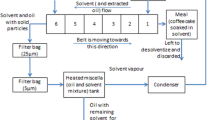
Abstract
Oil extracted from spent coffee ground (SCG) has been well known as a potential feedstock for high quality biodiesel production. This work was to investigate extraction, physical and chemical characterizations of Robusta coffee oil (CO) and its application for biodiesel production. Analysis of seven coffee ground (CG) samples showed that oil content in CGs depended on technique of the manufacturer. Morphological changes of CGs surface were recorded by FESEM technique which showed the particle size significantly increased with the oil loss. Infrared spectroscopies revealed absence of SCG oil in the de-oiled SCG, confirmed that soxhlet method in hexane was used efficiently for the oil extraction. Thermal properties of SCG oil, fresh coffee ground (FCG), SCG and de-oiled SCG samples were investigated by simultaneous TG–DTA measurement. The obtained data showed the oil content relating to thermal changes of SCG samples. Comparison between chemical components of Robusta coffee bean (RCB) and SCG reflected a fact that most of oil content in the SCG could be originated in manufacturing process of FCG. Quality biodiesel product has prepared from SCG oils via a two-step process. After pre-treatment process, transesterification of SCG oils was carried out with methanol (v/v, 30%) and NaOH (w/v, 1%) in yield 89.2%.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With increasing global energy demand, alternative renewable energy sources have attracted significantly attention due to the finite nature and emissions greenhouse gases of fossil fuel reserves. The geopolitical instability and security caused the volatility of oil prices [1, 2]. In order to cope with the oil prices rapidly rising, new renewable approaches of energy supply as a cost-competitive and stable solution are urgently required.
Coffee beans are produced in over 46 countries, provided about 8.60 million tons worldwide. There are ten producers of coffee beans worldwide, with Brazil and Vietnam accounted for nearly half of total production and RCB are produced globally with more 40% of the total bean types in 2015 as seen in Fig. 1 [3]. As the second largest producer, Vietnam exported 1.65 million tons in 2015, in which Robusta coffee accounts for 97% of total output due to the geographical condition of the country appropriate for Robusta’s growth [4, 5].
Total production of coffee by countries (a) and types of bean (b) in 2015 [3]
Biodiesel, a mixture of long chain fatty acids alkyl ester derived from vegetable oils or animal fats by transesterification with methanol or ethanol in presence of alkaline catalyst [6], has been particularly considered as an efficient alternative for fossil fuel due to very low greenhouse gas emissions [7, 8]. Unlike fossil diesel fuel, biodiesel as a non-toxic, biodegradable and environment-friendly fuel contains no aromatics and almost no sulfur which has been attributed to reducing negative impact to the life environment [9]. Despite this favourable impact, high price of biodiesel is the major obstacle toward its marketability. The critical reason for the failure of the biodiesel programme was inappropriate pricing policy of the feedstock [10]. In fact, prices of traditional biofuel feedstocks have been rapidly increased over recent years due to competition of food and feed production. For instant, price of virgin vegetable oils has increased nearly twice in several years (from early 2000s to 2007) [11]. As a result, investigating ways to minimum the cost of feedstock available for biodiesel manufacturing has being strongly interested during recent years. Among them, use of biomass residues such as waste cooking oils [12], animal fats [13] and by-products of industrial product [14] has been excellent solutions for commercially low-cost biodiesel production. A potential source of waste residues is well known as SCG. Assuming 10 wt% oil content in SCG, this can add approximately 0.8 million tons of biodiesel to the world’s fuel supply. Moreover, because SCG oil contained naturally very high content of antioxidant which was attributed to stabilization of the oil as well as its biodiesel, it has been considered as a high quality and cost-effective feedstock for biodiesel production in comparison with other waste sources [15, 16].
Although many techniques have been used to manufacture biofuel from waste oils such as pyrolytic distillation [17], catalytic cracking [18], and bio-hydrotreating diesel [19], the transesterification process to obtain high quality biodiesel has revealed as an efficiently low-cost method for biofuel production [12, 20]. The lipid oil amount in CGs varies 10–20 wt% depending upon types of beans and its origin. On average, its lipid composition contains 90% of glycerides which can be transesterified with methanol to produce biodiesel [11]. There are many transesterification techniques reported for biodiesel production from different lipid oils such as heterogeneous catalyzed transesterification [21], lipase catalyzed transesterification [22] or supercritical transesterification [23]. Each transesterification technique requires different oil feedstock characters. Therefore, understanding of SCG oil properties is particularly important to utilize effectively this waste sources for biofuel manufacturing. However, physicochemical data of SCG oil extracted from Vietnamese Robusta CGs have not been provided fully so far. In this work, we focus to investigate the oil extraction, physical properties and chemical compositions of Vietnamese Robusta coffee oil and its application in biodiesel manufacturing.
