Abstract
Changes in organic matter (OM) characteristics of wastewater sludge (WWS) submitted to three handling processes were monitored. Thermal drying, electron beam (e-beam) irradiation and anaerobic digestion were compared. The knowledge of the characteristics of the final residual biomass is essential to improve its valorization. The OM of WWS was investigated at the global scale using elemental analysis, infrared spectroscopy, thermogravimetric analysis and chemical fractionation. Double-shot thermochemolysis coupled with gas chromatography and mass spectrometry (GCMS) was used to compare the diversity and distribution of the molecular contents. A strong influence of thermal drying on lipids and humic-like substances contents was observed through fractionation, which traduced a weakening of the OM. The anaerobic digestion induced an increase in lipids for the hydrolysis phase followed by a decrease which correlates with the volume reduction of sludge by about 30%. E-beam induced change in the distribution of the different pools of organic matter depending on the irradiation dose. At the molecular scale, fatty acids, steroids and aromatics were the main thermochemolysis products in all the samples. The thermal drying induced an increase in fatty acids and in steroids, probably released from the refractory OM. Anaerobic digestion modified exclusively the amount and distribution of fatty acids while e-beam induced a decrease in all the identified compounds including aromatics. Finally double-shot thermochemolysis-GCMS demonstrated that the consequences of the handling process on the molecular contents of WWS should be taken into account for the choice of the final valorization pathway.
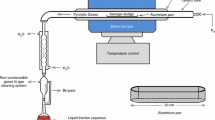
Graphical Abstract
Studied handling processes

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sludge management into a wastewater treatment plant (WWTP) represents major capital and operating expenditures and is an important technical challenge. The main limitation in sludge handling is dewatering operations consequently several advanced sludge treatments (AST) including thermal hydrolysis, chemical oxidation and biological digestion have been developed [1, 2]. In WWS, microorganisms are embedded and protected into a strongly hydrated matrix of extracellular polymeric substances (EPS) [3]. EPS mainly consisted of different classes of organic compounds such as proteins, polysaccharides, lipids and humic like substances [4–6]. It has been demonstrated that proteins and saccharides represent up to 80% of the total EPS and the remaining 20% might be attributed to humic compounds, uronic acid, nucleic and lipids [2, 7]. The main technical strategy to improve WWS dewatering operation is the degradation of EPS.
Compared to other AST techniques, advanced oxidation process seems the most promising results with reduced operating cost [8]. The most common radical species generated during the AOPs is the hydroxyl radical (HO•). Several AOPs were studied in the literature in order to improve sludge dewatering: Wet oxidation process, Ozonation, Fenton’s like reaction, etc… For example, Yin et al. (2007) found that an Ozone/hydrogen peroxide/microwave process could release more than 30% of total phosphor and 20% of the total kjeldahl nitrogen into the water phase of the sludge. In addition up to 37% of the total organic carbon was solubilized from the sludge matrix [9].
Among all existing AOPs, the electron beam (e-beam) irradiation process presents important advantages due to its high-flow rate treatment capacity and its small footprint [10]. E-beam irradiation might interact with the whole sludge matrix inducing strong modification into bacteria, organic compounds and micropollutants. It is commonly observed that an irradiation dose lower than 1 kGy (i.e. 1 kJ/m3) is sufficient to reach a high degree of disinfection [11]. Changqing et al. (2012) observed that the soluble chemical oxygen demand, soluble nitrogen and UV absorption intensity of the water phase of the sludge strongly increased while the mixed liquor suspended solids content considerably decreased with an increasing doses up to 20 kGy [12]. However, according to authors’ best knowledge no study has been reported on the characterization at molecular scale of bulk organic of WWS after e-beam treatment. Such work might improve the understanding of organic matter modification occurring during e-beam irradiation in order to optimize AST.
