Abstract
This study aims to compare the performance and kinetics between the single-stage anaerobic digestion (SAD) and the two-stage anaerobic digestion (TAD) of vegetable waste (VW). The SAD was performed using continuously stirred tank reactors. Meanwhile, the TAD experiment was set up using a combined system involving a continuously stirred tank for hydrolysis/acidogenesis and an upflow reactor for methanogenesis. The hydrolytic reactor operated as a batch process with a retention time (RT) of 9 days, while the methane reactor was a continuous process operation with RT of 20 days. Both TAD and SAD were controlled at a temperature of 36 °C. The SAD experiments lasted for 143 days, and were characterised by the kinetic rate constant k = 0.02 day−1 which was much lower than that for the TAD (k = 0.66 − 2.16 day−1). The SAD seemed to be inhibited by high concentration of free ammonia and low inoculum to substrate ratio; herein, only 17.8–22.3% of the initial carbon could be converted into biogas (equivalent to 91–110 Nml/g-VSadded) with low methane content (44.1–48.7%). Meanwhile, TAD converted 41.67% initial carbon to biogas (equivalent to 299.0–374.6 Nml/g-VSadded) with high methane content (71.68–81.0%). Moreover, methanogenesis in the TAD was highly stable which enabled the digestion process to return to normal state within a few days, even though the concentrations of the influent increased to double (6.5–24.5 g-COD/l). As per these results, the TAD was much more stable, faster, and stronger than the SAD.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
This paper provided a new approach for evaluations and comparisons between single-stage digestion and two-stage digestion. In which, the kinetic rate constant, which determines the rate of reaction processes, was used as a good indicator. Moreover, the fate of carbon, which reveals the proportion of biogas conversion, was employed for performance assessments. This study is also like a guideline for an in-depth evaluation of both single-stage digestion and two-stage digestion.
Introduction
Vegetable waste (VW) is generated daily in large amounts globally as a natural consequence of agricultural and food industrial activities [1, 2]. European Union alone annually produces 89 million tons of fruit and vegetable waste and this value is expected to further significantly increase [3]. It is highly biodegradable, and when discarded into landfills or dumping sites, it inevitably causes natural anaerobic digestion (AD) leading to various environmental and human health problems such as odour pollution, CH4 emission, groundwater contamination, surface water pollution, and spread of diseases [1, 2, 4, 5]. Therefore, AD systems for controlling AD of biodegradable waste, including VW, and the recovery of energy gas (CH4) have been developed [5].
The simplest system is the single-stage anaerobic digestion (SAD), which allows both steps, hydrolysis/acidogenesis and acetogenesis/methanogenesis, to occur in one reactor [6]. However, microbial growth characteristics between these digestion steps differ. Hydrolysis/acidogenesis is implemented by facultative anaerobes with the short minimum doubling time (30 min) and optimal pH in the range of 5.5–6.5 [7, 8]. Meanwhile, microorganisms performing acetogenesis/methanogenesis are the obligate anaerobes with the long minimum doubling time (several days) and an optimal pH of 7–8 [5, 7]. Moreover, the obligate anaerobes are sensitive to volatile fatty acids (VFAs) that are produced during the first step. Therefore, it is difficult to maintain balance of microbial growth between these steps at a high loading rate in an SAD system and its loading rate remains unsatisfactory [6]. Therefore, the idea of two-stage anaerobic digestion (TAD), which separates the first step and the second step in two different reactors, has been proposed to optimise every digestion step. By implementing TAD, the system can achieve more stable operation, higher organic loading capacity and a higher resistance toward toxicants and inhibiting substances [5, 6, 9]. However, the current TAD technology requires very high investment and operation cost; hence, the SAD systems are favoured for application [9]. The TAD is still being improved using different configurations to meet competition with SAD [10]. The comparisons between SAD and TAD pertaining to different aspects are necessary to orientate the future direction of technology development. Aslanzadeh et al. [6] performed AD of food waste and organic fraction of municipal solid waste (OFMSW). They reported that SAD could not handle an organic loading rate (OLR) > 2–3 g-VS/l/d. Meanwhile TAD run well with an OLR in the range of 8–12 g-VS/l/d with a lower RT. Xiao et al. [11] investigated AD of food waste at thermophilic temperature. In this study, CH4 yield observed in the SAD system (775 ml/g-VSadded) was lower than that in TAD (810 ml/g-VSadded). Schievano et al. [12] reported that there were no differences in the overall energy recovery between SAD and TAD of market bio-waste. However, the bacterial and archaeal microbial communities in both systems were considerably different, and a higher bacterial diversity was observed in SAD. Begum et al. [13] experimented with AD of landfill leachate and showed that COD removal in TAD was higher at 21% compared to SAD. Thus, majority of the comparisons have revolved around issues of biogas yield, effects of operating parameters, and microbial communities. Meanwhile, the kinetics of methanogenesis as a reliable indicator, that determines the speed of the processes, has not been used for comparison. Furthermore, the comparison between two digestion systems using VW is limited in the literature.
