Abstract
The production of cellulosic ethanol has been gaining attention in the industry sector because of the high availability of lignocellulosic biomass from agricultural and forestry activities. Pinus patula is one of the most typical softwood species in Colombia. The aim of this work is to evaluate the production of ethanol using Pinus patula as raw material using dilute acid pretreatment and enzymatic hydrolysis to produce sugars able to be used as substrate for the strain Saccharomyces cerevisiae. Three fermentation configurations were selected to evaluate the performance of the microorganism: configurations 1 and 2 used glucose in a percentage of 80%w/v and 70%w/v, respectively, as substrate to establish the adaptation requirements of the microorganism. The configuration 3 considered the use of concentrated P. patula hydrolysate. An experimental yield of 0.364 ± 0.009 g ethanol/g sugar (73% of the theoretical) was obtained. Additionally, the economic and energetic comparison between the biochemical (ethanol production through fermentation) and thermochemical (synthesis gas through gasification) pathways to produce bioenergy was performed through simulation approaches. As main results, a higher ethanol production cost (1.53 USD/L) was obtained in comparison to the market price (0.77 USD/L) and a low energy efficiency (20%). Different alternatives such as waste integration and energy incentives must be considered in order to produce ethanol in a feasible way.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
State of Novelty
Pinus patula is a softwood that is highly available in Colombia and other countries. Nevertheless, the application of this raw material has been limited to furniture making and direct energy production through combustion. The high cellulose and hemicellulose content of P. patula as well as its processing residues (having the same composition) evidences the possibility to obtain different added-value products through different platforms (i.e. sugar extraction and gasification). Therefore, this work presents a technical, economic and energetic assessment of the ethanol production using P. patula as raw material in order to determine the main technical concerns (experimental procedure), economic bottlenecks (processing costs) and energy constraints (valorization of residues).
Introduction
Pinus patula (PP) is one of the types of softwood with high production in Colombia because of its fast growth and high crop yield among the forest plantations [1]. Pinus patula has a high content of cellulose and hemicellulose, between 40 and 50% on dry basis [2]. However, one of the disadvantages of most softwoods is the high lignin content (approximately, 25% on dry basis) that hinders the application of this type of raw material to obtain bio-based materials. Consequently, the use of softwood as feedstock for the direct production of energy (thermochemical route) is very common due to the easy treatment requirements [3]. Thermochemical routes involve different processes such as pyrolysis, combustion, and gasification in order to produce different species that can be used as platform for different products such as electricity, steam and liquid fuels through the Fischer–Tropsch process [4]. On the other hand, the growing interest in the production of cellulosic ethanol is related to the advances in different pretreatment methods (chemical, enzymatic and physical) that allow the production of fermentable sugars, which are considered a promising substrate for microorganisms to produce different added-value products, such as ethanol [5–7]. The most studied application of softwood in fermentative processes is the production of ethanol on laboratory [8, 9] and commercial scales [10] due to the content of cellulose, hemicellulose and lignin. In fact, softwoods contain around 43–45% cellulose, 20–23% hemicellulose and 25–28% lignin. Theoretically, around 410 L of ethanol can be produced per metric ton of dry raw material using only the hexose fraction and 455 L if all carbohydrates are considered [8].
In this context, Söderström et al. [11] investigated the effect of the two-step steam pretreatment of SO2-impregnated softwood (Picea abies.) on the ethanol yield at different severities. Additionally, the effectiveness of the developed pretreatment was assessed by both enzymatic hydrolysis of the solids and simultaneous saccharification and fermentation (SSF) of the whole slurry. From the enzymatic hydrolysis, an overall sugar yield of 80% was reached. In the SSF configuration, an overall ethanol yield of 69% was achieved. Hoyer et al. [9] studied the production of ethanol using spruce as raw material. Based on the content of fermentable sugars in the fermenter, a theoretical yield of 81% was reached using a water-insoluble solids (WIS) content of 12% and SSF. Furthermore, Cellunolix® plant located in Kajaan (Finland) produces 10 million L of ethanol per year on an industrial scale using sawdust and recycled wood as raw material [12].
On the other hand, the thermochemical pathway comprises the direct conversion of the raw material into intermediate products (i.e. synthesis gas and bio-oil) that can be used as platform for the production of energy (heat and/or electricity). The most important thermochemical processes that have been used to evaluate the energy potential of softwood are pyrolysis, combustion, and gasification. Pyrolysis includes the conversion of biomass into char, gas and a liquid composed of a mixture of hundreds of oxygenated organic compounds, which is known as bio-oil [13,14,15]. Combustion involves three phenomena: evaporation of the water, volatilization at 200 °C and combustion at 500 °C [16, 17]. Instead, gasification converts biomass into a gaseous mixture (i.e., hydrogen, methane, carbon monoxide and carbon dioxide), small quantities of char and condensable compounds [18,19,20]. The use of softwood for one of these pathways will depend on different factors such as environmental friendliness, energy efficiency and economic profitability. In this paper, the main goal of the production of energy using P. patula will be the economic and energetic comparison of two technological routes (biochemical and thermochemical). Different authors have evaluated the economic performance of the ethanol and electricity production using softwood [21]. Moncada et al. [2] performed the techno-economic analysis of the ethanol production using P. patula as raw material, obtaining a production cost of 1.08 USD/L. Furthermore, other authors have evaluated the production cost of electricity through gasification (5 USD/kWh) and direct combustion (7.2 USD/kWh) using as raw material softwood [22].
The aim of this work is to evaluate the potential use of P. patula for the production of ethanol using two different approaches: experiments and simulation. First, a series of experiments were carried out involving dilute acid pretreatment followed by an enzymatic saccharification. Then, C6-sugar fermentation was carried out using the strain Saccharomyses cerevisiae to obtain ethanol. Additionally, the simulation of the ethanol production was performed using the software Aspen Plus v9.0. Subsequently, the economic assessment of the fermentative production of ethanol was evaluated at different process scales. Finally, the energy comparison between the biochemical and thermochemical pathways for energy production using P. patula as raw material was performed based on the energy potential of the produced ethanol and the electricity generation through gasification.