Materials and Methods
Materials and Measurement
Seven Robusta Fresh coffee ground (FCG) samples named from S1 to S7, which were collected from Vietnam market with 100% Robusta beans, were brewed with freshly boiled water throughout a coffee phin for 10 min. Then the coffee residue was dried to obtain the SCG samples. In order to study composition and origin of CO, the green RCB harvested from Lam Dong Province in Vietnam, was roasted without any additives and then grinded up powder. The moisture contents in FCG, SCG and RCB were recorded between 7.1 and 9.0%. All of the CG samples were dried in an oven at 70 °C for 24 h to reduce the moisture before using.
The morphology of CGs and de-oiled SCG was measured by field emission scanning electron microscopy (FESEM) using JSM7401F (Japan). FTIR spectra were recorded on a BRUKER EQUINOX 55 IR spectrophotometer. A LabSys evo S60/58988 Thermoanalyzer (Setaram, France) was used for simultaneous thermal analysis combining thermogravimetric (TG) analysis and differential thermal analysis (DTA) with a heating rate of 10 °C/min in the air atmosphere.
Oil Extraction
Coffee oil was extracted by using Soxhlet method within hexane (250 mL). Samples (RCB, SCGs and FCGs) were weighted accurately 15 g and placed in a thimble. The hexane was refluxed at boiling point for 4 h. After extraction had been accomplished, the solvent was evaporated under vacuum at 40 °C. The oil extraction yield for each case is calculated as follows:
Oil Properties
The oils collected from RCB and SCG were characterized by determining the viscosity, density, acid value, saponification, unsaponifiable matter and iodine value. The acid value, which reflects the total acidity or the amount of free fatty acid (FFA) not attached to a glycerol backbone, was defined as the weight (mg) of potassium hydroxide required to neutralize the free acid groups in oil [24]. Herein, the acid value was determined by indirect method of titration as described elsewhere [11]. Saponificated value was defined as the amount of required alkali to saponify a defined weight of sample and specified the free acid and bound acid, e.g. esters of glycerol. It is expressed in mg of KOH per gram sample [24]. The amount of the fatty acid (FA) and the number of ester bonds per g sample can be derived from the saponified value.
The FA composition in oils was determined through fatty acid methyl ester (FAME) contents of biodiesel products prepared by transesterification as described in the following section. The FAME content was estimated by GC–MS method [25]. In the instrument, the quadrupole mass analyzer was directly connected to capillary column and an electron multiplier detector. Helium was used as a carrier gas for GC system.
Transesterification
In order to reduce FFA value, the extracted oils (100 mL) was stirred with concentrated H2SO4 (0.1 mL), hexane (100 mL) and methanol (20 mL) for 2 h. The mixture was washed with water to pH 7.0 and dried by anhydrous Na2SO4. The solvent was evaporated in vacuum at 40 °C. The percent of FFA in oil was determined < 1%. The pre-treated oil was transferred into the reaction flask and esterified with methanol and KOH as the catalyst. The mixture was remained at 63 ± 2 °C within 2 h. The effect of catalyst and methanol on FAME yield was investigated by varying sodium hydroxide concentration and methanol volume to oil ratio. After completion of the esterification, the biodiesel layer was separated after 12 h and washed with water, brine and dried over anhydrous Na2SO4. The excess methanol in the biodiesel was evaporated under vacuum at 40 °C. The percent yield of biodiesel is calculated as follows [26]:
Biodiesel Analysis
Properties of coffee biodiesels were measured using the standard methods as described previously. Standard test methods included viscosity (mm2/s), ASTM D445; density (mg/mL), ASTM D1298; acid value (mg KOH/g), ASTM D664; free glycerol (mass %), ASTM D6584; total glycerol (mass %), ASTM D6584; methyl ester (mass %), EN 14103.