Therefore, this work aims to compare the single-stage and the two-stage digestion of VW based on the kinetics and performance of methanogenesis. Moreover, this study shows the fate of carbon in these AD processes, which have been scarcely reported in literature to date.
Materials and Methods
Analytical Methods
Carbon (C) and nitrogen (N) of the substrate were determined using a CHN analyser (2400 II, PerkinElmer, USA). Total solid (TS), volatile solid (VS), and pH were carried out according to standard methods [14]. Total carbon and inorganic carbon of the liquid samples were measured by a TOC-L analyser (Shimadzu, Japan). The liquid sample was filtered (0.45 μm) to obtain soluble carbon before measuring. Total chemical oxygen demand (TCOD) and total nitrogen (TN) were measured by a spectrophotometer (MD600, Lovibond, UK). Gas content (carbon dioxide, methane and other) was analysed according to the method described by Dinh et al. [15]. VFA components were determined by a GC-14A (Shimadzu, Japan) equipped with a flame ionization detector and a capillary column.
Substrate and Inoculum
Samples of VW and horse dung (HD) were collected from an agricultural field; HD played the role of inoculum source, and VW was substrate for anaerobic digestion. Characteristics of VW and HD were determined as described in the Sect. “Analytical Methods” and are listed in Table 1. Vegetable waste was cut into small particles (< 5 mm), stored in a refrigerator at a temperature below zero, and shredded with using a household blender before use. The feedstock for digestion consisted of a mixture of VW and HD in a ratio of 10:1.
Single-Stage Anaerobic Digestion
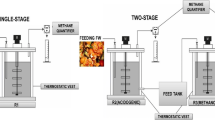
Two similar continuous stirred tank reactors (CSTRs) were employed in the batch anaerobic digestion with TS condition of 3% and 5%. The outline of the experiment is shown in Fig. 1a. The reactors were set briefly in a hot water tank with a controlled temperature 36 ± 1 °C. The substrate inside the reactors was mixed using a stirrer. In the first 10 days, the pH was maintained at pH 6.5 by a pH controller. The pH controller received signals from a pH probe to control (on/off) a switch pumping NaOH solution (10 M). Biogas was collected temporarily by a plastic bag for further analysis.
