Abstract
Oil palm trunk (OPT) is one of the richest biomass in Southern Thailand. The OPT residues (OPTr), obtained after separation of the sap, was hydrolyzed by the crude enzymes of the selected fungal isolates under solid-state fermentation (SSF) and submerged fermentation (SmF). From screening and identification studies, Ceratocystis paradoxa TT1 produced the highest CMCase activity (18.16 Unit gds−1) from SmF while Trichoderma koningiopsis TM3 exhibited the highest xylanase and FPase activities (56.46 and 2.13 Unit gds−1, respectively) from SSF. Enzymatic hydrolysis of OPTr using the crude enzymes of T. koningiopsis TM3 (25 Unit gds−1) at 50 °C for 15 h revealed the maximum reducing sugars of 11.92 g L−1 with the yield of 0.48 gg−1. Bioconversion of the OPTr hydrolysate to ethanol by Saccharomyces cerevisiae TISTR5055 could be increased (2.13-fold) by supplementation of YM nutrients. The effects of co-cultures (S. cerevisiae TISTR5055 and Acetobacter aceti) for production of co-products (ethanol and acetic acid) from the OPTr hydrolysate with and without YM nutrients addition was conducted under two-stage fermentation and simultaneous fermentation. Both fermentation types gave similar ethanol production (4.15 and 4.01 g L−1) but the ethanol productivity (0.34 g L−1 h−1) from two-stage fermentation was 1.55-fold higher than that from simultaneous fermentation. On the other hand, the maximum acetic acid production (2.12 g L−1) and productivity (0.09 g L−1 h−1) were achieved from the simultaneous fermentation of the co-cultures without nutrient addition. These two values were 1.7-fold higher acetic acid concentration and 4.4-folds higher productivity than the two-stage fermentation.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
Felled oil palm trunks (OPT) were plantation waste never been utilized before. This research work initiated the valorization of felled OPT to produce ethanol and acetic acid. No previous knowledge of suitable fermentation process of substrate derived from OPT. This work compares the two-stage and simultaneous fermentation. The results showed that the two-stage process is superior to the simultaneous process in ethanol production (4.15 g L−1), but not for acetic acid (2.12 g L−1 for simultaneous process). Combination of the naturally fermentation of the original OPT sap could increase the total acetic acid to 5.12 g L−1. This concentration level of acetic acid was reported to be effective for treatment of chronic wounds based on antibiofilm properties
Introduction
Lignocellulosic biomass is reported to be one of the alternative source for fossil fuels [1]. Utilization of biomass can address the environmental issues such as global warming, extreme climate change, acid rain and human health deterioration [2]. Thermochemical process is commonly used for hydrolysis of cellulosic biomass to produce sugars [3, 4] but it causes further conversion of the released sugars to other by-products such as furfural and 5-hydroxymethylfurral (5-HMF) that were reported to be inhibitors and interfered with microbial fermentation [5, 6]. Enzymatic hydrolysis of lignocelluloses has been discussed extensively in the literature [4, 7,8,9]. It is preferred to chemical hydrolysis because enzymes display the high substrate and reaction specificity, operate under mild conditions and do not generate by-products [10, 11]. The bioconversion of lignocellulose to fermentable sugars requires the synergistic action of complete cellulase system which act randomly on soluble and insoluble cellulose chains [12,13,14,15] and also for hemicellulose degradation [16]. Considering the potential for biotechnological applications of cellulases and xylanases, the prospect of new microbial source is important, especially regarding filamentous fungi that are excellent enzymes producers [17]. The successful strategy to produce lignocellulolytic enzymes can be achieved through microorganism selection and improved fermentation process conditions. These include screening for effective lignocellulolytic enzyme-producing microbes and developing pre-treatments that alter the cellulose lattice structure and increase enzyme accessibility. It is therefore necessary to search for microorganisms that have a high rate of lignocellulolytic enzymes production [18]. In this sense, fungi are a promising group considering that most genera have been little explored as hydrolases producers and they most likely produce enzymes with interesting characteristics in terms of access and attack to the polysaccharides of plant cell walls, since they have to invade and colonize plant tissues [19]. Most of the studies regarding the production of plant degrading enzymes by fungi involve cultivation on solid media and qualitative evaluation of substrates hydrolysis [20]. Quantitative analysis by submerged fermentation (SmF) is also cited [21]. However, solid-state fermentation (SSF) is advantageous in some aspects including higher yields in a shorter time and the use of lignocellulosic residues as substrates [22].
In Thailand, oil palm is one of the most important oil crops, the area of oil palm plantations was 882,389 ha, mainly in the South (767,677 ha) accounted for 87% of the total area [23]. Plantation area also generates large amounts of solid wastes such as oil palm trunk (OPT) and oil palm fronds (OPF) and they are cut and discarded or burnt at the plantation site [24]. To utilize the felled OPT for biofuel production, there are two sources of the substrates; the OPT sap and the OPT residues. Large quantities of OPT sap contains many simple sugars particularly xylose and glucose [24, 25]. Furthermore, the estimated cellulosic residue remaining after pressing of the trunks was approximately 30% (ww−1) of the trunk without bark [26, 27]. Three main lignocellulosic components of OPT contain 29–46% cellulose, 12–26% hemicelluloses and 10–24% lignin [28,29,30]. In term of its high content of holocellulose (41–72%), OPT is a potential source for production of lignocellulolytic enzymes [30] and fermentable sugar for production of biofuels. Besides ethanol, acetic acid is another important intermediate compound for industrial production of many chemicals such as vinyl acetate polymer, cellulose acetate, terephthalic acid, dimethyl terephthalate, acetic acid esters/acetic anhydride and calcium magnesium acetate. All these products are produced from petroleum-derived acetic acid [31]. The biological route via bacterial fermentation to produce acetic acid for food industry (i.e. vinegar) remains important as it can be used as flavoring agent and food preservation [32]. The production of acetic acid from sustainable biomass or biomass-based platforms has gained attention and extensively studied. Bioconversion of lignocellulosic sugars in hydrolysates from wheat straw, forest residues, switch grass and sugarcane straw to acetic acid by Moorella thermoacetica revealed that sugarcane straw hydrolysate gave the highest acetic acid yield (17 g L−1 from 24 g L−1 total sugars) [33] or 0.71 g g−1 total sugar. Typical acetic acid production in a batch fermentation using purified glucose (20 g L−1) is around 13 g L−1 [34] or 0.65 g g−1 glucose. To the best of our knowledge, the studies on conversion of OPT into ethanol, lactic acid, hydrogen and butanol have recently been reported [24, 25, 35, 36], there is no any report available for bioconversion of OPT to acetic acid.
The objectives of this study are (i) to screen the high enzymes producing fungi from the natural fermented felled oil palm trunk (OPT) through SSF and SmF, (ii) to study the enzymatic hydrolysis of OPT residues (OPTr) (iii) to investigate on the effects of fermentation type (two-stage fermentation vs simultaneous fermentation) and nutrient supplementation for production of co-products (ethanol and acetic acid), with acetic acid as main product, from the OPT hydrolysate using the co-cultures of S. cerevisiae TISTR5055 and Acetobacter aceti.