Anaerobic digestion process transforms organic matter into biogas. The latter consists mainly of methane (CH4) and carbon dioxide (CO2) gazes. The primary interest of anaerobic digestion of the sewage sludge is to reduce its volume and to value produced energy. The studies and projects dedicated to biogas are multiplying, thanks to the will of European states to support the production sectors of “green” energy through several new legislations. In France, the trend in the development of anaerobic digestion of sludge from sewage treatment plants comes from the development of bio-waste co-digestion (from the food industry, the separate collection of household waste, organic waste from kitchens, etc.). The biogas from WWTP sector would be a positive development in the number of facilities, increasing to 130 installations in 2020 [13]. Anaerobic digestion allows 40–50% reduction of the sludge organic matter (SOM) [14]. Biogas production is in the order of 0.8–1.2 Nm3/kg of consumed SOM. Anaerobic digestion is constituted with three biological and chemical stages: hydrolysis, acidogenesis (including acetogenesis), and methanogenesis. The DOM content and composition in the biogas reactor are influenced by these three stages. For example, insoluble organic polymers (insoluble in solvent), such as carbohydrates, are broken down to solvent soluble derivatives like sugar in the hydrolysis stage, which causes the increase in the DOM content. Meanwhile, methanogenesis converts the dissolved intermediate products to biogas (methane, carbon dioxide), decreasing DOM content [15]. However, it remains as a result of the process a significant amount of organic material to dispose of or to recover in agriculture through soil amendment. A better knowledge of this organic matter (OM) is essential to (i) estimate its interaction with micro-pollutants and soil OM as part of the amendment but also (ii) consider other recovery methods. Many researches were done to evaluate the impact of operating conditions on the quality and quantity of the biogas [15–21], but there is to author’s knowledge no study on molecular evolution of organic matter in the anaerobic digestion process of sewage sludge.
There are many factors affecting the biodigestion and the generation of gas, the pH, the temperature and the loading rate. A deviation from normal operating temperature and pH could induce a drop of digester performance due to an inhibition of mesophilic bacteria. The appropriate temperature and pH are usually set at 35 °C and 6.5–7.5, respectively [17, 18]. The loading rate of most municipal digester is ranged between 0.5 and 1.6 kg volatile solid per m3 of sludge per day. In addition, the carbon to nitrogen ratio (C/N) is often recommended to high values ranged from 20/1 to 30/1. Indeed, high concentration of nitrogen could decrease the methanogen activity, inhibiting the aerobic digestion process [19]. Methanation is often divided into three stages—the acidogenesis, acetogenesis and methanogenesis—although actually, they tend to occur simultaneously.
The objective of this study was to compare the influence of three different processes: thermal drying, electron beam (e-beam) irradiation and anaerobic digestion on the OM characteristics of WWS. Indeed the knowledge of the characteristics of this residual biomass is essential to improve their valorization. WWSs OM were investigated at the global scale using elemental analysis, attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR), thermogravimetric analysis (TGA-DSC) and OM fractionation. Double-shot thermochemolysis coupled with gas chromatography and mass spectrometry (GCMS) was used to compare the diversity and distribution of the molecular contents.
Materials and Methods
Wastewater Treatment Plants and Sludge Line
Floated sludge samples (LF-0) were collected from the wastewater treatment plant (WWTP) of Poitiers (semi-separative urban network, 152,200 population equivalent, sludge residence time: 48 h, sludge production: 1794 t dry matter a year) on April 2015.
The WWTP of Poitiers is based on an activated sludge process with an advanced dephosphatation treatment step. The secondary effluent (treated wastewater) is separated from the solid compounds (sludge) via a settling tank (FeCl3 is added to improve phosphate removal). While secondary effluent is directly rejected to the environment (if regulation limits are reached) sludge are subjected to further processes.
Thermal Drying
Dried sludge (LF-85) was produced at the WWTP of Poitiers thanks to large scale thin film conductive drier working at 85 °C (few minutes residence time). The 6 mm pellets (LF-120) are produced using a dryer-pelletizer working at 120 °C. The sludge pellets are then stored at ambient temperature before agricultural recycling or incineration. The total sludge production is equal to 1357 tons of DS/year. Before drying the concentrated sludge is mixed with a cationic polymer (ZETAG, BASF France) at a concentration of 15 kg per ton of DS. 68% of the total WWS amount is used in co-composting and 32% is transformed into pellets.