Two-Stage Anaerobic Digestion
The two-stage digestion system included a hydrolysis reactor (employing CSTR), a buffer tank, and a methane reactor (an upflow anaerobic sludge blanket—UASB), as shown in Fig. 1b. The hydrolytic reactor was controlled at a temperature of 36 ± 1 °C and pH 6.5. After hydrolysis, the products were filtered to collect the liquid fraction (hydrolysate) and then diluted using tap water (in the buffer tank). Finally, the liquid was pumped into the UASB reactor by a peristaltic pump. The operation of the methane reactor was divided into stage I, II, and III with a dilution rate of hydrolysate at 10, 5, and 2.5, respectively. Every stage was maintained for 24 days. Particularly stage III, the pH of the liquid hydrolysate was raised to pH 7.0 that is more suitable for methanogenesis. Prior to the experiment, the conditions inside the UASB reactor, suitable for the anaerobic bacterial cultures were maintained for three months. The temperature of UASB was maintained at 36 ± 1 °C by a hot water jacket. RT in the hydrolysis reactor, buffer tank, and UASB reactor was nine days, one day, and twenty days, respectively.
Kinetic Models
For the Batch SAD process, Dinh et al. [16] recently proposed a new kinetic biogas production model (the DINH model) that can overcome disadvantages of the conventional models. Therefore, it was employed to calculate the kinetic rate coefficient (km1 day−1), written as Eqs. 1 and 2 [15, 16].
where, Gt = accumulative methane yield (Nml/g-VS); A = me reactors leading to inhethane yield potential (Nml/g-VS); t = digestion time (day); to = time when the biogas rate gets maximum (day); m = intermediate coefficient; and km1 = average rate kinetic coefficient (day−1). The kinetic coefficients in Eq. 1 were determined by the least squares fitting method. Moreover, Eq. 1 was converted into a linear regression equation (Eq. 3) for comparison between experimental values and modelling data using the correlation of determination (r2).
For the two-stage digestion, the first-order model was applied to calculate the rate kinetics coefficient (km2 day−1) in the methane tank [17]. Under steady-state conditions, the model can be expressed by the following equation:
where, Si = concentration of the substrate in influent (mg-COD/L); Se = concentration of the substrate in effluent (mg-COD/L); and HRT = hydraulic retention time (day).
Results and Discussion
Single-Stage Anaerobic Digestion
Characteristics of biogas production and digestate from the single-stage digestion experiments are shown in Table 2. While the biogas yield and methane content in reactor with TS at 3% were respectively 207.7 Nml/g-VSadded and 44.1%, they were 226 Nml/g-VSadded and 48.7% in the TS 5% reactor. Ji et al. [1] reviewed a considerable literature pertaining to the digestion of fruit and vegetable waste (FVW), and reported the methane yield as ranging between 190 and 400 ml/g-VS. Recently, many authors have focused on the co-digestion of VW and other waste to improve methane production. Dinh et al. [15] experimented on the co-digestion of VW and food waste, and reported that the biogas yield was in the range of 431.6–596.9 Nml/g-VSadded (56.2–67.5% of CH4). Shen et al. [18] gained a biogas yield of 328–544 ml-CH4/g- g-VSadded when performing the co-digestion of VW and kitchen waste. Dinh et al. [19] used a mixture of FW, HD, and sludge, and obtained biogas yield in the range of 138–648 Nml/ g-VSadded (58–76% CH4). Compared to other studies, our current work showed a lower methane yield.
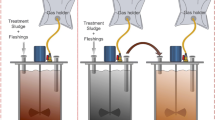
The fate of carbon in the SAD process is shown in Fig. 2. Here, only 18.7–22.3% of initial carbon in the feedstock was converted into the gaseous state, and 18–19% of carbon remained in the solid state. The SAD stopped while the carbon concentrations in the liquid and suspended matter were still high, which is a sign of inhibition. The free ammonia (FAN) concentrations obtained in TS 3% and TS 5% reactors were 128.5 and 218.9 mg/l, respectively. Several other studies also observed inhibition in the SAD around this FAN concentration range. Gallert, Winter [20] reported that 50% biogas production was inhibited when ammonia concentration was in the range of 3–3.7 mg-N/l (equivalent to FAN of 220–280 mg-N/l) at mesophilic temperature. Braun et al. [21] reported that the FAN levels of approximately 150 mg-N/l could cause methanogenic growth inhibition. Moreover, for digestion to proceed adequately, a sufficient amount of active inoculum was required. The inoculum to substrate (I/S) ratio should be high enough to catalyse a series of interdependent biochemical reactions [22]. Parawira et al. [23] showed that the increase in I/S ratio could help the system overcome inhibition. Meanwhile, the I/S ratio in the current study (2:5, VS basis) was lower than that has often been reported as being sufficient for SAD. Therefore, the inhibition could be attributed to both high FAN concentration and low I/S ratio.