Results and Discussion
Oil Extraction
All FCG samples were chosen from Vietnamese market with 100% of Robusta beans. Yields of oil extraction are presented in Fig. 2. Extraction yield of seven commercial CGs (S1–S7) ranged from 6.5 to 18.7% (w/w) with an average value of oil content at 12.0% (w/w) and a maximum value at 18.7% for FCG sample S1. In comparison with natural RCB oil content, the average oil content of CG samples was approximately double to the content of natural RCB (6.2%, (w/w)), even triple calculated for the sample S1 (Fig. 3). It facts that amount of CO was depended on technology of the coffee manufacturer, accounted variation of oil content in the previous reports despite using the same extraction technique [27,28,29,30].
On the other hand, oil amount of SCGs is slightly lower than that extracted from the FCGs, reflected that small amount of FCG oil moved into the hot water within the brewed process through the coffee phin. In the present work, we selected the sample S1 which contained the highest oil content for further studies.
FESEM Analysis
Solubilization compounds inducing changes of FCG surface were recently investigated by Mateus et al. [31]. However, effect of oil loss on the surface of CGs which can be observed by FESEM analysis has not reported. FESEM observation of FCG, SCG and de-oiled SCG samples (Fig. 4) exhibited coffee pores with diameter in arrange of 100–150 µm, in agreement with that of previous reports [31, 32]. The analysis of FCG and SCG samples (Fig. 4a, c) showed similar results of morphology and average particle size (35 µm), indicated that changes of CG surface in the brewing process is not very much. Clear changes of morphology however can be observed from FESEM images of SCGs (Fig. 4c, e). The oil loss might induce subversion to form great number of particles on de-oiled SCG surface. Particle size of SCG and de-oil SCG was calculated in range of 20–110 and 20–220 µm, respectively and the average size of de-oiled SCG particle (45 µm) was greater than that of SCG (35 µm). Thus, the oil extraction showed evident effect on the CGs surface.
FTIR Analysis
FTIR spectra were analyzed to characterize components of SCG oil and to determine efficiency of the oil extraction. The FTIR spectroscopies of SCG, SCG oil and de-oiled SCG are presented in Fig. 5. The SCG oil sample showed the peaks at 2925 and 2855 cm−1, assigned to asymmetric and symmetric stretching vibration of C–H bonds of aliphatic CH2 group of the fatty acid backbone, respectively. A shoulder at 3009 cm−1, characteristic to the C–H stretching vibration of cis double bonds showed presence of unsaturated fatty acids. Absorption band for ester bonds between glycerol and FAs was observed at 1745 cm−1, corresponding to stretching vibration of ester carbonyl group. Also, the wavenumber 1163 cm−1 was assigned to stretching vibration of C–O ester group [33].
The presence of oil in the SCG sample can be unambiguously observed from the FTIR spectra that exhibited all characteristic peaks of lipid compounds. The SCG sample showed a broad peak at 3384 cm−1 relating to stretching vibration of OH groups. The sharp peak at 1659 cm−1 was identified for carbonyl groups of caffeine which has been used as the determinant band in quantitative analysis of caffeine in roasted coffee samples [34]. The wavenumber range of 1400–900 cm−1 was characterized by vibrations of several types of bond, including C–H, C–O and C–N, attributed to absorption band of carbohydrates and several special compounds in the coffee beans (e.g. chlorogenic acids) as reported previously [35, 36]. The fact that most of the characteristic peaks for the lipid compounds were absent in the spectroscopy of de-oiled sample, indicated that coffee oil might be extracted completely in hexane solvent by soxhlet method.
Thermal Analysis
Simultaneous TG–DTA and DTG curves of de-oiled SCG, SCG and SCG oil in the air atmosphere are shown in Fig. 6 and their thermal data are listed in Table 1. The results of the SCG oil showed that mass loss did not occur at temperature below 230 °C, indicated that there is no solvent in the extracted oil. Thermal decomposition of the SCG oil occurs completely within three stages between 230 and 590 °C. Percent of mass loss of SCG oil in initial stages between 230 and 480 °C (85%) greater than that of SCG (69%), FCG (51%) and de-oiled SCG (54%). All the CGs showed loss of mass (3–4%) at the temperature range between 67 and 230 °C which corresponds to removal of volatile compounds and water molecules [37]. Moreover, it is clear that the thermal stability increases in the order of de-oiled SCG (550 °C), FCG (569 °C) and SCG (580 °C) attributed to effect of oil content in the SCG sample. The ash content of FCG (8%) remained from oxidation process at 700 °C is higher than that of SCG (2%) and de-oiled SCG (5%). It can be due to inorganic compounds of FCG samples solubilized in the brewing process. A similar trend from burning process was also observed in the previous report [38, 39].