Ionisation with an Electron Beam
Electron beam irradiation from a Van De Graaff accelerator (Vivirad, 3 MeV, 500 µA) available in our group was performed on fresh sampled sludge. The sludge samples were introduced in a vessel (layer <1 cm) placed on a conveyor belt under the scanned beam. The absorbed dose was controlled by the residence time (i.e. depending on the conveying rate) of the sludge exposed to the electron beam (set at 2.6 MeV, 75 µA). Under these experimental conditions, the absorbed dose rate was 870 Gy s−1, and the absorbed dose D (Gy i.e. J kg−1) is calculated as follows: \({D=\frac{{D^\circ l}}{r}}\).with D°: absorbed dose rate (Gy s−1); r: conveyor belt rate (cm s−1); l: length of the irradiation zone (l = 4 cm).
The effect of two absorbed doses, 1.15 (B1) and 50 kGy (B50) corresponding to one pass at r = 3 cm s−1 and 21 passes at 1.5 cm s−1 respectively, on the fresh sludge was studied. For these doses, the exposure time was 1.3 and 56 s.
After irradiation, the samples were frozen at −20 °C and lyophilised to enable their characterisation.
Digestion
Methanogenic bacteria were provided by the biogas plant of Thouars (Tiper-Methaneo, Deux-Sèvres, France). Methanogenic bacterias are kept under anaerobic conditions at 37 °C before the pilot startup to maintain their capacity and ensure their methanogenic activity.
The bioreactor used was a BioFlo Fermentors & CelliGen® Bioreactors 115 with a capacity of 7 L purchased from New Brunswick® from Eppendorf. The heating is controlled thanks to an external jacket around the feed glass tank. The feed tank is sealed with a steel lid equipped with nozzles for all accessories. The ventilation and evacuation tubing gas are equipped with sterile membrane filters to avoid any contamination. Four perilstatic pumps could be used to purge or feed the biological mix. Finally, several electrodes and sensors were used to control the temperature, pH.
Once the biological mix (sludge + inoculum, 3 and 2 L, respectively) was introduced into the bioreactor, the mixture was aerated 1 h with nitrogen to remove oxygen and to operate into anaerobic conditions. The mixture was kept under constant blade stirring at 200 rpm. The reactor temperature was maintained at 37 °C. The biogaz production was measured using a graduated cylinder filled with tap water. The glassware was connected to the bioreactor and to a separation funnel. The amount of biogaz produced was measured every 24 h according to the variation of the water level in the graduated cylinder. Every 7 days, 200 mL of digest were removed from the pilot via peristaltic pumps to maintain anaerobic conditions. The samples (D1, D2, D3, D4 corresponding to the first, second, third and fourth week of methanation, respectively) were frozen (−20 °C) and lyophilized (−50 °C; 0.034 mbar) and then stored at −20 °C until analysis.
pH Measurement
The sludge were separated by centrifugation into a supernatant and a pellet. The pH of the supernatant was measured directly. The pellet was diluted in ultra-pure water 5/1 in order to measure the pH of the suspension.
Elemental Analysis
The OM content (i.e.: mixed liquor volatile suspended solids) of WWS was determined from 3 g sample, by combustion at 500 °C for 4 h. Elemental analysis (C, H, N) was carried out on 1 mg sample using an elemental analyser (Thermo Electron Corporation Flash EA 1112 series) by catalytic combustion under oxygen at 970 °C. To determine the sulfur percentage, 1 mg of vanadium oxide was added to 1 mg of the raw sludge.
Attenuated Total Reflectance Fourier Transform Infrared spectroscopy (ATR-FTIR)
ATR-FTIR spectra were recorded on a Thermo Nicolet 6700 Fourier transform infrared (FTIR) spectrometer equipped with a diamond crystal. Spectra were taken between 4000 and 650 cm−1 with a resolution of 4 cm−1. 16 Scans were collected per spectrum. In an aim of consistency and to allow comparison, the spectra are normalized to the C–H band (2960 cm−1).
Chemical Fractionation
The OM was fractionated according to the International Humic Substances Society (IHSS) protocol. Lipids were extracted from 10 g WWS with 3 × 240 mL dichloromethane/methanol (2/1) using a Speed Extractor (Buchi). The extraction temperature was set to 80 °C, nitrogen pressure was 50 bars, and the solvent contact time with the sludge was 5 min. The solid remaining after extraction was the residue.
“Humic” and “fulvic acids” were extracted from the residue by 0.1 M NaOH (10 mL per g) under a nitrogen atmosphere in order to prevent OM oxydation. “Humic acids” were separated from “fulvic acids” by acidification to pH 1 (1 M HCl solution) and centrifugation (20 min, 8000 g). The alkaline-insoluble residue corresponded to “humin”.