Cumulative biogas production in the TS 3% and TS 5% reactors are shown in Fig. 3. In both reactors, while carbon dioxide was generated soon after the initiation of the reactions, methane production only was considerably delayed. So, there was a lag-time between methanogenesis and hydrolysis/acidogenesis, that was also reported by Dinh et al. [15], Boulanger et al. [22], and Chen et al. [24]. This phenomenon could be explained, firstly by the inhibition of methanogens caused by high FAN concentration and low I/S as mentioned above, and secondly by the fact that rapid growth facultative microorganisms caused correspondingly rapid accumulation of a high concentration of VFAs in the reactors leading to inhibition of methanogens [1]. Methanogenesis in both reactors was quite slow (143 days) and was characterised by the rate kinetic constants km1 = 0.02 day−1. Recently, Dinh et al. [15] also performed co-digestion of VW and horse dung at mesophilic temperature, using a higher inoculum (15%, wet basis), and obtained higher values of km1 (0.031–0.039 day−1) and lower lag-time of methanogenesis. At a higher I/S ratio (1:1, VS basis), Nielfa et al. [25] reported that the lag-time was zero and the kinetic constant was 0.39 day−1. Therefore, this study agrees with Boulanger et al. [22] that the I/S ratio significantly affects the kinetics of methanogenesis.
Two-Stage Anaerobic Digestion
After nine days of fermentation at pH 6.5, total VFAs and TCOD of the fermentative product was 29.6 g/l (equivalent to a yield of 484.4 g-VFAs/kg-VSadded) and 62.4 g/l, respectively. In which, VFA component included acetate, propionate, butyrate, and valerate accounted for 41.3%, 7.1%, 45.2%, and 6.4%, respectively. Ravi et al. [26] and Schievano et al. [12] also observed that acetate and butyrate were the main components in the fermentation of VW. However, total VFAs in these reports were significantly lower than the current study. While Ravi et al. [26] obtained 215.3 g-VFAs/g-VSadded at pH 5.5 (RT of 4 days) and 347.8 g-VFAs/g-VSadded at pH 6.0 (RT of 6 days), Schievano et al. [12] only acquired total VFAs of 136.5 g/g-VSadded with conditions of pH 5.5 and 3 days RT. These differences could be explained, firstly by the current study used a higher RT (9 days) compared to the others, and secondly by the effect of pH on the fermentation process, which was often reported in the literature. Using long RT as in the current study leads to requiring a big reactor, this disadvantage can be surmounted by increasing the I/S ratio to reduce RT as recommended by Boulanger et al. [22]. Characteristics of the fermentative product showed more clearly the operation of the methane reactor at different stages as shown in Table 3.
The results of the biogas yield and methane content from the start-up phase to stage I-III in the methane reactor are shown in Fig. 4. In the start-up phase, the biogas yield was 275.6 Nml/g-VSadded (71.1% CH4). After a sudden increase in COD of the influent, all subsequent stages included a shock period followed by a stable period. In stable periods, biogas yield obtained in stage I, II, and III was 299.0 Nml/g-VSadded (71.68% CH4), 373.9 Nml/g-VSadded (73.24% CH4), and 374.6 Nml/g-VSadded (81.0% CH4), respectively. The biogas yield tended to increase over time and stabilised in stage III. This result can be attributed to the microbiological consortium inside the reactor still in the process of stability.