Materials and Methods
In this section, two procedures are presented aiming to evaluate the production of ethanol using P. patula as raw material. The characterization of the raw material, the pretreatment (i.e. dilute acid hydrolysis and enzymatic saccharification) and the ethanol production (i.e. fermentation) are categorized in “Experimental Procedure” section. The main purpose of this section is to summarize the methods, protocols, and conditions used in each one of the experimental procedures. On the other hand, the simulation process (“Simulation Procedure” section) involves the use of the obtained results from the experimental assays as well as the reported data in the literature for ethanolic fermentation of softwood as input for the economic and energy assessment of the biochemical route for ethanol production. Additionally, the gasification of P. patula was simulated in order to compare the ethanol fermentation from the energetic point of view.
Experimental Procedure
Raw Material
Pinus patula (PP) was collected from a farm located in the central western region of Colombia (5°03′58″N 75°29′05″W) and dried at room temperature (18–23 °C). The wood was milled with an upper vibratory disk mill (Retsch SR 200) and sieved to a particle diameter of 400 µm. The chemical characterization of P. patula was taken from a previous work García et al. [23].
Methods
Reagents
For the pretreatment of P. patula, sulfuric acid 96% (MERCK) and distillate water were used. In the enzymatic hydrolysis, cellulases (Celluclast® 1.5 L), multienzyme complex (Viscozyme® L), citric acid (Disproalquimicos) and sodium citrate (CARLO ERBA) for the buffer solution, and sodium azide solution 2% were used. Freeze-dried Saccharomyces cerevisiae strain, d(+)-glucose (MERCK), ammonium sulfate (Panreac), potassium dihydrogen phosphate (MERCK), magnesium sulfate (MERCK), calcium chloride (Panreac) and distillate water were used in the fermentation. For the determination of sugars, 3,5-dinitrosalicylic acid (MERCK), sodium hydroxide (MERCK), potassium sodium tartrate (Panreac) and distillate water were employed.
Dilute-Acid Pretreatment of P. patula
The raw material was mixed with a dilute solution of sulfuric acid (2%v/v) in a solid to liquid (S/L) ratio of 1:10. The procedure was carried out in an autoclave at 121 °C and 15 psig during 90 min, which are typical operation conditions that ensures a high hemicellulose solubilization and low inhibitors production [24]. After the acid pretreatment, a vacuum filtration was performed aiming to separate the solid and liquid fraction (i.e., hydrolysate). The solid fraction was washed using tap water until reach a pH near to 4.8, which is the pH condition of the enzymatic hydrolysis. This fraction was not dried to avoid re-crystallization of amorphous cellulose and to ensure a high sugars yield from this process [25]. Instead, the liquid fraction from the dilute acid pretreatment was cooled to room temperature and stored until sugars analysis.
Enzymatic Saccharification
Enzymatic hydrolysis was carried out following the protocol NREL/TP-510-42629 [26] with a commercial cellulase (Celluclast® 1.5 L) and multienzyme complex (Viscozyme® L). The enzymatic hydrolysis was carried out using 30 g of the solid fraction from the dilute acid pretreatment. For this, it was used an enzyme dosage of 64 filter paper units (FPU) per g of cellulose and 60 p-nitrophenyl-β-glucoside units (pNPGU) per g of cellulose for Celluclast 1.5 L and Viscozyme L, respectively. The reaction volume was 2 L, the pH was adjusted using a 0.1 M citrate buffer solution (pH 4.8–5.0). Finally, a 2%v/v sodium azide solution was added to prevent the growth of organisms during the saccharification. The operative conditions for the enzymatic hydrolysis process were 72 h at 50 °C and 100 rpm. Afterward, the liquid C6-rich hydrolysate was separated by vacuum filtration. Then, this hydrolysate was used as substrate for the fermentation process.
Ethanolic Fermentation
Freeze-dried S. cerevisiae was used for the production of ethanol. The microorganism was able to intake C6 sugars from the saccharification process. Different fermentative configurations (three mediums) were prepared in 200 mL Erlenmeyer flasks (100 mL working volume): sugar concentration of 15 g/L (80% glucose and 20% hydrolysate), sugar concentration of 10 g/L (70% glucose and 30% hydrolysate) and total (100%) hydrolysate. The mediums were supplemented with minerals: 1.5 g/L \({\left( {{\text{N}}{{\text{H}}_4}} \right)_2}{\text{S}}{{\text{O}}_4}\), 1 g/L \({\text{K}}{{\text{H}}_2}{\text{P}}{{\text{O}}_4}\), 0.3 g/L \({\text{MgS}}{{\text{O}}_4}\) and 0.05 g/L \({\text{CaC}}{{\text{l}}_2}\). Experiments were carried out at 32 °C and 150 rpm in an incubator (BINDER Incubator BD056) with a shaker.
Analytical Quantification
The amount of sugar in the sample was determined using the dinitrosalicylic acid (DNS) method, where the absorbance was measured at a wavelength of 540 nm according to the procedure proposed by Miller [27]. The biomass concentration (g/L) was determined by dry weight of yeast. The concentration of ethanol was determined using a GC-2014 (Shimadzu) gas chromatograph equipped with a flame ionization detector (FID) and a capillary column Stabilwax. The operating conditions of the gas chromatograph were: injection volume 10 µL, injector temperature 220 °C, detector temperature 270 °C, nitrogen as carrier gas with a flow in the column of 0.5 mL/min and running time of 21.75 min.
Simulation Procedure
The production of ethanol using P. patula as raw material was simulated based on the results from the experimental section and complemented with data reported in the literature. For this purpose, the dilute acid pretreatment, enzymatic saccharification and ethanolic fermentation were conceptually designed using main simulation tools as software Aspen Plus V9.0 (Aspen Technology, Inc, USA), which allows calculating the mass and energy balances of the process scheme. The behavior of the pretreatment, saccharification and fermentation were described based on data reported in the literature from different authors (see, “Ethanolic Fermentation” section) [28, 29]. Then, the kinetic models of these stages were modeled using the computational tool Matlab (MathWorks, USA). The properties of missing components in the Aspen Plus Database were taken from external databases, especially the work developed by Wooley and Putsche [30]. The selection of the thermodynamic method was based on previous works [31], where the Non-Random Two Liquid (NRTL) model was used to describe the behavior of the liquid phase. For calculations, a mass flow rate of 3300 ton/day of P. patula (wet basis) and a moisture content of 40% were used in the simulation procedure.
The results from the simulation procedure were used as starting point for the economic and energetic analysis. In the economic analysis, the contribution of the total capital costs and the operating costs (variable and fixed) to the total production cost of the process was assessed. Furthermore, the ethanol production cost from the evaluated process was compared with other studies that deal with the production of ethanol using different pretreatment configurations and raw materials [32, 33]. Since most forest materials (hardwood and softwood) are commonly used for the direct production of energy through thermochemical processes (i.e. combustion and gasification), the energy analysis was focused on the potential energy used from P. patula for bioenergy production through a biochemical pathway (fermentation) and its comparison with a common thermochemical route (i.e. gasification). Therefore, the gasification of P. patula was also simulated but only for the energy comparison. The energy efficiency of both processes was calculated based on the relation between the energy content of the products (ethanol and synthesis gas) and the energy content of the feedstock [3]. A detailed description of the economic and energy assessment is presented in “Economic Assessment” and “Energy Analysis” section, respectively.
Ethanolic Fermentation
Pinus patula is initially chipped and dried in order to achieve the particle size and moisture content required for the fermentation (see, “Ethanolic Fermentation” section). Subsequently, the physical treated raw material is submitted to a two-step hydrolysis process in order to achieve the extraction of sugars from the lignocellulosic matrix. In the first stage, the hemicellulose fraction is hydrolyzed with dilute sulfuric acid (2% by weight) at a temperature of 121 °C. This stage was modeled using the kinetic model and the parameters reported by Rafiqul et al. [34] (see, Supplementary material Table S1). Moreover, the kinetic model was solved using the residence time specified in the experimental section (i.e., 90 min) aiming to obtain similar concentrations in comparison with those quantified in the experimental dilute acid pretreatment. Once the model was solved using the experimental conditions, the process was modeled in the Aspen Plus V9.0 simulation software and the flowsheet was completed. From this pretreatment, a non-converted solid fraction and a rich-pentose liquor are obtained. This stream is separated by filtration. The liquor is mainly composed of xylose and glucose. Although, this stream was not considered for fermentation since the strain S. cerevisiae used in this study was not able to degrade xylose. The solid fraction, rich in cellulose and lignin, is sent to an enzymatic saccharification process at 50 °C using cellulase as enzyme, which is able to convert the cellulose to glucose for further use as substrate in the ethanolic fermentation. The enzymatic hydrolysis was modeled based on the experimental yields and using the kinetic model and parameters reported by Khodaverdi et al. [35] and Kadam et al. [36] (see, Supplementary material Table S2). As in the case of the dilute acid pretreatment, the experimental conditions applied to the enzymatic saccharification were used as input data in the kinetic model. In fact, the cellulose, cellobiose and glucose concentration profiles were calculated up to a residence time of 72 h. Once the final yields of the saccharification process were obtained, these results were used to simulate the enzymatic hydrolysis in the Aspen Plus V9.0 software employing a RYield model. Then, the fermentation process is simulated using the S. cerevisiae as microorganism at 32 °C. The yields of the ethanolic fermentation were taken from the results of the experimental procedure previously described in “Gasification” section. Afterward, the cell biomass is separated from the culture broth through a centrifuge. The culture broth with an ethanol concentration of 7–10 wt% is taken to the separation stage that consists of two distillation columns and molecular sieves. In the first column, ethanol is concentrated nearly to 50–55% by weight [37]. This distillation column is designed with 20 trays, a reflux ratio of 1.5 and a distillate-to-feed ratio of 0.03. In the second column, the liquor is concentrated until the azeotropic point (96 wt%) using a 20-tray column with a reflux ratio of 1.5 and a distillate-to-feed ratio of 0.3. Subsequently, the azeotropic mixture is sent to the dehydration zone with molecular sieves to obtain ethanol at 99.7 wt% [38]. The process scheme for the ethanol production from P. patula is presented in Fig. 1.

Repoduced with permission from Referemce [31]
Scheme of the ethanol production from P. patula using S. cerevisiae as microorganism. Units (1) Acid Hydrolysis, (2) Filter, (3) Detoxification, (4) Filter, (5) Dewatering, (6) Enzymatic Saccharification, (7) Filter, (8) Dewatering, (9) Autoclave, (10) Ethanol Fermentation, (11) Centrifuge, (12) Distillation column, (13) Rectification column and (14) Ethanol dehydration.
Gasification
The simulation of the gasification was previously described by García et al. [39]. The raw material is first conditioned to the particle size and moisture content required for the gasification. For this purpose, chipping and drying processes are simulated in order to obtain a particle size between 0.5 and 1 cm and a moisture content of 20%. Subsequently, the chipped and dried raw material is submitted to a downdraft gasifier where different chemical reactions (devolatilization, combustion and reduction) take place. For simulation purposes, the downdraft gasification is divided into two steps: first, the devolatilization (pyrolysis) of the raw material is carried out at 600 °C in absence of air in order to obtain as main components carbon, hydrogen, oxygen, nitrogen, char and ash. The elemental analysis (raw material characterization) was used to determine the yields of carbon, hydrogen, oxygen and nitrogen; whereas the char and ash content were taken from the proximate analysis (raw material characterization). Then, the combustion and reduction are simulated using the Free Gibbs Energy minimization method to predict the composition of H2, CO2, CO, CH4, N2 in the synthesis gas [40, 41]. Ash and particulate matter are separated from the synthesis gas using a cyclone. This syngas can be used as fuel for the production of electricity using gas engines. The simulation of the engine was done based on the combustion reaction between the syngas and air, but also considering the electric efficiency of gas engines, which can vary between 40 and 45%.
Economic Assessment
The results of the mass and energy balances from the simulation procedure were used as starting point for the economic analysis. The total capital investment of the ethanolic fermentation was calculated based on the data provided by the complementary software Aspen Economic Analyzer v9.0 (Aspen Technologies, Inc., USA), which performs the “mapping” (sizing and costing) of the equipment. The operating costs were divided into two types: fixed and variable. The fixed operating costs are those related to the operation of equipment in the process such as labor, maintenance, fixed and general and plant overhead costs. These costs were calculated based on the estimation method reported by Peters and Timmerhaus [42]. Maintenance costs were estimated as the 6% of the total capital investment [42]. The labor costs were determined based on the number of employees-hour/day required for the operation of a 3300 ton/day facility according to the data reported by Peters and Timmerhaus [42]. Fixed charges were calculated as the 3% of the total capital investment, whereas the plant overhead and general costs were estimated as the 60% and 20% of the labor costs, respectively [42]. On the other hand, the variable operating costs are those associated with the raw material, reagents and utilities (heating and cooling) purchase costs. The raw material and reagent costs were calculated from the inflows of the process and the purchase prices (see, Table 1). The utility costs were calculated using the complementary software Aspen Energy Analyzer and data about the market prices (see, Table 1). This analysis was estimated in US dollars for a 10-year period at an annual interest rate of 17% (typical for the Colombian economy), considering the straight-line depreciation method and an income tax of 25%. Prices and economic data used in this analysis such as the costs of the raw materials and utilities, income tax, labor salaries, among others are summarized in Table 1.