DSC/ATG
DSC and TGA were carried out on a TA Instruments SDT Q600. WWSP was analyzed without any pre-treatment. The analysis was performed using platinum crucibles in air (combustion) atmosphere. The following conditions were employed: heating rate of 5 °C min−1 from 25 to 900 °C and an isotherm of 5 min at 900 °C. A flow-rate of 100 cm3 min−1 of air was maintained during the analysis.
Thermochemolysis (THM-GC/MS)
Thermochemolysis was done using tetramethyl ammonium hydroxide (TMAH) as alkylating agent using a temperature ramp from 100 to 350 °C with a temperature increase of 500 °C min−1. The pyrolyzer was a Frontier Lab EGA 2020 pyrolyzer equipped with an AS-1020E auto-shot sampler coupled with GCMS (Shimadzu QP 2010 Ultra). 0.5 mg of lipids were mixed with 5 µL of TMAH methanolic solution 50/50 (v/v) in methanol and then placed in an inox cup. GC separations were done using a capillary column (30 m long, 0.25 mm i.d., 0.25 µm phase thickness). The injector temperature was set at 250 °C. Column temperature was programmed from 50 to 300 °C at a rate of 5 °C min−1 and then kept at 300 °C for 9 min. The detector was a quadrupolar mass spectrometer. The ionization mode was electron impact (70 eV) and the source temperature was 220 °C.
The organic compounds were identified on the basis of their GC retention times and by comparison of their mass spectra with those of standards and library data (NIST).
Quantification was done using calibration standards (hexadecanoic acid and coprostanol) as describe by Collard et al. [22].
Results and Discussion
Bulk Properties
The elemental composition (Table 1) and ash content (Table 2) of the floated sludge LF-0 are in agreement with those previously determined for activated sludge.
ATR and diffuse reflectance FTIR spectroscopy have been used to characterize or monitor the transformations of different fractions of OM of environmental samples such as composts or sewage sludge [23]. ATR-FTIR spectra (Fig. 1) of sludge samples exhibited the following peaks wave numbers: 3270 cm−1 (OH stretch), 3180 cm−1 (NH2 stretch of amides) [24], 2925 cm−1 and 2855 cm−1 (aliphatic C–H stretch), 1620 cm−1 (C=C of aromatics), 1540 cm−1 (C=O of amides) [25], 1250 cm−1 (C–O of carboxylic acids or C–N of amides), 1030 cm−1 (C–O stretch of polysaccharides) [22, 26].
The DSC curves (Fig. 2) show two exothermic phenomena corresponding respectively to volatilization of light compounds such as aliphatic molecules or carbohydrates and to oxidation of high molecular weight components [27]. The endotherm observed between 25 and 150 °C is mainly related to dehydration reactions. The two exothermic phenomena corresponding to the OM decomposition are observed in the 200–600 °C range. The first one, associated with desorption of aliphatic compounds, is observed between 200 and 350 °C. The second one, associated with the degradation of more complex aromatic structures, is observed between 400 and 550 °C.
The chemical fractionation according to the IHSS protocol was used to monitor transformation such as complexification or weakening of the organic matter. Four fractions of OM are obtained according to their solubility [28]. The relative abundances of each fraction are reported Table 2.
Thermal Drying
The LF-0 sludge was dried at 85 °C (LF-85) to reduce water content then pelletised at 120 °C (LF-120). The C/N ratio which is linked with biodegradability was 5.8 and remained stable (Table 1) during the thermal drying process [29]. According to the C–O/C–H ratio slightly decrease during thermal drying suggesting a reduction process of OM (data not shown).
The ash content (Table 2) remained constant during the process which shows that the thermal treatment does not induce a loss of total OM. However the fractionation of OM after thermal drying showed strong differences between LF-0 and LF-120. Indeed lipids, fulvic acids and humic acids increased respectively of 83, 60 and 71% (Table 2). In parallel, a strong decrease (76%) in humin is observed. These changes are probably due to a weakening of OM. Lipidic compounds which were bound to the macromolecular network via ester, or ether bonds were probably released during this period thus increasing the extractable fractions [30]. