpert, J., Herman, J., Weiss, A.: Simulation-based ongoing professional practice evaluation in psychiatry: a novel tool for performance assessment. Jt. Comm. J. Qual. Patient Saf. 39, 319–323 (2013)
Kim, S.B., You, S.J., Kim, Y.T., Lee, S.M., Lee, H., Park, K., Park, E.D.: Dehydration of d-xylose into furfural over H-zeolites. Korean J. Chem. Eng. 28, 710–716 (2011). https://doi.org/10.1007/s11814-010-0417-y
Antunes, M.M., Lima, S., Fernandes, A., Pillinger, M., Ribeiro, M.F., Valente, A.A.: Aqueous-phase dehydration of xylose to furfural in the presence of MCM-22 and ITQ-2 solid acid catalysts. Appl. Catal. A 417–418, 243–252 (2012). https://doi.org/10.1016/j.apcata.2011.12.046
Deng, A., Lin, Q., Yan, Y., Li, H., Ren, J., Liu, C., Sun, R.: Bioresource Technology A feasible process for furfural production from the pre-hydrolysis liquor of corncob via biochar catalysts in a new biphasic system 216, 754–760 (2016). https://doi.org/10.1016/j.biortech.2016.06.002
Hua, D.R., Wu, Y.L., Liu, Y.F., Chen, Y., De Yang, M., Lu, X.N., Li, J.: Preparation of furfural and reaction kinetics of xylose dehydration to furfural in high-temperature water. Pet. Sci. 13, 167–172 (2016)
Shi, X., Wu, Y., Yi, H., Rui, G., Li, P., Yang, M., Wang, G.: Selective preparation of furfural from xylose over sulfonic acid functionalized mesoporous Sba-15 materials. Energies. 4, 669–684 (2011). https://doi.org/10.3390/en4040669
Dashtban, M., Technologies, A., Dashtban, M.: Production of furfural : overview and production of furfural : overview and challenges. J. Sci. Technol. For. Prod. Process. 2, 44–53 (2012)
Bhaumik, P., Dhepe, P.L.: Solid acid catalyzed synthesis of furans from carbohydrates. Catal. Rev. Sci. Eng. 58, 36–112 (2016). https://doi.org/10.1080/01614940.2015.1099894
Smallwood, I.M.: Solvent Recovery Handbook, 2nd edn. Blackwell Science, Oxford (2002)
Gairola, K., Smirnova, I.: Hydrothermal pentose to furfural conversion and simultaneous extraction with SC-CO2: kinetics and application to biomass hydrolysates. Bioresour. Technol. 123, 592–598 (2012). https://doi.org/10.1016/j.biortech.2012.07.031
Sako, T., Sugeta, T., Nakazawa, N., Okubo, T., Sato, M., Hiaki, T., Taguchi, T.: Kinetic study of furfural formation accompanying supercritical carbon dioxide extraction. J. Chem. Eng. Japan. 25, 372–377 (1992). https://doi.org/10.1252/jcej.25.372
Sangarunlert, W., Piumsomboon, P., Ngamprasertsith, S.: Furfural production by acid hydrolysis and supercritical carbon dioxide extraction from rice husk. Korean J. Chem. Eng. 24, 936–941 (2007). https://doi.org/10.1007/s11814-007-0101-z
Khosravi-Darani, K., Vasheghani-Farahani, E.: Application of supercritical fluid extraction in biotechnology. Crit. Rev. Biotechnol. 25, 231–242 (2005). https://doi.org/10.1080/07388550500354841
Han, X., Poliakof, M.: Continuous reactions in supercritical carbon dioxide: problems, solutions and possible ways forward. Chem. Soc. Rev. 41, 1428–1436 (2012). https://doi.org/10.1039/c2cs15314a
Montastruc, L., Ajao, O., Marinova, M., Barreto, C., Carmo, D.O., Domenech, S.: Hemicellulose biorefinery for furfural production : energy requirement analysis and minimization 1, 48–53 (2011)
Xin, K., Song, Y., Dai, F., Yu, Y., Li, Q.: Liquid–liquid equilibria for the extraction of furfural from aqueous solution using different solvents. Fluid Phase Equilib. (2016). https://doi.org/10.1016/j.fluid.2016.06.040
De, S., Dutta, S., Saha, B.: Catalysis Science & Technology biomass valorization : current thrust and emerging. Catal. Sci. Technol. 6, 7364–7385 (2016). https://doi.org/10.1039/c6cy01370h
Li, X., Yang, J., Xu, R., Lu, L., Kong, F., Liang, M., Jiang, L.: Industrial Crops & Products Kinetic study of furfural production from Eucalyptus sawdust using H-SAPO- 34 as solid Brønsted acid and Lewis acid catalysts in biomass-derived solvents. Ind. Crop. Prod. 135, 196–205 (2019). https://doi.org/10.1016/j.indcrop.2019.04.047
Chen, Z., Bai, X., Lusi, A., Jacoby, W.A., Wan, C.: One-pot selective conversion of lignocellulosic biomass into furfural and co-products using aqueous choline chloride/methyl isobutyl ketone biphasic solvent system. Bioresour. Technol. 289, 121708 (2019)
Hoydonckx, H.E., Van Rhijn, W.M., Van Rhijn, W., De Vos, D.E., Jacobs, P.A.: Furfural and derivatives. Ullmann's Encycl. Ind. Chem. 16, 285–313 (2000). https://doi.org/10.1002/14356007.a12_119.pub2
Malinowski, A., Wardzińska, D.: The catalytic conversion of furfural towards fuel biocomponents science technique. Angew. Chemie Int. Ed. 66, 982–990 (2012)