According to Chernicharo, Augusto [4], methanogenesis primarily occurs in two different ways: (1) acetotrophic bacteria ferment acetic acid to CH4 and CO2; (2) hydrogenotrophic methanogens synthesise CH4 from CO2. These bacterial groups respond differently to the change in environmental conditions [4, 5], that influence the reaction rates of (1) and (2) causing a change in CH4 percentage in biogas. This study observed an increase in the percentage of CH4 and a significant reduction in biogas production when the COD input concentration doubled (shock period). These results reflected that both hydrogenotrophic and acetotrophic methanogens had been shocked. However, slight increase in CH4 concentration indicated that hydrogenotrophic methanogens were affected less than the acetotrophic methanogens were. It is reasonable because hydrogenotrophic bacteria grow at a faster rate than acetotrophic bacteria [5]. Overall, the shock periods passed over shortly after 4–6 days due to the high adaptability of the reactor.
Different models relating to the two-stage anaerobic digestion of vegetable have been studied. Ganesh et al. [10] used two complete mixed reactors for both phases with the batch operation mode at 35 °C. They centrifuged the hydrolysate; the solid part was returned to the hydrolytic reactor, while the liquid was taken to the methane reactor with an RT of 30 days, and biogas yield of 300 ml-CH4/g-VSadded was obtained. Wu et al. [27] also used centrifugation method to remove particles of the hydrolysate. However, they employed the configuration of CSTR/UASB for hydrolysis/methanogenesis with the continuous operation mode. They observed a lower CH4 yield (244.2 ml/g-VSadded). In fact, using the centrifugation method to remove solid materials out of the hydrolysate is a complicated approach for a full-scale treatment plant. Therefore, Raynal et al. [28] used a 0.5 mm iron sieve instead of using the centrifugation method to deal with the hydrolysate. A sequencing batch reactor (SBR) was employed for hydrolysis and an anaerobic filter (AF) reactor for methanogenesis. They obtained 240 ml-biogas/g-CODadded in the methane reactor. The above results together with the current research result showed that both the configuration and operation of TAD systems significantly affected biogas production, which was also confirmed by Van et al. [5].
Stage III achieved the highest efficiency between the three stages, and its carbon flow is shown in Fig. 5. Solid fractions accounted for 26% after fermentation. This result is reasonable because VW was often reported to contain a large number of fibrous materials [1, 10]. This non-hydrolysable carbon was higher than that was collected in the SAD process. Therefore, if hydrolytic time is increased to more than one hundred days, the hydrolytic ratio will only increase by 6–7%, which is considered to be non-economical. The amount of carbon converted into biogas in the two-stage digestion system was 41.67%. This result indicates that TAD was much more effective than the SAD. However, a large amount of carbon in the feedstock was hydrolysed but could not be converted into biogas (see Fig. 5). It was a significant energy loss. So, further study on how to transform this amount of carbon into biogas should be done. The carbon in the effluent (21.75% of initial carbon) was mainly in the inorganic form leading to release low greenhouse gas emission values, even not. Meanwhile, the effluent of the anaerobic digestion process contains high concentrations of nitrogen, phosphorus, and minerals [29]. Thus, it might be used directly as a fertiliser source for agricultural activities. According to Romli et al. [30], the loss of mass in the anaerobic digestion process accounted for approximately 5% initial carbon. Therefore, only a small portion of the feedstock was converted into microbial biomass (10.6 − 5% = 5.6%), which then constituted the excess sludge of the methane reactor. Compared to the aerobic process, a huge amount of feedstock are often converted into residual sludge biomass (50–60%) leading to sludge treatment which is a cost burden for the overall treatment process [4]. The creation of less residual sludge is, therefore, a great advantage of anaerobic treatment compared to the aerobic process.