Materials and Methods
Chemical Composition of Oil Palm Trunk (OPT) and Preparation of OPT Residues (OPTr)
Oil palm tree, approximately 25 years old, was obtained from local oil palm plantation at Khao Phanom District, Krabi Province, Thailand. The old or felled OPT (9 m long) was cut into five parts based on its height;top end (or tip) (a), top (b), middle (c), bottom (d), and bottom end (e), as shown in Fig. 1. The strong outer part of the OPT (bark) was removed and only the soft inner part of each part of OPT except the bottom end part was ground (using a grinder) and used for determination of OPT composition (as shown in Table 1).
The OPTr was prepared by grinding the selected part of OPT (top (b) and middle part (c); mixed in the ratio 1:1 (ww−1)) and soaking in distilled water in the ratio of 1:1 (w v−1) for 1 h, then pressed through a screw press to obtain OPT sap (or OPS) and OPT residues (OPTr) [28]. The OPTr were sun-dried for 1 day, then dried in a hot air oven (68 °C) for 3 days until obtain constant weight. They were kept as feedstock in sealed plastic containers at room temperature (30 ± 2 °C) until used.
Isolation, Selection and Identification of the High Enzyme Producing Fungi
Ground samples from each part of OPT and surface scrapped residues of OPT plank (1.5 inch thick × 4 inch wide × 2 m long) were naturally fermented (without inoculum added) at room temperature (30 ± 2 °C) for about 3 days or until growth of microorganisms appeared. All samples were added with 0.1% Tween 80 (10 mL g−1 of fermented substrate) and the mixture was shaken (150 rpm) for 30 min [37], then made dilution to 10−6–10−7. The diluted culture suspension (0.1 mL) was inoculated onto PDA plates using spread-plate technique and incubated at room temperature (30 ± 2 °C). The fungal isolates were purified through repeated streaking on fresh PDA plates and pure cultures were maintained on PDA slant.
Screening was based on the ability of the fungal isolates to utilize ground OPTr (1% w v−1) as substrate for growth and enzymes production under solid-state fermentation (SSF) and submerged fermentation (SmF) using the modified CDM [38] with and without agar respectively. The fungal isolates were streaked on the agar plates and incubated at room temperature (30 ± 2 °C) for 5 days. The selected fungal isolates were subcultured on fresh PDA slant. They were cultivated under SSF using OPTr as substrate with supplementation of CDM nutrients solution (5 mL g−1 dry substrate (gds)). After sterilization, spore suspension at 106 spores gds−1 was inoculated and the moisture content was adjusted to 60% [39,40,41], then incubated for 4 days at room temperature (30 ± 2 °C). Crude enzymes were extracted from the OPTr culture, filtered to remove the mycelium by using cheesecloth [42] and centrifuged (1789×g for 10 min) to obtain a clear supernatant [43]. Aliquots of the supernatant was diluted to appropriate concentration for determination of enzymes activity of cellulase (CMCase), xylanase and FPase.
For submerged fermentation (SmF), spore suspension (106 spores gds−1) was inoculated into the modified CDM containing 5% (wv−1) of OPTr and incubated at room temperature (30 ± 2 °C) for 4 days. Samples were taken for determination of enzymes activities (as described above).The isolates producing the highest enzymes activity were selected for further studies. The selected strains were identified by 18S rRNA gene sequence [44] and the sequences were BLAST searched against the National Center for Biotechnology Information (NCBI) database.
Enzymes Production from the Three Selected Strains and the Mixed Cultures
Time course on enzymes production from the three selected fungal strains and the mixed-cultures cultivated in MMS medium [45] using OPTr as a carbon source was conducted under SSF and SmF at room temperature (30 ± 2 °C) for 7 days. The strain and the fermentation type (either SmF or SSF) giving the highest enzymes activity were selected for further studies.
The enzyme production from the selected strain was carried out under SSF condition (as described above). The spore suspension was inoculated to OPTr supplemented with MMS medium (ratio 1:1 wv−1) in 250 ml Erlenmeyer flasks. Samples were taken and centrifuged to remove the cells, then extracted the enzymes from the supernatant by adding cold acetone (4 °C) at the ratio of 1:4 wv−1 (supernatant: acetone) and allowed precipitation to occur at − 20 °C overnight [46]. The precipitate was dissolved in minimal amount of 50 mM citrate buffer (pH 4.8) and its enzyme activity was determined.
Effect of Enzyme Loading During the Hydrolysis of OPTr
The 2.5% (wv−1) ground OPTr was added into the citrate buffer (50 mM, pH 4.8) and sterilized (121 °C for 15 min). After cooling, the enzymes at various concentrations were added to obtain the enzyme loading of 0–40 Unit g−1OPTr. The enzyme–substrate mixtures were incubated at 50 °C for 24 h under constant shaking (150 rpm) condition. Samples were taken at time interval and determined for sugar concentration using HPLC [24] and reducing sugar using DNS method [47].
Two-Stage and Simultaneous Fermentation of OPTr Hydrolysate for Production of Ethanol and Acetic Acid Using Saccharomyces cerevisiae and Acetobacter aceti
The OPTr hydrolysate with and without supplementation of YM nutrients (yeast extract 3.0 g L−1, malt extract 3.0 g L−1 and peptone 5.0 g L−1) was used for ethanol and acetic acid production under two-stage fermentation and simultaneous fermentation using S. cerevisiae TISTR5055 and A. aceti. In two-stage process, the starter culture of S. cerevisiae (OD600 = 0.5) was inoculated (10% v v−1) into 250 mL Erlenmeyer flask containing the OPTr hydrolysate (total working volume of 50 mL), with and without addition of nutrients and incubated at room temperature (30 ± 2 ̊C) for 24 h and sample (5 mL) was taken to determine for ethanol production. After that, the starter culture of A. aceti (OD600 = 0.5) was inoculated (10% v v−1) for acetic acid production in the second stage and incubated until 60 h. Samples were taken at time interval (every 6 h) and the supernatant was analyzed for sugars, ethanol and acetic acid concentration using HPLC [24].
For simultaneous fermentation, starter cultures of S. cerevisiae and A. aceti (OD600 = 0.5) were mixed in 1:1 ratio (vv−1)) and inoculated (10% v v−1) into 250 Erlenmeyer flask containing the OPTr hydrolysate (total working volume of 50 mL), with and without addition of YM nutrients. They were incubated at room temperature (30 ± 2 °C) for 36 h. During fermentation, samples were withdrawn (every 6 h) and its supernatant was analyzed for sugar, ethanol and acetic acid concentration using HPLC [24].
Analytical Methods
Enzyme Activity Assay
Carboxymethylcellulase (CMCase) was assayed in reaction containing 1% (wv−1) of CMC (0.5 mL) in 50 mM citrate buffer pH 4.8 and appropriate diluted enzyme (0.5 mL). After 30 min incubation at 50 °C, reducing sugar was measured by 3,5-dinitrosalicylic acid method [44] with glucose was used as standard [48, 49]. One unit (U) of CMCase activity is defined as the amount of enzymes that liberates 1 µmol of glucose equivalents per minute.
Xylanase activity was assayed in reaction containing 1% (wv−1) of oat spelt xylan (0.5 mL) in 50 mM citrate buffer pH 4.8 and appropriate diluted enzyme (0.5 mL). After 10 min incubation at 50 °C, reducing sugar was measured by 3,5-dinitrosalicylic acid (DNS) method [44] with xylose was used as standard [48, 49]. One unit (U) of xylanase activity is defined as the amount of enzymes that liberates 1 µmol of xylose equivalents per minute.
Exoglucanase (FPase) assay was carried out by incubating 0.5 ml suitably diluted crude enzyme with 1 mL citrate buffer (50 mM, pH 4.8) containing Whatman Filter paper (No.1) strip (1 cm × 6 cm, 50 mg) and incubation at 50 °C for 60 min. Then, reducing sugar was measured by 3,5-dinitrosalicylic acid (DNS) method [44] with glucose used as standard. One unit of FPase activity correspondent to 1 µmol of glucose released per minute [30].
Determination of Sugars, Ethanol and Acetic Acid Concentration
The concentrations of hexose (glucose), pentose (xylose, arabinose), ethanol and acetic acid were determined using high performance liquid chromatography (HPLC) (Agilent 1200) equipped with a HPX-87H (300 mm × 7.8 mm) column (Bio-Rad, Hercules, CA) and a refractive index (RI) detector. The sample was diluted with deionized water, filtered through 0.22 µm, 13 mm Nylon membrane filter (Sartorius, Goettingen, Germany) and then injected in the chromatograph under the following conditions: column temperature at 65 °C, 5 mM sulfuric acid as mobile phase at a flow rate of 0.6 mL min−1, and an injection volume of 20 µL. The concentration of these compounds was calculated using calibration curves obtained from standard solutions [24]. Data shown were the average of three replicated assessments.
Statistical Analysis
The data presented were analyzed using SPSS (SPSS Inc., version 15.0). One-way analysis of variance (ANOVA) was carried out to compare the means of different treatment where significant F value was obtained. Differences between individual means were tested using Duncan’s Multiple Range Test (DMRT) at 0.05 significant levels. The data was performed in triplicate.
Results and Discussion
Chemical Composition of OPT and Preparation of OPTr
The chemical composition of cross section of OPT was previously reported [1, 25, 28] but not yet the composition along its length. In this study, the chemical composition of 9 m long OPT (weight of 1786 kg) divided into five parts (see Fig. 1) and its four parts were analyzed (Table 1). The OPT was found to accumulate starch with the highest quantity at the top end (tip) part (1 m long, diameter 42 cm) (3.79% ww−1) followed by the top part (2 m long, diameter 45 cm) (2.78% w w−1). The values were similar to the average starch content of the middle part of the whole log (2.5 m long and 36–41 cm in diameter) (3.5% of dried solid OPT disc) [50]. The moisture content of OPT decreased along its height with the highest value at the bottom part (about 65%) was lower than that reported by Noparat et al. [28] (about 75%). In addition, the total amount of lignocelluloses of OPT was also highest at the bottom part (87.71%) and decreased along its height (59.10, 37.16 and 26.15%, respectively). The holocellulose content of OPT (13–55% cellulose, 12–20% hemicellulose) was comparable with that reported by Ang et al. [30] (45.81% cellulose, 17.74% hemicellulose) but the lignin content was much lower (1.30–13.00% compared to 24.49%).Based on the composition of high holocellulose with low lignin content, the top and middle parts of felled OPT were selected as feedstock for enzyme production.
The OPT sap contained glucose as the dominant sugar (4.96–20.13 g L−1) followed by fructose (3.04–6.02 g L−1) (Table 1). The highest values of glucose (20.13 g L−1) was 2.5 times lower while fructose (6.02 g L−1) was two times higher than the values of Malaysian oil palm sap (50.17 g L−1 glucose and 3.07 g L−1 fructose, respectively) [25]. The amount of glucose in this study was about 1.3 times higher than that reported by Noparat et al. [28] (15.72 g L−1). Arabinose was present only in trace amount (0.43–0.84 g L−1) in the top end and top part of OPT. In contrast to OPT sap, the sap of Raphia palm (Raphia hookeri) contained sucrose as the dominant sugar [51]. The discrepancy may be due to the difference in varieties, species and/or cultivating conditions.
Isolation, Selection and Identification of the High Enzyme Producing Fungi
A total of 20 fungal strains were isolated from the natural fermented OPT and the plank samples. Eight out of the 20 fungal isolates were able to grow on CDM containing ground OPTr as carbon source and encoded as the isolate TT1, TT2, TT3, TT4, TT5, TM1, TM2 and TM3.They were screened for their ability to produce cellulase and xylanase under SSF and SmF (Fig. 2). The isolate TT1 exhibited the highest CMCase activity under SmF (12.20 Unit gds−1with 2.3 times higher than its highest value under SSF (5.37 Unit gds−1). The isolate TM3 gave the highest xylanase activity under SmF (23.06 Unit gds−1) which was 1.7 times higher than under SSF (13.91 Unit gds−1). The isolates TT2 gave the highest FPase activities both under SSF (1.66 Unit gds−1) and SmF (1.54 Unit gds−1). Based on these results, the isolates TT1, TM3 and TT2 were selected as the producer of CMCase (12.20 Unit gds−1 from SmF), xylanase (23.06 Unit gds−1 from SmF) and FPase (1.66 Unit gds−1 from SSF), respectively.
Comparison on enzymes production from the eight selected fungal strains cultivation in CDM medium containing oil palm trunk residues (OPTr) as a carbon source under solid-state fermentation (SSF) (a, c, e) and submerged fermentation (SmF) (b, d, f) after cultivation at room temperature (30 ± 2 °C) for 4 days
The three isolates possessing high enzyme activity were identified using 18S rRNA gene sequence and NCBI blast search (Fig. 3). It showed a 100% sequence identity of the isolate TT1 with Ceratocystis paradoxa (KJ881375) that was reported as a sugarcane phytopathogen [52] causing black seed rot disease in oil palm sprouted seeds [53] as well as bud and trunk rots affecting almost all species of palm [54, 55].The isolate TM3 exhibited 98% sequence identity with Trichoderma koningiopsis T-404 (JQ278019). Strains of Trichoderma can accumulate high activities of endo- and exo-glucanases, but are poor in β-glucosidases [56]. Additionally, T. koningiopsis Th003 was able to induce the activity of β-1,3-glucanase and endo-chitinases to control different pathogens including the system tomato-Fusarium oxysporum and stimulate growth in many crops [57]. The growth of some phytopathogenic fungi could be inhibited by the chemicals (volatile substances such as alkanes, monoterpenes and arenes, heterocycles, and aldehydes) produced by T. koningiopsis YIM PH30002 [58]. These chemical compounds possessed the antifungal activity, nitric oxide inhibition and anticoagulant activity [59]. The isolate TT2 exhibited 95% sequence identity with Hypocrea nigricans NBRC 30611 (JN941681). H. nigricans is an anamorph of Trichoderma sp., by nature a wood decaying fungus and a common fungal species of moist forests [60]. In addition, Hypocrea species was used in the biological control of plant pathogenic fungi; many species have the ability to break down cellulosic materials. This ability has led to the commercial exploitation of some Hypocrea and Trichoderma species in production of cellulolytic enzymes used in manufacture of denims, animal feed and bio fuels [60, 61]. Under the optimum condition, H. nigricans produced higher endo- and exo-β-1,4-D-glucanases (from 3.9 to 11.89 Ug−1 and 2.98 to 11.89 Ug−1, respectively) [60]. Besides the three selected isolates (TT1, TM3 and TT2), the other five isolates were identified;TT3, TT5 and TM1 were belonged to the genus T. asperellum and the isolate TM2 and TT4 were with the genus Aspergillus niger and A. tubingensis, respectively.