The results from the software Aspen Process Economic Analyzer and the estimation method reported by Peters and Timmerhaus [42] were used to determine the production cost of 1 L of ethanol using the dilute-acid hydrolysis coupled to the ethanolic fermentation and P. patula as raw material. The calculated production costs of the proposed process scheme were compared to the reported data in the literature from different authors that have evaluated the production of ethanol using different raw materials and pretreatments.
Energy Analysis
As mentioned in the methodology (“Materials and Methods” section), the energy comparison of the biochemical and thermochemical pathways was carried out using ethanol and syngas as energy carriers, respectively. The criteria selected for this purpose was the energy efficiency of both processes, which was calculated following the methodology proposed by García et al. [3]. The energy efficiency was evaluated considering the gross energy content of the P. patula\(\left( {{E_{{\text{biomass}}}}} \right)\) and the main products \(\left( {{E_{{\text{products}}}}} \right)\) of each pathway, as presented in Eq. 1.
The energy content of the biomass was calculated based on the mass flow (\(\dot {m}\)) and the lower heating value (\(LH{V_B}\)) of the P. patula reported by García et al. [23] (see, Eq. 2).
In the same way, the energy content of the products was calculated based on the mass flow rates and their respective heating values (see, Eq. 3, 4). The flow rates of ethanol and syngas were obtained from the simulation procedure in the software Aspen Plus. On the other hand, the heating values were taken from different sources: the lower heating value (LHV) of ethanol was 26.95 MJ/kg [48], whereas the lower heating value (LHV) of the synthesis gas was calculated considering the mass composition and the heating content of the main gaseous species in the synthesis gas (hydrogen, methane and carbon monoxide). The LHV of the hydrogen, methane and carbon monoxide were 119.96, 50 and 10.11 MJ/kg, respectively [48]. The calculated value for the synthesis gas was 6.77 MJ/kg.
Results and Discussion
Dilute Acid Pretreatment and Enzymatic Saccharification
Dilute-acid hydrolysis was used as the first stage for the pretreatment of the lignocellulosic biomass (P. patula) in order to improve the efficiency of the enzymatic hydrolysis. The quantification of the total reducing sugars was 25.37 g/L, which is higher than the results reported by Moncada et al. [2] (20 g/L) using P. patula bark. Additionally, Kim [49] and Bösch et al. [50] reported yields of 39.3 g of sugars per 100 g of dry hemlock sawdust and 26.3 g of sugars per 100 g of softwood spruce, respectively. The results obtained in this work are acceptable in comparison to those reported in the literature, despite the use of one-step dilute acid hydrolysis.
The solid fraction from the dilute acid hydrolysis was used as input for the saccharification process. The highest amount of reducing sugars in the liquid fraction from this process was 20 g of reducing sugars per 100 g of dry feed (3.02 g/L). The results obtained in this work are in agreement with those reported by other authors such as Söderström et al. [11] (17 g of sugars per 100 g of Picea abies) and Shinozaki et al. [51] (14 g of reducing sugars per 100 f of ensiled rice straw). From the enzymatic saccharification, a cellulose conversion of 67% (w/w) was achieved.
The enzymatic hydrolysate was used as carbon substrate for the production of ethanol using S. cerevisiae as microorganism. The low concentration of reducing sugars in the hydrolysate can be explained due to the high amount of water used in the enzymatic hydrolysis. In this sense, the hydrolysate was concentrated until a concentration of 13.5 g/L.
Ethanolic Fermentation
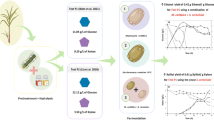
The main objective of the experimental procedure was to evaluate the potential use of P. patula for the production of ethanol. For this purpose, the ability of the microorganism to directly degrade the hydrolysate was evaluated. Microorganisms can adapt to environmental perturbations, such as changes in the osmotic pressure, temperature, and depletion of nutrients [52]. Nevertheless, if the changes are not progressive, the microorganism is submitted to stress, which leads to a decrease in the cell growth or prolonged lag phases. In order to reduce the stress of the microorganism, three fermentation configurations were proposed to evaluate the effect of the substrate concentration and type in the ethanol production (see, Table 2).
Figure 2 presents the consumption of total reducing sugars during 69 h of fermentation. As a result, a substrate consumption of 8.185, 7.996 and 6.295 g/L was obtained from the fermentation configurations 1, 2 and 3, respectively. In the fermentation configuration 1, a relatively high consumption of substrate was achieved; whereas the final concentration of sugars in the configurations 2 and 3 were 50% of the initial concentration. It is important to clarify that in the present study, inhibitors were not quantified. However, previous studies have demonstrated that lignocellulosic biomass (i.e. wood wastes) generates hydrolysates that contain inhibitors such as furfural, HMF and aromatic-lignin compounds that limit the microbial growth, microorganism metabolism in the fermentation and therefore, the consumption of the substrate [53]. In this context, Qian et al. evaluated the dilute-acid hydrolysis of the softwood aiming to obtain a hydrolysate composed of glucose and xylose, and its further fermentation to ethanol by co-cultures [54]. In this study, two fermentation configurations were studied: (i) detoxified hydrolysate by a combined method and (ii) hydrolysate without detoxification. The use of the softwood hydrolysate without detoxification evidenced the same behavior as the present study, where 50% of the substrate was not consumed, which indicates the possible presence of inhibitors in the hydrolysate. Therefore, the hydrolysate should be detoxified in order to increase the substrate consumption and ethanol production. However, the dilute-acid pretreatment generates lower degradation products than the use of concentrated-acid pretreatments. Additionally, high hydrolysis yields have been reported when pretreating lignocellulosic materials with diluted H2SO4 [53]. Fermentation configuration 3 showed a substrate consumption similar to configuration 2, which was rich in glucose (15 g/L). Consequently, the microorganism does not require an adaptation stage, so it is possible to use the microorganism in the hydrolysate.
Additionally, Table 2 presents the ethanol concentration in each fermentation. An experimental yield of 0.364 ± 0.009 g ethanol/g sugar (73% of the theoretical) was obtained, which it is similar to the yields reported by other authors Chacha [55] and Hawkins and Doran-Peterson [56]. Nguyen et al. [57] used softwood chips through direct impregnation with sulfuric acid and steam explosion as pretreatment to produce ethanol using S. cerevisiae. As a result, ethanol yields between 74 and 89% of the theoretical were obtained. Qian et al. [54] studied the fermentation of the dilute-acid softwood hydrolysate to produce ethanol using co-cultures of adapted S. cerevisiae and Escherichia coli. The highest ethanol yield from the experimental procedure was 0.45 g/g total sugar. The low concentration of ethanol in this work can be explained due to the use of substrates with low sugar content, which suggests that the hydrolysate must be concentrated to obtain a higher ethanol concentration.
Furthermore, fermentation 1 evidenced the lowest concentration of biomass (3.34 g/L), as shown in Fig. 3. While Fermentation 2 (highest concentration of glucose) produced a high concentration of biomass (4.51 g/L) after 69 h of fermentation. The biomass concentration in the concentrated hydrolysate showed an intermediate value between the two previous experiments (3.7 g/L). It should be noted that it is very important to guarantee aseptic conditions (sterilization of the medium and sampling) since the microorganism is susceptible to external agents. This contamination can cause reduction in the cell growth and undesirable products such as lactic and acetic acid. In this work, these products were not detected during the quantification of the samples.
Techno-Eeconomic Analysis (TEA) of the Ethanolic Fermentation
The total production costs of the ethanolic fermentation were classified into four categories: raw material costs, utility costs, fixed operating costs and total investment costs. Figure 4 presents the share distribution of these costs, evidencing the effect of the raw material costs (63.4%) in the total ethanol production cost. The utility costs account for 22.7% of the total production costs due to the high heating requirements of the downstream processing of the fermentation broth. Besides, the purchase of equipment contributes up to 12% of the total production costs. These results are in agreement with the data reported by Mesa et al. [58] who evaluated the production of ethanol through a dilute-acid pretreatment using sugarcane straw as raw material. The authors reported that the costs of raw materials (sugarcane straw and enzymes) had the most significant impact on the total production costs. The same behavior was evidenced in this work as observed in Fig. 5, where the share contribution of the main raw materials costs is presented. In this case, the P. patula and enzyme costs account for 59% of the raw materials costs, whereas sulfuric acid accounts for 34%.
The moisture content of the P. patula has a significant impact on the purchase price of this feedstock. According to Forest Fuels [43], raw materials with high moisture content (40%) have a market price of 60 USD/ton, whereas raw materials with low moisture (20%) content have a higher market price 160 USD/ton.
Several authors have studied different methods to improve the economic feasibility of the cellulosic ethanol production by means of the on-site enzyme production. Liu et al. [45] studied the influence of the enzyme cost on the cellulosic ethanol production considering different scenarios where the enzyme was commercially purchased or on-site produced. The authors concluded that the on-site enzyme production can significantly reduce the enzyme cost, providing a promising option for the large-scale cellulosic ethanol production.
On the other hand, the high contribution to the total costs of the sulfuric acid is related to the high liquid-to-solid ratio used in the acid hydrolysis. From the experimental procedure, a dilute sulfuric acid solution (2%v/v) in a liquid-to-solid ratio of 1:10 was used. Considering the large processing capacity of the evaluated process (3300 ton/day), the required amount of sulfuric acid is too high and thus, it has a great influence in the economic performance of the ethanol production. Therefore, the amount of sulfuric acid used for the pretreatment of P. patula should be reduced in order to increase the profits of the process. One of the possibilities is to reduce the concentration of sulfuric acid and the liquid-to-solid ratio. However, these changes can affect the sugar yields of the process and thus other variables must be considered such as temperature and pretreatment time. Parajó et al. [59] have evaluated these parameters in order to determine the best conditions for the dilute acid hydrolysis in order to use low amount of reagents and relative high pretreatment times.
Based on the information provided by the economic assessment, the production costs of 1 L of ethanol were calculated and compared with literature reports of different raw materials that use the dilute-acid hydrolysis as pretreatment. Figure 6 presents the comparison between the ethanol production costs of the evaluated process, some literature reports, and the market price. In this work, the ethanol production cost was 1.53 USD/L, which is higher than the market price (0.77 USD/L). However, the source of the raw material has an important effect on the feasibility of the ethanol production as evidenced by Daystar et al. [60] who evaluated the ethanol production cost from different raw materials (i.e. pine, eucalyptus, switchgrass and sweet sorghum) using the dilute-acid pretreatment. When forest biomass (pine) was used as raw material, the ethanol production costs was 2.25 USD/L, but if agricultural crops such as sweet sorghum are used as feedstock, the ethanol production cost drops to 0.5 USD/L. Zhao et al. [61] studied the effect of the dilute acid pretreatment in the ethanol production using corn stover. As a result, an ethanol production cost of 1.6 USD/L was obtained if no incentives were considered; however, the ethanol cost can be reduced up to 1.24 USD/L if tax exemptions and Feed-in-tariff are considered.
Energetic Comparison of Biochemical and Thermochemical Pathways for Energy Production Using P. patula
Table 3 presents the results from the energy analysis of both biochemical and thermochemical processes for bioenergy production. It is evidenced that the energy yield of the ethanolic fermentation is lower than the gasification of P. patula. As a result, the net energy efficiency of both processes was 20% and 42% for the ethanol and synthesis gas production, respectively. The energy efficiency of the ethanolic fermentation is lower than the data reported in the literature for different raw materials [62,63,64] since the valorization of the xylose rich-liquor and the lignin was not considered in this work. Additionally, the stillage from the first distillation column can be used as input for cogeneration systems, which can reduce the heating requirements (steam) of the process and thus, improve the overall energy efficiency. Despite the high pretreatment requirements of P. patula for sugar extraction and the relatively low energy efficiency, the production of ethanol through fermentation can promote the valorization of the lignocellulosic matrix (cellulose, hemicellulose and lignin) of this raw material to obtain different platforms in comparison to the direct conversion to electricity. Electricity cannot be considered an added-value product due to the low market price and the diversity of methods to produce it, especially in Colombia where hydropower plants are used to produce more than 60% of the electricity required in the country [65]. On the other hand, the increment of the earth temperature has increased the concern of using fossil fuels as energy carriers due to the global warming. In this sense, ethanol is gaining an important place as a future alternative to fossil fuels, especially, in the automotive sector. In order to fulfill the agreement in the COP21, blends between fossil fuels and ethanol have been implemented aiming to reduce the emissions from their combustion. This scenario provides an opportunity not only to evaluate different technologies to produce energy but also to consider other raw materials that can be transformed into added-value products such as ethanol.