The values of kinetic rate constant (k) were 0.67, 1.26 and 2.18 day−1 with CODinfluent of 6.5, 12.2 and 24.5 g/l respectively. They were remarkably higher than those obtained in the SAD systems. This result indicated that the biodegradation rate of substrate in TAD was much higher than in the SAD. In relation to using the first-order kinetic model to evaluate the methane reactor in the two-stage digestion of VW, the values of k in the current study are significantly lower than those reported by Diamantis, Aivasidis [31] (19–23 day−1) who also used UASB to serve methanogenesis at mesophilic temperature. Math-Alvarez et al. [32] even observed a lower value of k (0.176 day−1) compared to the current study. They also used a TAD system but operated the methane reactor by the batch process. These differences can be attributed to the different testing conditions (wastewater pre-acidification, biomass concentration, the concentration of the influent) and especially, the organic loading rate. In the current study, the requirements of adding alkaline to control pH, water for dilution, and removing particles were great disadvantages. Fortunately, the demand of water and alkali consumption can be significantly reduced by using the effluent of the methane reactor for the dilution [5]. However, recirculating the effluent can cause accumulation of inhibitors; hence, monitoring the level of inhibitors to ensure that they are below toxic levels is essential [33]. Particle removal can be easily attained by using filter machines that are popular in the sludge treatment such as filter press and screw press. Therefore, the disadvantages of the TAD system can be addressed easily using the current technologies.
Conclusions
This study proved that the TAD has several outstanding advantages compared to the SAD in the treatment of VW. The conversion proportion of carbon into biogas in the TAD process was much higher than that in the SAD process. The kinetics of methanogenesis in the TAD was also faster than those in the SAD. Besides, the TAD process produced much higher CH4 content than the SAD process. Both hydrogenotrophic and acetotrophic bacteria had good resistance to the concentration change of the influent; however, the former depicted higher resistance. In the TAD, a large amount of initial carbon was hydrolysed but could not be converted into biogas, leading to significant energy loss. Hence, further studies should be conducted pertaining to the transformation of unutilised carbon into biogas.
References
Ji, C., Kong, C.-X., Mei, Z.-L., Li, J.: A review of the anaerobic digestion of fruit and vegetable waste. Appl. Biochem. Biotechnol. 183(3), 906–922 (2017). https://doi.org/10.1007/s12010-017-2472-x
Lin, J., Zuo, J., Gan, L., Li, P., Liu, F., Wang, K., Chen, L., Gan, H.: Effects of mixture ratio on anaerobic co-digestion with fruit and vegetable waste and food waste of China. J. Environ. Sci. 23(8), 1403–1408 (2011). https://doi.org/10.1016/S1001-0742(10)60572-4
Plazzotta, S., Manzocco, L., Nicoli, M.C.: Fruit and vegetable waste management and the challenge of fresh-cut salad. Trends Food Sci. Technol. 63, 51–59 (2017). https://doi.org/10.1016/j.tifs.2017.02.013
Chernicharo de Lemos, C.A.: Anaerobic reactors, vol. 4. Biological Wastewater Treatment Series. IWA Publishing, London (2007)
Van, D.P., Fujiwara, T., Tho, B.L., Toan, P.P.S., Minh, G.H.: A review of anaerobic digestion systems for biodegradable waste: configurations, operating parameters, and current trends. Environ. Eng. Res. (2019). https://doi.org/10.4491/eer.2018.334
Aslanzadeh, S., Rajendran, K., Taherzadeh, M.J.: A comparative study between single-and two-stage anaerobic digestion processes: effects of organic loading rate and hydraulic retention time. Int. Biodeterior. Biodegrad. 95, 181–188 (2014). https://doi.org/10.1016/j.ibiod.2014.06.008
Ramos-Suárez, J., Arroyo, N.C., González-Fernández, C.: The role of anaerobic digestion in algal biorefineries: clean energy production, organic waste treatment, and nutrient loop closure. In: Singh, B., Kuldeep, B., Faizal, B. (eds.) Algae and Environmental Sustainability, pp. 53–76. Springer, India (2015)
Mao, C., Feng, Y., Wang, X., Ren, G.: Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 45, 540–555 (2015). https://doi.org/10.1016/j.rser.2015.02.032
Trzcinski, A.P., David, C.S.: Microbial biomethane from solid wastes: principles and biotechnogical processes. In: Harzevili, F.D., Serge, H. (eds.) Microbial Fuels. pp. 77–151. CRC Press, Boca Raton (2017)
Ganesh, R., Torrijos, M., Sousbie, P., Lugardon, A., Steyer, J.P., Delgenes, J.P.: Single-phase and two-phase anaerobic digestion of fruit and vegetable waste: comparison of start-up, reactor stability and process performance. Waste Manag. (Oxford) 34(5), 875–885 (2014).