DTA curve of SCG oil showed that exothermic reactions occur at maximum peak of 365 °C and Texo values depend on content of coffee oil. After the exothermic reaction, the final oxidations of the matter were found at three Texo values, 416, 473 and 534 °C. Also, the presence of oil was attributed to increase of Texo values of the SCG (347, 518 and 545 °C) and FCG (351, 500 and 561 °C) samples in comparison with that of de-oiled SCG (316, 450 and 474 °C).
Oil Properties
The properties of the extracted oil from RCB and SCG are shown in Table 2. Both the kinematic viscosity (74.15 mm2/s at 30 °C) and density (0.92 g/mL) of RCB were slightly higher than the SCG oil viscosity (62.80 mm2/s at 30 °C) and density (0.89 g/mL). Remarkably, the FFA value of SCG oil is as high as 3.07%, over double to that of RCB (1.41%). This value suggested that the transesterification of SCG and RCB oils should be carried out according to a two-step process which is presented thereafter.
Fatty Acid Composition
Since Jenkins et al. [40] showed that the FA composition of the both FCG and SCG oils including materials from Vietnam is almost similar, this work only focuses on comparison of FA composition between natural RCB and SCG oils. The results are shown in Table 3. The palmitic acid (C16:0) and steric acid (C18:0) contents are not much different in the both types of oil while the RCB oil contains oleic acid content (12.22%) much lower than linoleic acid (42.13%) which was also observed by other research groups on RCB samples from other countries [41, 42]. In contrast, the RCG oil obtained by the same extraction exhibits oleic acid content (30.90%) being greater than linoleic acid content (24.34%). This result is in agreement with Jenkins’ study on Vietnamese commercial CG samples [40]. The oleic acid content, which was found popularly in animal and vegetable fats and oils, increasing highly in SCG oil compared with natural RCB oil reflects that the lipid sources containing high oleic acid content could be added into the coffee beans in production process. The addition could purpose to improve taste of drinking Robusta coffee.
Transesterification
The oil extracted from SCG was found FFA value as high as 3.07% which is above the satisfactory limit of the transesterification reaction using alkaline catalyst. In order to avoid a higher degree of oxidation and hydrolysis reactions during the process [43], transesterification of the SCG oil to biodiesel was carried out using the two-step method [44]. FFA should be initially converted into ester in a pre-treatment process with a solvent mixture of hexane and methanol using acid sulfuric as a catalyst. Presence of hexane can improve the miscibility between oil and methanol during the esterification process. After percent of FFA in oil determined < 1% (w/w), the pre-treated oil is completely transesterified using KOH as a catalyst. Because the transesterification is almost complete within 2 h at boiling point of methanol 63 ± 2 °C, two critical parameters, NaOH catalyst and methanol concentration, which strongly affect to biodiesel yield of coffee oil, were focused to investigate in the present work. The results presented in Fig. 7. When concentration of NaOH was varied from 0.5 to 1.5% (w/v) at 35% (v/v) methanol concentration (molar ratio of oil to methanol 1:3.5) the yield of biodiesel increased up to 88.2% for 1.0% NaOH concentration and then the yield dropped. A similar trend is also observed in the case of methanol concentration as showed in the Fig. 7b. When concentration of methanol varied from 20 to 50% (v/v) (molar ratio of oil to methanol ranged from 1:2 to 1:5) within 1% (w/v) NaOH concentration, the highest biodiesel yield was achieved at 89.2% for 30% methanol (molar ratio of oil to methanol 1:3). Also, the yield dramatically declines with increase of methanol amount. It can be because the separation of biodiesel becomes difficult with an excess of methanol [26].
Biodiesel Properties
The properties of biodiesel fuel prepared from SCGs are analyzed by ASTM analysis. The data is showed in Table 4 where displays > 97% of FAME content. It revealed that biodiesel from Vietnamese Robusta SCGs is a strong candidate as an alternative to diesel.
Conclusion
We have demonstrated that oil of Robusta CG was originated from production process and SCGs can be used as a potential feedstock to produce qualitative biodiesel. The SCG with average oil content approximately double to oil content of RCB and the significant difference between chemical components of RCB and SCG oils was evidence that CGs oil could be originated within manufacturing process. The thermal properties of CG samples significantly depend on content of the oil in CGs. DTA curve of coffee oil showed three exothermic peaks which were attributed to the oxidation reactions of oil. Oil content in SCG induced increase of both the decomposition and exothermic temperatures. The SCG oil was converted into high quality biodiesel via a two-step process with yield of 89.2%. We strongly believe that cost of biodiesel feedstock from SCG reduces significantly when this material source is utilized efficiently.
Abbreviations
- SCG:
-
Spent coffee ground
- CO:
-
Coffee oil
- RCB:
-
Robusta coffee bean
- FCG:
-
Fresh coffee ground
- TG:
-
Thermogravimetric
- DTA:
-
Differential thermal analysis
- FA:
-
Fatty acid
- FFA:
-
Free fatty acid
- FAME:
-
Fatty acid methyl ester
References
Luque, R., Lovett, J.C., Datta, B., Clancy, J., Campelo, J.M., Romero, A.A.: Biodiesel as feasible petrol fuel replacement: a multidisciplinary overview. Energy Environ. Sci. 3, 1706–1721 (2010). https://doi.org/10.1039/C0EE00085J
Goodwin, A.R.H.: The future of oil and gas fossil fuels. In: Letcher, T. M. (ed.) Future Energy Improved, Sustainable and Clean Options for Our Planet, pp. 3–24. Elsevier, New York (2008)
International Coffee Organization (ICO): Total production by all exporting countries. http://www.ico.org. Accessed 10 September 2016
Marsh, A.: Diversification by Smallholder Farmers: Viet Nam Robusta Coffee. FAO, Rome (2007)
Dhaeze, D., Deckers, J., Raes, D., Phong, T.A., Loi, H.V.: Environmental and socio-economic impacts of institutional reforms on the agricultural sector of Vietnam: land suitability assessment for Robusta coffee in the Dak Gan region. Agric. Ecosyst. Environ. 105, 59–76 (2005). https://doi.org/10.1016/j.agee.2004.05.009
Graboski, M.S., McCornick, R.L.: Combustion of fat and vegetable oil derived fuels in diesel engines. Prog. Energy Combust. Sci. 24, 125–164 (1998). https://doi.org/10.1016/S0360-1285(97)00034-8
Marchetti, J.M., Miguel, V.U., Errazu, A.F.: Possible methods for biodiesel production. Renew. Sustain. Energy Rev. 11, 1300–1311 (2007). https://doi.org/10.1016/j.rser.2005.08.006
Balat, M.: Production of biodiesel from vegetable oils: a survey. Energy Source A 29, 895–913 (2007). https://doi.org/10.1080/00908310500283359
Norjannah, B., Ong, H.C., Masjuki, H.H., Juan, J.C., Chong, W.T.: Enzymatic transesterification for biodiesel production: a comprehensive review. RSC Adv. 6, 60034–60055 (2016). https://doi.org/10.1039/C6RA08062F
Antolin, G., Tinaut, F.V., Briceno, Y., Castrano, V., Perez, C., Ramirez, A.I.: Optimisation of biodiesel production by sunflower oil transesterification. Bioresour. Technol. 83, 111–114 (2002). https://doi.org/10.1016/S0960-8524(01)00200-0
Al-Hamamre, Z., Foerster, S., Hartmann, F., Kroger, M., Kaltschmitt, M.: Oil extracted from spent coffee grounds as a renewable source for fatty acid methyl ester manufacturing. Fuel 96, 70–76 (2012). https://doi.org/10.1016/j.fuel.2012.01.023
Phan, A.N., Phan, T.M.: Biodiesel production from waste cooking oils. Fuel 87, 3490–3496 (2008). https://doi.org/10.1016/j.fuel.2008.07.008
Bankovic-Ilic, I.B., Stojkovic, I.J., Stamenkovic, O.S., Veljkovic, V.B., Hung, Y.T.: Waste animal fats as feedstocks for biodiesel production. Renew. Sustain. Energy Rev. 32, 238–254 (2014). https://doi.org/10.1016/j.rser.2014.01.038
Rodriguez, R.P., Melo, E.A.: Conversion of by-products from the vegetable oil industry into biodiesel and its use in internal combustion engines: a review. Braz. J. Chem. Eng. 31, 287–301 (2014). https://doi.org/10.1590/0104-6632.20140312s00002763
Yanagimoto, K., Ochi, H., Lee, K.G., Shibamoto, T.J.: Antioxidative activities of fractions obtained from brewed coffee. J. Agric. Food Chem. 52, 592–596 (2004). https://doi.org/10.1021/jf030317t
Oliveira, L.S., Franca, A.S., Camargos, R.R.S., Ferraz, V.P.: Coffee oil as a potential feedstock for biodiesel production. Bioresour. Technol. 99, 3244–3250 (2007). https://doi.org/10.1016/j.biortech.2007.05.074
Arpa, O., Yumrutas, R., Demirbas, A.: Production of diesel-like fuel from waste engine oil by pyrolitic distillation. Appl. Energy 87, 122–127 (2010). https://doi.org/10.1016/j.apenergy.2009.05.042
Chen, J., Jiang, J.C., Nie, X.A., Xu, J.M., Chang, X., Li, K.: Diesel-like fuel production from catalytic cracking and esterification of waste oil. J. Renew. Sustain. Energy 5, 052004 (2013). https://doi.org/10.1063/1.4822035
Phimsen, S., Kiatkittipong, W., Yamada, H., Tagawa, T., Kiatkittipong, K., Laosiripojana, N., Assabumrungrat, S.: Oil extracted from spent coffee grounds for bio-hydrotreated diesel production. Energy Convers. Manag. 126, 1028–1036 (2016). https://doi.org/10.1016/j.enconman.2016.08.085
Go, A.W., Sutanto, S., Ong, L.K., Tran-Nguyen, P.L., Ismadji, S., Ju, Y.H.: Developments in in-situ (trans) esterification for biodiesel production: a critical review. J. Renew. Sustain. Energy 60, 284–305 (2016). https://doi.org/10.1016/j.rser.2016.01.070
Borges, M.E., Díaz, L.: Recent developments on heterogeneous catalysts for biodiesel production by oil esterification and transesterification reactions: a review. Renew. Sustain. Energy Rev. 16, 2839–2849 (2012). https://doi.org/10.1016/j.rser.2012.01.071
Bajaj, A., Lohan, P., Jha, P.N., Mehrotra, R: Biodiesel production through lipase catalyzed transesterification: an overview. J. Mol. Catal. B 62, 9–14 (2010). https://doi.org/10.1016/j.molcatb.2009.09.018
Laosiripojana, N., Kiatkittipong, W., Sutthisripok, W., Assabumrungrat, S.: Synthesis of methyl esters from relevant palm products in near-critical methanol with modified-zirconia catalysts. Bioresour. Technol. 101, 8416–8423 (2010). https://doi.org/10.1016/j.biortech.2010.05.076
Wrolstad, R.E., Acree, T.E., Decker, E.A., Penner, M.H., Reid, D.S., Schwartz, S.J., Shoemaker, C.F., Smith, D., Sporns, P.: Hand Book of Food Analytical Chemistry Water, Proteins, Enzymes, Lipids and Carbohydrates. Wiley, Hoboken (2000)
Jain, Z., Xuanjun, W., Qilong, H., Mingjun, H., Shuyan, L.: Physicochemical properties, combustion and emission performance of a novel Zanthoxylum bungeanum seed oil methylic ester biodiesel. Int. J. Green Energy 12, 1255–1262 (2015). https://doi.org/10.1080/15435075.2014.892492
Poojary, S., Rao, C.V., Venkatesh, K.H.: Scleropyrum pentandrum (Dennst.) mabb—oil as a feedstock for biodiesel production—engine performance and emission studies. Int. J. Green Energy 14, 279–288 (2017). https://doi.org/10.1080/15435075.2016.1254637
Kondamudi, N., Mohapatra, S.K., Misra, M.: Spent coffee grounds as a Versatile source of green energy. J. Agric. Food Chem. 56, 11757–11760 (2008). https://doi.org/10.1021/jf802487s
Banerjee, A., Singh, V., Solanki, K., Mukherjee, J., Gupta, M.N.: Combi-protein coated microcrystals of lipases for production of biodiesel from oil from spent coffee. Sustain. Chem. Process. 1, 1–9 (2013). https://doi.org/10.1186/2043-7129-1-14
Haile, M., Asfaw, A., Asfaw, N.: Investigation of waste coffee ground as a potential raw material for biodiesel production. Int. J. Renew. Energy Res. 3, 854–860 (2013)