Girisuta, B.: Levulinic acid from lignocellulosic biomass. Doctoral dissertation, University of Groningen (2007)
Mariscal, R., Ojeda, M.: Environmental science molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 9, 1144–1189 (2016). https://doi.org/10.1039/C5EE02666K
Daengprasert, W., Boonnoun, P., Laosiripojana, N., Goto, M., Shotipruk, A.: Application of sulfonated carbon-based catalyst for solvothermal conversion of cassava waste to hydroxymethylfurfural and furfural. Ind. Eng. Chem. Res. 50, 7903–7910 (2011). https://doi.org/10.1021/ie102487w
Li, W., Zhu, Y., Lu, Y., Liu, Q., Guan, S., Min Chang, H., Jameel, H., Ma, L.: Enhanced furfural production from raw corn stover employing a novel heterogeneous acid catalyst. Bioresour. Technol. 245, 258–265 (2017). https://doi.org/10.1016/j.biortech.2017.08.077
Qing, Q., Guo, Q., Zhou, L., Wan, Y., Xu, Y., Ji, H., Gao, X., Zhang, Y.: Catalytic conversion of corncob and corncob pretreatment hydrolysate to furfural in a biphasic system with addition of sodium chloride. Bioresour. Technol. 226, 247–254 (2017). https://doi.org/10.1016/j.biortech.2016.11.118
Li, H., Deng, A., Ren, J., Liu, C., Lu, Q., Zhong, L., Peng, F., Sun, R.: Catalytic hydrothermal pretreatment of corncob into xylose and furfural via solid acid catalyst. Bioresour. Technol. 158, 313–320 (2014). https://doi.org/10.1016/j.biortech.2014.02.059
Li, H., Ren, J., Zhong, L., Sun, R., Liang, L.: Production of furfural from xylose, water-insoluble hemicelluloses and water-soluble fraction of corncob via a tin-loaded montmorillonite solid acid catalyst. Bioresour. Technol. 176, 242–248 (2015). https://doi.org/10.1016/j.biortech.2014.11.044
Bhaumik, P., Dhepe, P.L.: Efficient, stable, and reusable silicoaluminophosphate for the one-pot production of furfural from hemicellulose. ACS Catal. 3, 2299–2303 (2013). https://doi.org/10.1021/cs400495j
Dias, A.S., Lima, S., Brandão, P., Pillinger, M., Rocha, J., Valente, A.A.: Liquid-phase dehydration of D-xylose over microporous and mesoporous niobium silicates. Catal. Letters. 108, 179–186 (2006). https://doi.org/10.1007/s10562-006-0046-6
Lessard, J., Morin, J.F., Wehrung, J.F., Magnin, D., Chornet, E.: High yield conversion of residual pentoses into furfural via zeolite catalysis and catalytic hydrogenation of furfural to 2-methylfuran. Top. Catal. 53, 1231–1234 (2010). https://doi.org/10.1007/s11244-010-9568-7
Moreau, C., Durand, R., Peyron, D., Duhamet, J., Rivalier, P.: Selective preparation of furfural from xylose over microporous solid acid catalysts. Ind. Crops Prod. 7, 95–99 (1998). https://doi.org/10.1016/S0926-6690(97)00037-X
Antunes, M.M., Lima, S., Fernandes, A., Pillinger, M., Ribeiro, M.F., Valente, A.A.: Aqueous-phase dehydration of xylose to furfural in the presence of MCM-22 and ITQ-2 solid acid catalysts. Appl. Catal. A 417–418, 243–252 (2012). https://doi.org/10.1016/j.apcata.2011.12.046
Takagaki, A., Ohara, M., Nishimura, S., Ebitani, K.: One-pot Formation of Furfural from xylose via isomerization and successive dehydration reactions over heterogeneous acid and base catalysts. Chem. Lett. 39, 838–840 (2010). https://doi.org/10.1246/cl.2010.838
Mazzotta, M.G., Gupta, D., Saha, B., Patra, A.K., Bhaumik, A., Abu-Omar, M.M.: Efficient solid acid catalyst containing lewis and brønsted acid sites for the production of furfurals. ChemSusChem 7, 2342–2350 (2014). https://doi.org/10.1002/cssc.201402007
Laohapornchaiphan, J., Smith, C.B., Smith, S.M.: One-step preparation of carbon-based solid acid catalyst from water hyacinth leaves for esterification of oleic acid and dehydration of xylose. Chem. Asian J. 12, 3178–3186 (2017). https://doi.org/10.1002/asia.201701369
Lima, S., Antunes, M.M., Fernandes, A., Pillinger, M., Ribeiro, M.F., Valente, A.A.: Acid-catalysed conversion of saccharides into furanic aldehydes in the presence of three-dimensional mesoporous Al-TUD-1. Molecules 15, 3863–3877 (2010). https://doi.org/10.3390/molecules15063863
Acknowledgements
The authors acknowledge the School of Chemical and Bioengineering, Addis Ababa Institute of Technology, Addis Ababa University for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dulie, N.W., Woldeyes, B., Demsash, H.D. et al. An Insight into the Valorization of Hemicellulose Fraction of Biomass into Furfural: Catalytic Conversion and Product Separation. Waste Biomass Valor 12, 531–552 (2021). https://doi.org/10.1007/s12649-020-00946-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-00946-1