Enzymes Production from the Selected Strains and the Mixed Cultures
Improvement in lignocellulolytic enzymes production could be achieved via the mixture of different fungi. Mixed-culture is beneficial in lignocellulolytic enzymes production via SSF as the fungi are normally co-existed symbiotically on solid substrates in nature [62, 63]. Besides, mixed-culture also offers advantages such as higher productivity, adaptability and substrate utilization compared to pure and monoculture [64]. In this study, time course on enzymes production from the three selected fungal strains and the mixed-cultures (TT1:TT2:TM3 in the ratio of 1:1:1) cultivated in MMS medium using OPTr as a carbon source under SSF and SmF at room temperature (30 ± 2 °C) for 7 days was conducted. The maximum enzymes activities of all three strains cultivated under SSF and SmF were summarized in Table 2. C. paradoxa TT1 showed the highest CMCase, xylanase and FPase of 18.16, 36.99 and 1.64 Unit gds−1at 4 days cultivation under SmF. These were 1.5, 1.6 and 2.3 times higher than those cultivated in CDM medium. C. paradoxa was reported to show the highest xylanase activity when grown on wheat bran (12,728 IU mL−1) and β-glucosidases when grown on steam-treated bagasse (1068 IU mL−1) [49]. T. koningiopsis TM3 gave the highest xylanase and FPase (56.46 and 2.13 Unit gds−1 at 3 days cultivation). Therefore, C. paradoxa TT1 was cellulase producer while T. konigiopsis TM3 was xylanase (and FPase) producer. The lower enzymes activities of the mixed cultures than those from the monoculture may be due to the undesirable competition between the fungal strains [65]. Trichoderma species were reported to produce both volatile and nonvolatile metabolites that adversely affect growth of different fungi such as the fungistatic effects on the growth of C. paradoxa [53]. In addition, the inoculation sequence of different fungal strains might play a significant role in stimulating the enzyme production [66] or imposing any significant negative effect on the growth of each other [67]. Furthermore, the inoculating time of the fungal strains also had an impact on enzyme production. For examples, the co-cultivation of T. reesei RUT-30 and Phanerochaete chrysosporium exhibited the maximum cellulase activity when inoculation time was delayed for 1.5 day which correlated to the higher saccharide yields than those from monoculture [68]. Similarly, the maximum cellulase activity (3.2 IU g−1) was obtained when A. oryzae was co-cultured on soybean fiber with T. reesei and P. chrysosporium after 36 h incubation [69].
Comparison on enzymes production from this study to the other fungal strains is given in Table 3. It should be mentioned that the different values of enzyme activity were partly due to the difference in enzyme activity assay. Considering among oil palm wastes, C. paradoxa TT1 produced 2.23-fold higher CMCase activities than that of Botryosphaeria sp. (8.13 Unit g−1) [43] but lower than that of A. turingensis TSIP9 (26.10 Unit g−1) [63]. T. koningiopsis TM3 produced 1.60-fold higher xylanase (56.46 Unit gds−1) than Aspergillus niger USM Al 1 (35 Unit gds−1) [70] and slightly lower than A. turingensis TSIP9 (59.3 Unit gds−1) [71]. In this present study, T. koningiopsis TM3 was the most suitable for lignocellulase enzymes production from the OPTr with higher enzymes production (7.13 Unit g−1 of CMCase, 56.46 Unit g−1 of xylanase and 2.13 Unit g−1 of FPase activity) than those selected strains.
Effect of Enzyme Loading During the Hydrolysis of OPTr
The crude enzymes from the supernatant of T. koningiopsis TM3 was concentrated using acetone which was reported to be better than ammonium sulphate [46]. The concentrated enzyme TM3 possessed CMCase and xylanase activities of 3.22 and 54.14 Unit mL−1, respectively, with the yields of 60.04% and 68.74%, respectively (data not shown).
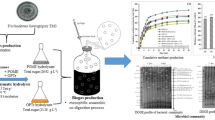
The effect of enzymes TM3 concentrations (0–40 Unit g−1OPTr) and reaction times (0–24 h) for hydrolysis of OPTr was studied. Figure 4 shows the optimum enzyme concentration at 25 Unit g−1 OPTr and 15 h reaction time, giving the highest reducing sugars concentration of 11.92 g L−1 with the yield of 476.8 mg g−1 OPTr. The increase of enzymes concentration from 5 to 25 Unit g−1 substrate resulted in the increase of reducing sugar conversion from 13.7% to 42.9% and dropped to 33.7% and 37.8% conversion at 30 and 40 Unit g−1 OPTr, respectively. Similar pattern was reported by Zulkefli et al. [1] that the hydrolysis of OPT did not improve the conversion further when loading the high amount of enzymes. The maximum concentration of this study (11.92 g L−1) was about 4.5 times higher than that from hydrolysis of pretreated empty fruit bunch (EFB) (2.4 g L−1 after 24 h reaction time) using the ratio of cellulase to β1-4 glucosidase of 5:1 while the productivity was times lower when higher β 1-4 glucosidase has been added into the reaction mixture (ratio 1:5) [73]. Higher enzyme loading giving lower reducing sugar conversion may be caused by the decrease of adsorption efficiency due to the saturation of enzyme on cellulose surface [74]. However, some studies showed the increase of sugar production at higher enzyme loading [75, 76]. Therefore, the enzyme hydrolysis reaction should be performed under the optimum condition (enzyme concentration and reaction time) and based on the cost. The enzymes from this strain exhibited higher saccharification efficiency on the OPTr than those from many brown rots fungi reported earlier (Table 4). This result showed that the OPTr was a better substrate than the other agricultural wastes (rice straw, wheat straw, etc.) as it gave the highest yield of reducing sugar without addition of the commercial enzyme (i.e. Novozyme). The sugar yield of this strain was almost 15 times higher than that of Formitoplis palustris acting on Avicel (32 mg g−1 after 43 h) [77].
Two-Stage and Simultaneous Fermentation of OPTr Hydrolysate to Ethanol and Acetic Acid Using S. cerevisiae and A. aceti
Ethanol production from plant biomass has received considerable attention because of the expectation that bioethanol will alleviate demands for petroleum-based fuels [80]. The hydrolysis of lignocellulosic biomass liberates sugars, primarily glucose and xylose, which are subsequently converted to ethanol by microbial fermentation [81]. S. cerevisiae has been used in the industrial bioethanol production due to its robustness and high ethanol productivity. However, this yeast strain cannot naturally ferment xylose [82] while Candida shehatae can utilize both glucose and xylose as substrates for ethanol fermentation [83]. However, our preliminary studies on ethanol production from OPTr hydrolysate using the co-culture of S. cerevisiae TISTR5055 and C. shehatae TISTR5843 produced lower ethanol concentration than the mono-culture (data not shown). As a consequence, only S. cerevisiae TISTR5055 was employed for ethanol production.