Atabani, A.E., Mercimek, S.M., Arvindnarayan, S., Shobana, S., Kumar, G., Cadir, M., Al-Muhatseb, A.H.: Valorization of spent coffee grounds recycling as a potential alternative fuel resource in Turkey: an experimental study. J. Air Waste Manag. Assoc. (2017). https://doi.org/10.1080/10962247.2017.1367738
Mateus, M.L., Rouvet, M., Gumy, J.C., Liardon, R.: Interactions of water with roasted and ground coffee in the wetting process investigated by a combination of physical determinations. J. Agric. Food Chem. 55, 2979–2984 (2007). https://doi.org/10.1021/jf062841g
Anderson, B.A., Shimoni, E., Liardon, R., Labuza, T.P.: The diffusion kinetics of carbon dioxide from fresh roasted and ground coffee. J. Food. Eng. 59, 71–78 (2003). https://doi.org/10.1016/S0260-8774(02)00432-6
Raba, D.N., Poiana, M.A., Borozan, A.B., Stef, M., Radu, F., Popa, M.V.: Investigation on crude and high-temperature heated coffee oil by ATR-FTIR spectroscopy along with antioxidant and antimicrobial properties. PLoS ONE 10, e0138080 (2015). https://doi.org/10.1371/journal.pone.0138080
Garrigues, J.M., Bouhsain, Z., Garrigues, S., Guardia, M.D.L.: Fourier transform infrared determination of caffeine in roasted coffee samples. Fresenius J. Anal. Chem. 366, 319–322 (2000). https://doi.org/10.1007/s002160050063
Wang, J., Jun, S., Bittenbender, H.C., Gautz, L., Li, Q.X.: Fourier transform infrared spectroscopy for Kona coffee authentication. J. Food Sci. 74, C385–C391 (2009). https://doi.org/10.1111/j.1750-3841.2009.01173.x
Lyman, D.J., Benck, R., Dell, S., Merle, S., Murray-Wijelath, J.: FTIR-ATR analysis of brewed coffee: effect of roasting conditions. J. Agric. Food Chem. 51, 3268–3272 (2003). https://doi.org/10.1021/jf0209793
Todaka, M., Kowhakul, W., Masamoto, H., Shigematsu, M.: Thermal analysis and dust explosion characteristics of spent coffee grounds and jatropha. J. Loss Prev. Process Ind. 44, 538–543 (2016). https://doi.org/10.1016/j.jlp.2016.08.008
Silva, M.A., Nebra, S.A., Silva, M.J.M., Sanchez, C.G.: The use of biomass residues in the Brazilian soluble coffee industry. Biomass Bioenergy 14, 457–467 (1998). https://doi.org/10.1016/S0961-9534(97)10034-4
Somnuk, K., Eawlex, P., Prateepchaikul, G.: Optimization of coffee oil extraction from spent coffee grounds using four solvents and prototype-scale extraction using circulation process. Agric. Nat. Resour. 51, 181–189 (2017). https://doi.org/10.1016/j.anres.2017.01.003
Jenkins, R.W., Stageman, N.E., Fortune, C.M., Chuck, C.J.: Effect of the type of bean, processing and geographical location on the biodiesel produced from waste coffee grounds. Energy Fuels 28, 1166–1174 (2014). https://doi.org/10.1021/ef4022976
Vila, M.A., Andueza, S., Pena, M.P.D., Cid, C.: Fatty acid evolution during the storage of ground, roasted coffees. J. Am. Oil Chem. Soc. 82, 639–646 (2005). https://doi.org/10.1007/s11746-005-1122-1
Speer, I.K., Speer, K.: The lipid fraction of the coffee bean. Braz. J. Plant. Physiol. 8, 201–216 (2006). https://doi.org/10.1590/S1677-04202006000100014
Knothe, G.: Some aspects of biodiesel oxidative stability. Fuel Process Technol. 88, 669–677 (2007). https://doi.org/10.1016/j.fuproc.2007.01.005
Burton, R., Fan, X., Austic, G.: Evaluation of two-step reaction and enzyme catalysis approaches for biodiesel production from spent coffee grounds. Int. J. Green Energy 7, 530–536 (2010). https://doi.org/10.1080/15435075.2010.515444
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dang, CH., Nguyen, TD. Physicochemical Characterization of Robusta Spent Coffee Ground Oil for Biodiesel Manufacturing. Waste Biomass Valor 10, 2703–2712 (2019). https://doi.org/10.1007/s12649-018-0287-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-018-0287-9