Xiao, B., Qin, Y., Wu, J., Chen, H., Yu, P., Liu, J., Li, Y.-Y.: Comparison of single-stage and two-stage thermophilic anaerobic digestion of food waste: performance, energy balance and reaction process. Energy Convers. Manag. 156, 215–223 (2018). https://doi.org/10.1016/j.enconman.2017.10.092
Schievano, A., Tenca, A., Scaglia, B., Merlino, G., Rizzi, A., Daffonchio, D., Oberti, R., Adani, F.: Two-stage vs single-stage thermophilic anaerobic digestion: comparison of energy production and biodegradation efficiencies. Environ. Sci. Technol. 46(15), 8502–8510 (2012). https://doi.org/10.1021/es301376n
Begum, S., Anupoju, G.R., Sridhar, S., Bhargava, S.K., Jegatheesan, V., Eshtiaghi, N.: Evaluation of single and two stage anaerobic digestion of landfill leachate: effect of pH and initial organic loading rate on volatile fatty acid (VFA) and biogas production. Bioresour. Technol. 251, 364–373 (2018). https://doi.org/10.1016/j.biortech.2017.12.069
APHA: Standard Methods for the Examination of Water and Wastewater. In. USA: Washington, D.C, (2012)
Dinh, P.V., Hoang, M.G., Pham Phu, S.T., Fujiwara, T.: Kinetics of carbon dioxide, methane and hydrolysis in co-digestion of food and vegetable wastes. Glob. J. Environ. Sci. Manag. 4(4), 401–412 (2018a). https://doi.org/10.22034/GJESM.2018.04.002
Dinh, P.V., Hoang, M.G., Pham Phu, S.T., Fujiwara, T.: A new kinetic model for biogas production from co-digestion by batch mode. Glob. J. Environ. Sci. Manag. 4(3), 251–262 (2018b). https://doi.org/10.22034/GJESM.2018.03.001
Basu, D., Asolekar, S.R.: Evaluation of substrate removal kinetics for UASB reactors treating chlorinated ethanes. Environ. Sci. Pollut. Res. 19(6), 2419–2427 (2012). https://doi.org/10.1007/s11356-012-0754-y
Shen, F., Yuan, H., Pang, Y., Chen, S., Zhu, B., Zou, D., Liu, Y., Ma, J., Yu, L., Li, X.: Performances of anaerobic co-digestion of fruit & vegetable waste (FVW) and food waste (FW): single-phase vs. two-phase. Bioresour. Technol. 144, 80–85 (2013). https://doi.org/10.1016/j.biortech.2013.06.099
Dinh, P.V., Fujiwara, T., Phu, S.T.P., Hoang, M.G.: Kinetic of biogas production in co-digestion of vegetable waste, horse dung, and sludge by batch reactors. In: IOP Conference Series: Earth and Environmental Science, vol. 1, p. 012041. IOP Publishing, Bristol (2018)
Gallert, C., Winter, J.: Mesophilic and thermophilic anaerobic digestion of source-sorted organic wastes: effect of ammonia on glucose degradation and methane production. Appl. Microbiol. Biotechnol. 48(3), 405–410 (1997). https://doi.org/10.1007/s002530051071
Braun, R., Huber, P., Meyrath, J.: Ammonia toxicity in liquid piggery manure digestion. Biotechnol. Lett. 3(4), 159–164 (1981). https://doi.org/10.1007/BF00239655
Boulanger, A., Pinet, E., Bouix, M., Bouchez, T., Mansour, A.A.: Effect of inoculum to substrate ratio (I/S) on municipal solid waste anaerobic degradation kinetics and potential. Waste Manag. (Oxford) 32(12), 2258–2265 (2012). https://doi.org/10.1016/j.wasman.2012.07.024