Conclusions
It was evidenced that the P. patula hydrolysate can be used as substrate for the production of ethanol. The microorganism did not require a prior adaptation period to the hydrolysate. Despite the low substrate consumption, an experimental yield of 0.364 ± 0.009 g ethanol/g sugar (73% theoretical yield) was obtained. Nevertheless, it is highly recommended to concentrate the hydrolysate due to the low solid concentration in the enzymatic hydrolysis (15 g/L). From the simulation procedure, the costs associated with the raw materials (P. patula, enzymes and sulfuric acid) have a significant influence in the ethanol production costs and thus, different scenarios should be analyzed in order to reduce the ethanol production costs such as on-site enzyme production and low sulfuric acid concentration. The calculated ethanol production costs (1.55 USD/L) were higher than the market price (0.77 USD/L); however, different alternatives can be considered in the future aiming to improve the feasibility of the ethanol production from forest biomass: integration of waste streams and incentives for bioenergy production. The lower energy efficiency (22%) of the biochemical route in comparison to the thermochemical pathway endorse the necessity to implement the previous alternatives in order to consider the P. patula as a potential raw material to produce added-value products such as ethanol.
References
Ford, C.M., Jones, N.B., Chirwa, P.W.: Pinus patula and pine hybrid hedge productivity in South Africa: a comparison between two vegetative propagation systems exposed to natural infection by Fusarium circinatum. J. For. Sci. 2620, 1–9 (2014). https://doi.org/10.2989/20702620.2014.916501
Moncada, J., Cardona, C.A., Higuita, J.C., Vélez, J.J., López-Suarez, F.E.: Wood residue (Pinus patula bark) as an alternative feedstock for producing ethanol and furfural in Colombia: experimental, techno-economic and environmental assessments. Chem. Eng. Sci. 140, 309–318 (2016). https://doi.org/10.1016/j.ces.2015.10.027
García, C.A., Betancourt, R., Cardona, C.A.: Stand-alone and biorefinery pathways to produce hydrogen through gasification and dark fermentation using Pinus patula. J. Environ. Manage. (2015). https://doi.org/10.1016/j.jenvman.2016.04.001
Damartzis, T., Zabaniotou, A.: Thermochemical conversion of biomass to second generation biofuels through integrated process design—a review. Renew. Sustain. Energy Rev. 15, 366–378 (2011). https://doi.org/10.1016/J.RSER.2010.08.003
Johnson, E.: Integrated enzyme production lowers the cost of cellulosic ethanol. Biofuels Bioprod. Biorefin. 10, 164–174 (2016). https://doi.org/10.1002/bbb
Soudham, V.P., Raut, D.G., Anugwom, I., Brandberg, T., Larsson, C., Mikkola, J.P.: Coupled enzymatic hydrolysis and ethanol fermentation: ionic liquid pretreatment for enhanced yields. Biotechnol. Biofuels. 8, 135 (2015). https://doi.org/10.1186/s13068-015-0310-3
Daza Serna, L.V., Orrego Alzate, C.E., Cardona Alzate, C.A.: Supercritical fluids as a green technology for the pretreatment of lignocellulosic biomass. Bioresour. Technol. 199, 113–120 (2016)
Galbe, M., Zacchi, G.: A review of the production of ethanol from softwood. Appl. Microbiol. Biotechnol. 59, 618–628 (2002). https://doi.org/10.1007/s00253-002-1058-9
Hoyer, K., Galbe, M., Zacchi, G.: Production of fuel ethanol from softwood by simultaneous saccharification and fermentation at high dry matter content. J. Chem. Technol. Biotechnol. 84, 570–577 (2009). https://doi.org/10.1002/jctb.2082
St1 Biofuels Oy: Cellunolix® ethanol plant to be built in Finland. https://www.st1.eu/cellunolix-ethanolplant-to-be-built-in-finland
Söderström, J., Pilcher, L., Galbe, M., Zacchi, G.: Two-step pretreatment of softwood with SO2 two-step steam pretreatment of softwood with SO2 impregnation for ethanol production. Appl. Biochem. Biotechnol. 98–100, 5–21 (2002)
St1 Biofuels Oy: St1´s and SOK´s joint venture NEB plans 50-million-litre Cellunolix® bioethanol plant in Pietarsaari. https://www.st1.eu/st1s-and-soks-joint-venture-neb-plans-50-million-litre-cellunolix-bioethanolpla
Aho, A., Kumar, N., Eränen, K., Holmbom, B., Hupa, M., Salmi, T., Murzin, D.Y.: Pyrolysis of softwood carbohydrates in a fluidized bed reactor. Int. J. Mol. Sci. 9(9), 1665–1675 (2008)
Garcìa-Pérez, M., Chaala, A., Pakdel, H., Kretschmer, D., Roy, C.: Vacuum pyrolysis of softwood and hardwood biomass: comparison between product yields and bio-oil properties. J. Anal. Appl. Pyrolysis. 78, 104–116 (2007). https://doi.org/10.1016/J.JAAP.2006.05.003
Oasmaa, A., Solantausta, Y., Arpiainen, V., Kuoppala, E., Sipilä, K.: Fast pyrolysis bio-oils from wood and agricultural residues. Energy Fuels. 24, 1380–1388 (2010). https://doi.org/10.1021/ef901107f
Amaral, S., De Carvalho Junior, A., Costa, M.A.M., Neto, T.G.S., Dellani, R., Leite, L.H.S.: Comparative study for hardwood and softwood forest biomass: chemical characterization, combustion phases and gas and particulate matter emissions. Bioresour. Technol. 164, 55–63 (2014). https://doi.org/10.1016/j.biortech.2014.04.060
Roy, M.M., Corscadden, K.W.: An experimental study of combustion and emissions of biomass briquettes in a domestic wood stove. Appl. Energy. 99, 206–212 (2012). https://doi.org/10.1016/J.APENERGY.2012.05.003
García, C.A., Morales, M., Quintero, J., Aroca, G., Cardona, C.A.: Environmental assessment of hydrogen production based on Pinus patula plantations in Colombia. Energy. 139, 606–616 (2017). https://doi.org/10.1016/j.energy.2017.08.012