In this study, ethanol and acetic acid were produced by two-stage fermentation and simultaneous fermentation of the OPTr hydrolysate, with and without addition of nutrients in YM medium (Fig. 5). In two-stage process, ethanol production by S. cerevisiae increased and rather stable during 12–24 h, then A. aceti was inoculated. The ethanol production was continued and reached the highest value of 2.87 g L−1 at 36 h. A. aceti assimilated ethanol and started to produce acetic acid after 30 h and reached the highest value (1.23 g L−1) at 54 h with simultaneous depletion of ethanol (Fig. 5a). With nutrients supplementation (Fig. 5b), ethanol production increased sharply and reached the maximum value (4.15 g L−1) at 12 h fermentation with the yield of 0.35 g ethanol g−1 sugar consumed. Hence, the ethanol production increased by 1.43-folds while its productivity increased by 4.27-folds. This was due to the influence of some nutrients that were essential for cell growth and metabolite production [84]. Higher ethanol production was reported from using empty fruit bunch (EFB)-derived sugar by S. cerevisiae (12.13 g L−1) [85] and from crude glycerol supplemented with yeast extract by Kluyvera cryocrescens (about 11 g L−1) [86]. In addition, the ethanol yield from this study (0.35 g g−1) was slightly lower than those produced from EFB hydrolysate (via dilute-acid hydrolysis) fermented by S. cerevisiae (0.46 g g−1) and Mucor indicus (0.45 g g−1) [87]. In two-stage process, there was no production of acetic acid which may be due to the growth inhibition of A. aceti by too high concentration of ethanol.
Time course of ethanol and acetic acid production by Saccharomyces cerevisiae TISTR5055 and Acetobacter aceti under two-stage (a, b) and simultaneous (c, d) fermentation from oil palm trunk hydrolysate, with (a, c) and without addition of nutrients (b, d) in shake-flask (150 rpm) culture at room temperature (30 ± 2 °C)
For simultaneous fermentation of OPTr, the maximum values of ethanol and acetic acid were 2.87 g L−1 at 18 h and 2.12 g L−1 at 24 h, respectively, with the productivity of 0.16 and 0.09 g L−1h−1 (Fig. 5c). The maximum acetic acid concentration and productivity were 1.7-fold and 4.0-fold higher than those from the two-stage fermentation. Addition of nutrients to the OPTr hydrolysate also exhibited strong influence on ethanol production (4.01 g L−1 at 18 h) and acetic acid production (1.81 g L−1 at 36 h) (Fig. 5d). Therefore, co-culture of S. cerevisiae and A. aceti in the OPTr hydrolysate without nutrients supplementation could increase the ethanol production from monoculture of S. cerevisiae by only 7% (from 2.68 to 2.87 g L−1) but gave 2.15-fold increase in ethanol productivity (0.074 to 0.159 g L−1 h−1). The production efficiency (productivity) was considered from the cultivation time required to reach the maximum values (co-culture reached within 18 h while the monoculture required 36 h). Nutrient supplementation to the monoculture could further enhance by 1.4-fold both the ethanol concentration (from 2.87 to 4.01 g L−1) and ethanol productivity (from 0.159 to 0.223 g L−1 h−1). However, the nutrients supplementation had an adverse effect on acetic acid production as it decreased the acetic acid concentration by 1.2-fold (from 2.12 to 1.81 g L−1) and also the productivity (from 0.09 to 0.05 g L−1 h−1). Acetic acid production, on the other hand, reduced ethanol production as the ethanol was oxidized to acetic acid. However, the decrease of acetic acid production may be due to the sharp increase of ethanol within the first 6 h (corresponding to the rapid consumption of glucose) and also the higher concentration of ethanol. These factors may affect the growth of acetic acid bacteria. Therefore, simultaneous fermentation using co-culture of S. cerevisiae and A. aceti could increase the ethanol productivity as well as acetic acid productivity by supplementation of nutrients to the system. The co-culture was more efficient in conversion of reducing sugars to ethanol. Furthermore, for efficient bioconversion of biomass-derived sugars to acetic acid, it is crucial to have proper balance of sugars in the hydrolysate [33].
Conclusion
The OPTr is a potential feedstock for enzymes, ethanol and acetic acid production. OPTr was used as substrate for production of lignocellulolytic enzymes by various isolated fungal strains through SSF and SmF. C. paradoxa TT1and T. koningiopsis TM3 produced the highest CMCase from SmF and xylanase from SSF, respectively. Then the OPTr was hydrolyzed using the crude enzyme TM3 (25 Unit g−1) and incubated at 50 °C for 15 h. The OPTr hydrolysate with nutrients supplementation showed the maximum ethanol production (4.1 g L−1) under the two-stage fermentation. The maximum acetic acid concentration (2.12 g L−1) and productivity (0.09 g L−1h−1) was achieved from the simultaneous fermentation without nutrient addition. The integration of OPTr utilization for production of both enzymes and biofuel will not only reduce the enzyme cost but also generate ethanol and acetic acid as its additional valuable products.