Parawira, W., Murto, M., Zvauya, R., Mattiasson, B.: Anaerobic batch digestion of solid potato waste alone and in combination with sugar beet leaves. Renew. Energy 29(11), 1811–1823 (2004). https://doi.org/10.1016/j.renene.2004.02.005
Chen, S., Zhang, J., Wang, X.: Effects of alkalinity sources on the stability of anaerobic digestion from food waste. Waste Manag. Res. 33(11), 1033–1040 (2015). https://doi.org/10.1177/0734242X15602965
Nielfa, A., Cano, R., Vinot, M., Fernández, E., Fdz-Polanco, M.: Anaerobic digestion modeling of the main components of organic fraction of municipal solid waste. Process Saf. Environ. Prot. 94, 180–187 (2015). https://doi.org/10.1016/j.psep.2015.02.002
Ravi, P.P., Lindner, J., Oechsner, H., Lemmer, A.: Effects of target pH-value on organic acids and methane production in two-stage anaerobic digestion of vegetable waste. Bioresour. Technol. 247, 96–102 (2018). https://doi.org/10.1016/j.biortech.2017.09.068
Wu, Y., Wang, C., Liu, X., Ma, H., Wu, J., Zuo, J., Wang, K.: A new method of two-phase anaerobic digestion for fruit and vegetable waste treatment. Bioresour. Technol. 211, 16–23 (2016). https://doi.org/10.1016/j.biortech.2016.03.050
Raynal, J., Delgenes, J., Moletta, R.: Two-phase anaerobic digestion of solid wastes by a multiple liquefaction reactors process. Bioresour. Technol. 65(1–2), 97–103 (1998). https://doi.org/10.1016/S0960-8524(98)00009-1
Möller, K., Müller, T.: Effects of anaerobic digestion on digestate nutrient availability and crop growth: a review. Eng. Life Sci. 12(3), 242–257 (2012). https://doi.org/10.1002/elsc.201100085
Romli, M., Greenfield, P., Lee, P.: Effect of recycle on a two-phase high-rate anaerobic wastewater treatment system. Water Res. 28(2), 475–482 (1994). https://doi.org/10.1016/0043-1354(94)90285-2
Diamantis, V., Aivasidis, A.: Kinetic analysis and simulation of UASB anaerobic treatment of a synthetic fruit wastewater. Glob. NEST J. 12(2), 175–180 (2010). https://doi.org/10.30955/gnj.000562
Math-Alvarez, J., Viturtia, A.M., Llabres-Luengo, P., Cecchi, F.: Kinetic and performance study of a batch two-phase anaerobic digestion of fruit and vegetable wastes. Biomass Bioenergy 5(6), 481–488 (1993). https://doi.org/10.1016/0961-9534(93)90043-4
Gerardi, M.H.: The Microbiology of Anaerobic Digesters. Wiley-Interscience, New Jersey, USA (2003)
Acknowledgements
The authors would like to thank the Okayama University (Japan) and National University of Civil Engineering (vietnam) for their financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pham Van, D., Takeshi, F., Hoang Minh, G. et al. Comparison Between Single and Two-Stage Anaerobic Digestion of Vegetable Waste: Kinetics of Methanogenesis and Carbon Flow. Waste Biomass Valor 11, 6095–6103 (2020). https://doi.org/10.1007/s12649-019-00861-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-019-00861-0