Waldner, M.H., Vogel, F.: Renewable production of methane from woody biomass by catalytic hydrothermal gasification. Ind. Eng. Chem. Res. 44, 4543–4551 (2005). https://doi.org/10.1021/ie050161h
Mandl, C., Obernberger, I., Scharler, I.R.: Characterisation of fuel bound nitrogen in the gasification process and the staged combustion of producer gas from the updraft gasification of softwood pellets. Biomass Bioenergy 35, 4595–4604 (2011). https://doi.org/10.1016/J.BIOMBIOE.2011.09.001
Moncada, J., Tamayo, J., Cardona, C.A.: Evolution from biofuels to integrated biorefineries: techno-economic and environmental assessment of oil palm in Colombia. J. Clean. Prod. 81, 51–59 (2014)
Chum, H., Faaij, A., Moreira, J.: Bioenergy. In: Edenhofer, O., Pichs-Madruga, R., Sokona, Y. (eds.) Renewable Energy Sources and Climate Change Mitigation. pp. 46–55. Intergovernmental Panel on Climate Change (IPCC), Geneva (2011)
García-Velásquez, C.A.: Hydrogen production through gasification and dark fermentation. (2016). http://www.bdigital.unal.edu.co/54321/
Timung, R., Mohan, M., Chilukoti, B., Sasmal, S., Banerjee, T., Goud, V.V.: Optimization of dilute acid and hot water pretreatment of different lignocellulosic biomass: a comparative study. Biomass Bioenergy 81, 9–18 (2015). https://doi.org/10.1016/j.biombioe.2015.05.006
Karimi, K., Taherzadeh, M.J.: A critical review of analytical methods in pretreatment of lignocelluloses: composition, imaging, and crystallinity. Bioresour. Technol. 200, 1008–1018 (2016). https://doi.org/10.1016/j.biortech.2015.11.022
Selig, M., Weiss, N., Ji, Y.: Enzymatic Saccharification of Lignocellulosic Biomass, pp. 1–5. National Renewable Energy Laboratory, Golden (2008)
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428 (1959). https://doi.org/10.1021/ac60147a030
Jensen, J., Morinelly, J., Aglan, A., Mix, A., Shonnard, D.: Kinetic characterization of biomass dilute sulfuric acid hydrolysis: mixtures of hardwoods, softwood, and switchgrass. Environ. Energy Eng. 54, 1637–1645 (2008). https://doi.org/10.1002/aic
Esteghlalian, A., Hashimoto, A.G., Fenske, J.J., Penner, M.H.: Modeling and optimization of the dilute-sulfuric-acid pretreatment of corn stover, poplar and switchgrass. Bioresour. Technol. 59, 129–136 (1997). https://doi.org/10.1016/S0960-8524(97)81606-9
Wooley, R.J., Putsche, V.: Development of an ASPEN PLUS physical property database for biofuels components, pp.1–38. National Renewable Energy Laboratory, Golden (1996)
García, C.A., Peña, Á, Betancourt, R., Cardona, C.A.: Energetic and environmental assessment of thermochemical and biochemical ways for producing energy from agricultural solid residues: coffee cut-stems case. J. Environ. Manage. (2017). https://doi.org/10.1016/j.jenvman.2017.04.029
Quintero, J.a., Moncada, J., Cardona, C.a.: Techno-economic analysis of bioethanol production from lignocellulosic residues in Colombia: a process simulation approach. Bioresour. Technol. 139, 300–307 (2013). https://doi.org/10.1016/j.biortech.2013.04.048
Moncada, J., Tamayo, J.A., Cardona, C.A.: Integrating first, second, and third generation biorefineries: incorporating microalgae into the sugarcane biorefinery. Chem. Eng. Sci. 118, 126–140 (2014). https://doi.org/10.1016/j.ces.2014.07.035
Rafiqul, I.S.M., Mimi Sakinah, aM.: Kinetic studies on acid hydrolysis of Meranti wood sawdust for xylose production. Chem. Eng. Sci. 71, 431–437 (2012). https://doi.org/10.1016/j.ces.2011.11.007
Khodaverdi, M., Karimi, K., Jeihanipour, A., Taherzadeh, M.J.: Kinetic modeling of rapid enzymatic hydrolysis of crystalline cellulose after pretreatment by NMMO. J. Ind. Microbiol. Biotechnol. 39, 429–438 (2012). https://doi.org/10.1007/s10295-011-1048-y
Kadam, K.L., Rydholm, E.C., McMillan, J.D.: Development and validation of a kinetic model for enzymatic saccharification of lignocellulosic biomass. Biotechnol. Prog. 20, 698–705 (2004). https://doi.org/10.1021/bp034316x
Quintero, J.A., Cardona, C.A.: Process Simulation of Fuel Ethanol Production from Lignocellulosics using Aspen Plus. Ind. Eng. Chem. Res. 50(10), 6205–6212 (2011)
Pitt, W.W., Haag, G.L., Lee, D.D.: Recovery of ethanol from fermentation broths using selective sorption-desorption. Biotechnol. Bioeng. 25, 123–131 (1983). https://doi.org/10.1002/bit.260250110
García, C.A., Moncada, J., Aristizábal Marulanda, V., Cardona, C.A.: Techno-economic and energetic assessment of hydrogen production through gasification in the Colombian context: coffee cut-stems. Int. J. Hydrogen Energy. 42, 5849–5864 (2017). https://doi.org/10.1016/j.ijheatmasstransfer.2017.09.038
Melgar, A., Pérez, J.F., Laget, H., Horillo, A.: Thermochemical equilibrium modelling of a gasifying process. Energy Convers. Manag. 48, 59–67 (2007). https://doi.org/10.1016/j.enconman.2006.05.004
Jarungthammachote, S., Dutta, A.: Equilibrium modeling of gasification: Gibbs free energy minimization approach and its application to spouted bed and spout-fluid bed gasifiers. Energy Convers. Manag. 49, 1345–1356 (2008). https://doi.org/10.1016/j.enconman.2008.01.006
Peters, M.S., Timmerhaus, K.D., West, R.E.: Plant Design and Economics for Chemical Engineers. McGraw-Hill, New York (2004)
Fuels, F.: Wood chip and wood pellet price comparison. https://www.forestfuels.co.uk/wood-fuel-pricecomparison/
Kemcore: Reagents Market Price. https://www.kemcore.com/sulphuric-acid-98.html
Liu, G., Zhang, J., Bao, J.: Cost evaluation of cellulase enzyme for industrial-scale cellulosic ethanol production based on rigorous Aspen Plus modeling. Bioprocess. Biosyst. Eng. 39, 133–140 (2016). https://doi.org/10.1007/s00449-015-1497-1
Federación Nacional de Biocombustibles de Colombia—Fedebiocombustibles: Biofuel prices in Colombia 2016–2017