References
Zulkefli, S., Abdulmalek, E., Rahman, M.B.A.: Pretreatment of oil palm trunk in deep eutectic solvent and optimization of enzymatic hydrolysis of pretreated oil palm trunk. Renew. Energy 107, 36–41 (2017)
Tan, K.T., Lee, K.T., Mohamed, A.R.: Role of energy policy in renewable energy accomplishment: the case of second-generation bioethanol. Energy Policy 36, 3360–3365 (2008)
Almeida, J.R., Modig, T., Petersson, A., Hahn-Hagerdal, B., Liden, G., Gorwa-Grauslund, M.F.: Increases tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J. Chem. Technol. Biotechnol. 82, 340–349 (2007)
Hassan, O., Ling, T.P., Maskat, M.Y., Illias, R.M., Badri, K., Jahim, J.: Optimization of pretreatments for the hydrolysis of oil palm empty fruit bunch fiber (EFBF) using enzyme mixtures. Biomass Bioenerg. 56, 137–146 (2013)
Panagiotou, G., Olsson, L.: Effect of compounds released during pretreatment of wheat straw on microbial growth and enzymatic hydrolysis rates. Biotechnol. Bioeng. 96(2), 250–258 (2007)
Lenihan, P., Orozco, A., O’Neill, E., Ahmad, M.N.M., Rooney, D.W., Walker, G.M.: Dilute acid hydrolysis of lignocellulosic biomass. Chem. Eng. J. 156, 395–403 (2010)
Cui, X., Zhao, X., Zeng, J., Loh, S.K., Choo, Y.M., Liu, D.: Robust enzymatic hydrolysis of formiline-pretreated oil palm empty fruit bunches (EFB) for efficient conversion of polysaccharide to sugars and ethanol. Bioresour. Technol. 166, 584–591 (2014)
Maitan-Alfenas, G.P., Visser, E.M., Guimarães, V.M.: Enzymatic hydrolysis of lignocellulosic biomass: converting food waste in valuable products. Curr. Opin. Food Sci. 1, 44–49 (2015)
Palamae, S., Dechatiwongse, P., Choorit, W., Chisti, Y., Prasertsan, P.: Cellulose and hemicelluloses recovery from oil palm empty fruit bunch (EFB) fibers and production of sugars from the fibers. Carb. Polymer. 155, 491–497 (2017)
Micard, V., Renard, C.M.G.C., Thibault, J.-F.: Enzymatic saccharification of sugar beet pulp. Enzyme Microb. Technol. 19, 162–170 (1996)
Zieminski, K., Romanowska, I., Kowalska, M.: Enzymatic pretreatment of lignocellulosic wastes to improve biogas production. Waste Manag 32, 1131–1137 (2012)
Milala, M.A., Shugaba, A., Gidado, A., Ene, A.C., Wafer, J.A.: Studies on the use of agricultural wastes for cellulase enzyme productions by Aspergillus niger. Res. J. Agr. Biol. Sci. 1, 325–328 (2005)
Bansal, N., Tewari, R., Gupta, J.K., Soni, S.K., Soni, R.: A novel strain of Aspergillus niger producing a cocktail of industrial deploy merising enzymes for the production of second generation biofuels. Bio Res. 6, 552–569 (2011)
Deswal, D., Khasa, Y.P., Kuhad, R.C.: Optimization of cellulose production by a brown rot fungus Fomitopsis sp RCK2010 under solid state fermentation. Bioresour. Technol. 102(10), 6065–6072 (2011)
Bansal, N., Tewari, R., Soni, R., Soni, S.K.: Production of cellulases from Aspergillus niger NS-2 in solid state fermentation on agriculture and kitchen waste residues. Waste Manag 32, 1341–1346 (2012)
Kumar, R., Singh, S., Singh, O.V.: Bioconversion of lignocellulosic biomass: biochemical and molecular perspectives. J. Ind. Microbiol. Biotechnol. 35(5), 377–391 (2008)
Marques, N.P., Pereiar, J.C., de Gomes, E., Silva, R., Araujo, A.R., Ferreira, H., Rodrigues, A., Dussan, K.J., Bocchini, D.A.: Cellulase and xylanases production by endophytic fungi by solid state fermentation using lignocellulosic substrates and enzymatic saccharification of pretreated sugarcane bagasse. Ind. Crop. Prod. 122, 66–75 (2018)
Sánchez, C.: Lignocellulosic residues: biodegradation and bioconversion by fungi. Biotechnol. Adv. 27(2), 185–194 (2009)
Corrêa, R.C.G., Rhoden, S.A., Mota, T.R., Azevedo, J.L., Pamphile, J.A., de Souza, C.G.M., Polizeli, M.D.L.T.D.M., Bracht, A., Peralta, R.M.: Endophytic fungi: expanding the arsenal of industrial enzyme producers. J. Ind. Microbiol. Biotechnol. 41, 1467–1478 (2014)
Sunitha, V.H., Devi, D., C, Nirmala Srinivas: Extracellular enzymatic activity of endophytic fungal strains isolated from medicinal plants. World J. Agric. Sci. 9(1), 01–09 (2013)
Katoch, M., Salgotra, A., Singh, G.: Endophytic fungi found in association with Bacopamonnieri as potential producers of industrial enzymes and antimicrobial bioactive compounds. Braz. Arch. Biol. Technol. 57, 714–722 (2014)
Ghoshal, G., Basu, S., Shivhare, U.S.: Solid state fermentation in food processing. Int. J. Food Eng. 8(3), 1246–1253 (2012)
Department of Agriculture Extension, Bangkok, Thailand: Guide to Office of Agricultural Economics, Agriculture situation. (2017) http://www.oae.go.th/assets/portals/1/fileups/prcaidata/files/oilpalm60.pdf
Noparat, P., Prasertsan, P., O-Thong, S.: Potential for using enriched cultures and thermotolerant bacteria isolates for production of biohydrogen from oil palm sap and microbial community analysis. Int. J. Hydrog. Energy 37, 16412–16420 (2012)
Kosuki, A., Tanaka, R., Magara, K., Murata, Y., Arai, T., Suliman, O., Hashim, R., Abdul Hamid, Z.A., Azri Yahya, M.K., Mohd Yusof, M.N., Ibrahim, W.A., Mori, Y.: Ethanol and lactic acid production using sap squeezed from old oil palm trunks felled for replanting. J. Biosci. Bioeng. 110, 322–325 (2010)
NoorMohd, A.M., Mohd, A.M.D., Islam, M.N., Mehat, N.A.: Physico-chemical properties of oil palm trunk starch. Starch/Stärke 51, 293–301 (1999)
Prawitwong, P., Kosugi, A., Arai, T., Deng, L., Lee, K.C., Ibrahim, D., Murata, Y., Sulaiman, O., Hashim, R., Sudesh, K., Ibrahim, W.A., Saito, M., Mon, Y.: Efficient ethanol production from separated parenchyma and vascular bundle of oil palm trunk. Bioresour. Technol. 125, 37–42 (2012)
Noparat, P., Prasertsan, P., O-Thong, S.: Isolation and characterization of high hydrogen-producing strain Clostridium beijerinckii PS-3 from fermented oil palm sap. Int. J. Hydrog. Energy 36, 14086–14089 (2011)
Khalil, A. H. P. S., Jawaid, M., Hassan, A., Paridah, M. T., Zaido, A.: Chapter 8: Oil palm biomass fibres and recent advancement in oil palm biomass fibres based hybrid biocomposites. In Composites and Their Applications, edited by Ning Hu. INTECH. (http://dx.doi.org/10.5772/48235) (2012)
Ang, S.K., Shaza, E.M., Adibah, Y., Suraini, A.A., Madihah, M.S.: Production of cellulases and xylanase by Aspergillus fumigatus SK1 using untreated oil palm trunk through solid state fermentation. Process Biochem. 48(9), 1293–1302 (2013)
Awad, H.M., Diaz, R., Malek, R.A., Othman, N.Z., Aziz, R.A., Enshasy, H.A.: Efficient production process for food grade acetic acid by Acetobacter aceti in shake flask and in bioreactor cultures. E-J Chem. 9(4), 2275–2286 (2012)
Vidra, A., Nemeth, A.: Bio-produced acetic acid: a review. Period. Polytech. Chem. Eng. 62(3), 245–256 (2018)
Ehsanipour, M., Suko, A.V., Bura, R.: Fermentation of lignocellulosic sugars to acetic acid by Moorellathermoacetica. J. Ind. Microbiol. Biotechnol. 43, 807–816 (2016)
Sugaya, K., Tuse, D., Jones, J.L.: Production of acetic acid by Clostridium thermoaceticum in batch and continuous fermentation. Biotechnol. Bioeng. 28, 678–683 (1986)
Khamtib, S., Plangklang, P., Reungsang, A.: Optimization of fermentative hydrogen production from hydrolysate of microwave assisted sulfuric acid pretreated oil palm trunk by hot spring enriched culture. Int. J. Hydrog. Energy 36, 14204–14216 (2011)
Komonkiat, I., Cheirsilp, B.: Felled oil palm trunk as a renewable source for biobutanol production by Clostridium spp. Bioresour. Technol. 146, 200–207 (2013)
Dhillon, G.S., Oberoi, H.S., Kaur, S.: Value-addition of agriculture wastes for augmented cellulase and xylanase production through solid-state tray fermentation employing mixed-culture of fungi. Ind. Crop. Prod. 34, 1160–1167 (2011)
Singh, A., Bajar, S., Bishnoi, N.R., Singh, N.: Laccase production by Aspergillus heteromorphus using distillery spent wash and lignocellulosic biomass. J. Hazard. Mater. 176, 1079–1082 (2010)
Prasertsan, P., Kittikul, A., Kunghae, A., Maneesri, J., Oi, S.: Optimization for xylanase and cellulase production from Aspergillus niger ATCC6275 in palm oil mill wastes and its application. World J. Microbiol. Biotechnol. 13(5), 555–559 (1997)
Prasertsan, P., H-Kittikun, A., Chantapaso, S.: Factors affecting treatment of palm oil mill effluent by enzyme from Aspergillus niger ATCC 6275 cultivated on palm cake. Songklanakarin J. Sci. Technol. 23, 797–806 (2001)
Delabona, P.S., da Pirota, R.D.P., Codima, C.A., Tremacoldi, C.R., Rodrigues, A., Cristiane, S.F.: Using amazon forest fungi and agricultural residues as a strategy to produce cellulolytic enzymes. Biomass Bioenergy 37, 243–250 (2012)
Prasertsan, P., Oi, S.: Production of cellulolytic enzymes from fungi and use in the saccharification of palm cake and palm fibre. World J. Microbiol. Biotechnol. 8(5), 536–538 (1992)
Bahrin, E.K., Seng, P.Y., Abd-Aziz, S.: Effect of oil palm empty fruit bunch particle size on cellulase production by Botryosphaeria sp. under solid state fermentation. Aust. J. Basic Appl. Sci. 5(3), 276–280 (2011)
White, T.J., Bruns, T., Lee, S., Taylor, J.W.: Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis, M.A., Gelf, J., Sninsky, J., White, T.J. (eds.) PCR Protocols: A Guide to Methods and Applications, pp. 315–322. Academic Press Inc, San Diego (1990)
Mandel, M., Weber, J.: The production of cellulose. Cellulose and their applications. American Chemistry Society. Adv. Chem. Ser. 95, 391–398 (1996)
Adeleke, E.O., Omafuvbe, B.O., Adewale, I.O., Bakara, M.K.: Purification and characterization of a cellulase obtained from cocoa (Theobroma cacao) pod-degrading Bacillus coagulans Co4. Turk. J. Biochem. 37(2), 222–230 (2012)
Miller, G.L.: Use of dinitrosalicyl acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428 (1959)
Bailey, Michael J., Peter, B., Kaisa, P.: Interlaboratory testing of methods for assay of xylanase activity. J. Biotechnol. 23(3), 257–270 (1992)
Ncube, T., Howard, R.L., Abotsi, E.K., van Rensburg, E.L.J., Ncube, I.: Jatropha curcas seed cake as substrate for production of xylanase and cellulase by Aspergillus niger FGSCA733 in solid-state fermentation. Ind. Crop. Prod. 37(1), 118–123 (2012)
Yamada, H., Tanaka, R., Sulaiman, O., Hashim, R., Hamid, Z.A.A., Yahya, M.K.A., Kosugi, A., Arai, T., Murata, Y., Nirasawa, S., Yamamoto, K., Ohara, S., Yusof, M.N.M., Ibrahim, W.A., Mori, Y.: Old oil palm trunk: a promising source of sugars for bioethanol production. Biomass Bioenerg. 34, 1608–1613 (2011)
Obahiagbon, F.I., Osagie, A.U.: Sugar and macrominerals composition of sap produced by Daphnia hookeri palms. Afr. J. Biotechnol. 6, 744–750 (2007)
Barros, R.R., Oliveira, R.A., Gottschalk, L.M., Bon, E.P.: Production of cellulolytic enzymes by fungi Acrophialophora nainiana and Ceratocystis paradoxa using different carbon sources. Appl. Biochem. Biotechnol. 161(1–8), 448–454 (2010)
Eziashi, E.I., Uma, N.U., Adekunle, A.A., Airede, C.E.: Effect of metabolites produced by Trichoderma species against Ceratocystis paradoxa in culture medium. Afr. J. Biotechnol. 5, 703–706 (2006)
Garofalo, J.F., McMillan, R.T.: Thielaviopsis diseases of palms. Proc. Fla. Hortic. Soc. 117, 324–325 (2004)
Girard, J.C., Rott, P.: Pineapple disease. In: Rott, P., Bailey, R.A., Comstock, J.C., Croft, B.J., Saumtally, A.S. (eds.) A guide to sugarcane diseases, pp. 131–135. Librairie du Cirad, Montpellier (2004)
Brijwani, K., Oberoi, H.S., Vadlani, V.: Production of a cellulolytic enzyme system in mixed-culture solid-state fermentation of soybean hulls supplemented with wheat bran. Process Biochem. 45(1), 120–128 (2010)
Moreno, C.A., Castillo, F., Gonzalez, A., Bernal, D., Jaimes, Y., Chaparro, M., Gonzalez, C., Rodriguez, F., Restrepo, S., Cotes, A.M.: Biological and molecular characterization of the response of tomato plants treated with Thichoderma koningiopsis. Physiol. Mol. Plant P. 74, 111–120 (2009)
Chen, J.L., Liu, K., Miao, C.P., Sun, S.Z., Chen, Y.W., Xu, L.H., Guan, H.L., Zhao, L.X.: Salt tolerance of endophytic Trichoderma koningiopsis YIM PH30002 and its volatile organic compounds (VOCs) allelopathic activity against phytopathogens associated with Panax notoginseng. Ann. Microbiol. 66, 981–990 (2016)
Liu, K., Yang, Y., Chen, J.L., Miao, C.P., Wang, Q., Zhou, H., Chen, Y.W., Li, Y.Q., Ding, Z.T., Zhao, L.X.: Koninginins N-Q, Polyketides from the endophytic fungus Trichoderma koningiopsis harbored in Panax notoginseng. Nat. Prod. Bioprospect. 6, 49–55 (2016)