Ulrich, G.D., Vasudevan, P.T.: How to estimate utility costs. Chem. Eng. 113, 66–69 (2006)
Boundy, B.: Biomass Energy Data Book, 4 ed, p. 254. Deparment of Energy, Washington DC, (2011)
Kim, K.H.: Two-stage dilute acid-catalyzed hydrolytic conversion of softwood sawdust into sugars fermentable by ethanologenic microorganisms. J. Sci. Food Agric. 85, 2461–2467 (2005). https://doi.org/10.1002/jsfa.2268
Bösch, P., Wallberg, O., Joelsson, E., Galbe, M., Zacchi, G.: Research impact of dual temperature profile in dilute acid hydrolysis of spruce for ethanol production. Biotechnol. Biofuels. 3, 15 (2010). https://doi.org/10.1186/1754-6834-3-15
Shinozaki, Y., Kitamoto, H.K.: Ethanol production from ensiled rice straw and whole-crop silage by the simultaneous enzymatic saccharification and fermentation process. J. Biosci. Bioeng. 111, 320–325 (2011). https://doi.org/10.1016/j.jbiosc.2010.11.003
Dinh, T.N., Nagahisa, K., Hirasawa, T., Furusawa, C., Shimizu, H.: Adaptation of Saccharomyces cerevisiae cells to high ethanol concentration and changes in fatty acid composition of membrane and cell size. PLoS ONE. 3, e2623 (2008). https://doi.org/10.1371/journal.pone.0002623
Alvira, P., Tomás-Pejó, E., Ballesteros, M., Negro, M.J.: Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour. Technol. 101, 4851–4861 (2010). https://doi.org/10.1016/j.biortech.2009.11.093
Qian, M., Tian, S., Li, X., Zhang, J., Pan, Y., Yang, X.: Ethanol production from dilute-acid softwood hydrolysate by co-culture. Appl. Biochem. Biotechnol. (2006). https://doi.org/10.1385/ABAB:134:3:273
Nyangi Chacha: Comparison of Escherichia coli KO11 and Saccharomyces cerevisiae ATCC 96581 in fermenting Pinus patula hydrolysate pretreated at different steam explosion severity. Afr. J. Biotechnol. (2012). https://doi.org/10.5897/AJB11.3498
Hawkins, G.M., Doran-Peterson, J.: A strain of Saccharomyces cerevisiae evolved for fermentation of lignocellulosic biomass displays improved growth and fermentative ability in high solids concentrations and in the presence of inhibitory compounds. Biotechnol. Biofuels. 4, 49 (2011). https://doi.org/10.1186/1754-6834-4-49
Nguyen, Q.A., Tucker, M.P., Keller, F.A., Beaty, D.A., Connors, K.M., Eddy, F.P.: Dilute acid hydrolysis of softwoods. Appl. Biochem. Biotechnol. (1999). https://doi.org/10.1385/ABAB:77:1-3:133
Mesa, L., Martínez, Y., Barrio, E., González, E.: Desirability function for optimization of dilute acid pretreatment of sugarcane straw for ethanol production and preliminary economic analysis based in three fermentation configurations. Appl. Energy. 198, 299–311 (2017). https://doi.org/10.1016/j.apenergy.2017.03.018
Parajó, J.C., Vázquez, D., Alonso, J.L., Santos, V., Dominguez, H.: Prehydrolysis of Eucalyptus wood with dilute sulphuric acid: operation at atmospheric pressure. Holz als Roh- und Werkst. 51, 357–363 (1993). https://doi.org/10.1007/BF02663809
Daystar, J., Treasure, T., Gonzalez, R., Reeb, C., Venditti, R., Kelley, S.: The NREL biochemical and thermochemical ethanol conversion processes: financial and environmental analysis comparison. BioResources. 10, 5096–5116 (2015). https://doi.org/10.15376/biores.10.3.5096-5116
Zhao, L., Zhang, X., Xu, J., Ou, X., Chang, S., Wu, M.: Techno-economic analysis of bioethanol production from lignocellulosic biomass in china: dilute-acid pretreatment and enzymatic hydrolysis of corn stover. Energies. 8, 4096–4117 (2015). https://doi.org/10.3390/en8054096
Foust, T.D., Aden, A., Dutta, A., Phillips, S.: An economic and environmental comparison of a biochemical and a thermochemical lignocellulosic ethanol conversion processes. Cellulose. 16, 547–565 (2009). https://doi.org/10.1007/s10570-009-9317-x
Piccolo, C., Bezzo, F.: A techno-economic comparison between two technologies for bioethanol production from lignocellulose. Biomass Bioenergy 33, 478–491 (2009). https://doi.org/10.1016/j.biombioe.2008.08.008
Wingren, A., Söderström, J., Galbe, M., Zacchi, G.: Process considerations and economic evaluation of two-step steam pretreatment for production of fuel ethanol from softwood. Biotechnol. Prog. 20, 1421–1429 (2004). https://doi.org/10.1021/bp049931v
Unidad de Planeación Energética (UPME): Informe Mensual De Variables De Generación Y Del Mercado Eléctrico Colombiano., Bogotá, Colombia (2015)
Acknowledgements
The authors express their acknowledgments to the Centro de Bioinformática y Biología Computacional (BIOS) for the financial support through the project entitled “Fortalecimiento de CTEI en biotecnologia para el departamento de Caldas apoyado por infraestructura computacional avanzada y trabajo colaborativo (CALDAS BIOREGION)” Grant No. 08112013-0621. The authors also express their gratitude to the Universidad Nacional de Colombia Sede Manizales through the Projects entitled “Development of modular small-scale integrated biorefineries to produce an optimal range of bioproducts from a variety of rural agricultural and agroindustrial residues/wastes with a minimum consumption of fossil energy—SMIBIO” from ERANET LAC 2015 Grant No. 202010011331 and the Project “Techno-economic and environmental evaluation of a biorefinery using the residues from the Coffee Crop” Grant No. 202010014230.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
García-Velásquez, C.A., Carmona-Garcia, E., Caballero, A.S. et al. Fermentative Production of Ethanol Using Pinus patula as Raw Material: Economic and Energy Assessment. Waste Biomass Valor 11, 1777–1788 (2020). https://doi.org/10.1007/s12649-018-0494-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-018-0494-4