Myla, S.K., Parapatla, H.R., Mekala, C.D., Kasireddy, H.R.: Production of endo-1,4-β-D-glucanasse and exo-1,4-β-D-glucanase on cellulosis substrate in solid state fermentation by Hypocrea nigricans. Int. J. Adv. Res. 4(5), 288–299 (2016)
Bhat, M.K., Bhat, S.: Cellulose-degrading enzymes and their potential applications. Biotechnol. Adv. 15(3), 583–620 (1997)
Hölker, U., Höfer, M., Lenz, J.: Biotechnological advantages of laboratory-scale solid-state fermentation with fungi. App. Microbiol. Biotechnol. 64(2), 175–186 (2004)
Kitcha, S., Cheirsilp, B.: Bioconversion of lignocellulosic palm byproducts into enzymes and lipid by newly isolated oleaginous fungi. Biochem. Eng. J. 88, 95–100 (2014)
Dashban, M., Qin, W.: Overexpression of an exotic thermotolerant β-glucosidase in Tricoderma reesei and it significant increase in cellulolytic activity and sacchaification of barley straw. Microb. Cell Fact. 11, 63 (2012)
Yoon, L.W., Ang, T.N., Ngoh, G.C., Chua, A.S.M.: Fungal solid-state fermentation and various methods of enhancement in cellulase production. Biomass Bioenerg. 67, 319–338 (2014)
Lio, J.Y., Wang, T.: Solid-state fermentation of soybean and corn processing coproducts for potential feed improvement. J. Agri. Food Chem. 60(31), 7702–7709 (2012)
Hu, H.L., Van den Brink, J., Gruben, B.S., Wosten, H.A.B., Gu, J.D., De Vries, R.P.: Improved enzyme production by co-cultivation of Aspergillus niger and Aspergillus oryzae and with other fungi. Int. Biodeter. Biodegr. 65, 248–252 (2011)
Yang, R., Meng, D., Hu, X., Ni, Y., Li, Q.: Saccharification of pumpkin pesidues by coculturing of Trichoderma reesei RUT-C30 and Phanerochaete chrysosporium burdsall with delayed inoculation timing. J. Agric. Food Chem. 61, 9192–9199 (2013)
Lin, L., Rong, Y., Yongqiang, L., Wenju, J.: In-depth investigation of enzymatic hydrolysis of biomass wastes based on three major components: cellulose Hemicellulose and Lignin. Bioresour. Technol. 101(21), 8217–8223 (2010)
Kheng, P.P., Omar, I.C.: Xylanase production by a fungal isolate, Aspergillus niger USM Al 1 via solid state fermentation using palm kernel cake (PKC) as substrate. Songklanakarin J. Sci. Technol. 27, 325–336 (2005)
Shenef, A., Ei-Tanash, A., Atia, N.: Cellulase production by Aspergillus fumigatus grown on mixed substrate of rice straw and wheat bran. Res. J. Microbiol. 5, 199–211 (2010)
Kalogeris, E., Christakopoulos, P., Katapodis, P., Alexiou, A., Vlachou, S., Kekos, D., Macris, B.J.: Production and characterization of cellulolytic enzymes from the thermophilic fungus Thermoascus aurantiacus under solid state cultivation of agricultural wastes. Process Biochem. 38(7), 1099–1104 (2003)
Hamzah, F., Tdris, A., Shuan, T.K.: Preliminary study on enzymatic hydrolysis of treated oil palm (Elaeis) empty fruit bunches fibre (EFB) by using combination of cellulose and β 1-4 glucosidase. Biomass Bioeng. 35, 1055–1059 (2013)
Vlaseenko, E.Y., Ding, H.: Enzymatic hydrolysis of pretreated rice straw. Bioresour. Technol. 59, 109–119 (1997)
Qiu, G.M., Aita, M., Walker, S.: Effect of ionic liquid pretreatment on thechemical composition, structure and enzymatic hydrolysis of energy canebagasse. Bioresour. Technol. 117, 251–256 (2012)
Ramachandriya, K.D., Wilkins, M.R., Hiziroglu, S., Dunford, N.T., Atiyeh, H.K.: Development of an efficient pretreatment process for enzymatic saccharification of Eastern redcedar. Bioresour. Technol. 136, 131–139 (2013)
Yoon, J.J., Kim, Y.K.: Degradation of crystalline cellulose by the brown-rot basidiomycete Fomitopsispalustris. J Microbiol. 43(6), 487–492 (2005)
Lee, J.W., Kim, H.Y., Koo, B.W., Choi, D.H., Kwon, M., Choi, I.G.: Enzymatic saccharification of biologically pretreated Pinusdensiflora using enzymes from brown rot fungi. J. Biosci. Bioeng. 106, 162–167 (2008)
Gupta, R., Khasa, Y.P., Kuhad, R.C.: Evaluation of pretreatment methods in improving the enzymatic saccharification of cellulosic materials. Carb. Polymer 84, 1103–1109 (2011)
Sakihama, Y., Hasunuma, T., Kondo, A.: Improved ethanol production from xylose in the presence of acetic acid by the overexpression of the HAA1 gene in Saccharomyces cerevisiae. J. Biosci. Bioeng. 119(3), 297–302 (2015)
Hasunuma, T., Kondo, A.: Development of yeast cell factories for consolidated bioprocessing of lignocellulose to bioethanol through cell surface engineering. Biotechnol. Adv. 30, 1207–1218 (2012)
Hahn-Hagerdal, B., Wahlbom, C.F., Gardonyi, M., van Zyl, W.H., Cordero Otero, R.R., Jonsson, L.J.: Metabolic engineering of Saccharomyces cerevisiae for xylose utilization. Adv. Biochem. Eng. Biotechnol. 73, 53–84 (2001)
Yuvadetkun, P., Boonmee, M.: Ethanol production capability of Candida shehatae in mixed sugars and rice straw hydrolysate. SainsMalaysiana 45(4), 581–587 (2016)
Choi, W.J., Hartono, M.R., Chen, W.H., Yeo, S.S.: Ethanol production from biodiesel-derived crude glycerol by newly isolated Kluyveracryocrescens. Appl. Microbiol. Biotechnol. 89, 1255–1264 (2011)
Nurul Adela, B., Nasrin, A.B., Loh, S.K., Choo, Y.M.: Bioethanol production by fermentation of oil palm empty fruit bunches pretreated with combined chemicals. J. Appl. Environ. Biol. Sci. 4(10), 234–242 (2014)
Choi, W.J., Hartono, M.R., Chen, W.H., Yeo, S.S.: Ethanol production from biodiesel-derived crude glycerol by newly isolated Kluyvera cryocrescens. Appl. Microbiol. Biotechnol. 89, 1255–1264 (2011)
Millati, R., Wikandari, R., Trihandayani, E.T., Cahyanto, M.N., Taherzadeh, M.J., Niklasson, C.: Ethanol from oil palm empty fruit bunch via dilute-acid hydrolysis and fermentation by Mucorindicus and Saccharomyces cerevisiae. Agric. J. 6, 54–59 (2011)
Acknowledgements
This research work was financially supported by Agricultural Research Development Agency (ARDA) (Grant No. CRP5605021180), Thailand Research Fund (Grant No. RTA6080010) and the PSU-Ph.D. Scholarship, Graduate School, Prince of Songkla University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nutongkaew, T., Prasertsan, P., Leamdum, C. et al. Bioconversion of Oil Palm Trunk Residues Hydrolyzed by Enzymes from Newly Isolated Fungi and Use for Ethanol and Acetic Acid Production Under Two-Stage and Simultaneous Fermentation. Waste Biomass Valor 11, 1333–1347 (2020). https://doi.org/10.1007/s12649-019-00678-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-019-00678-x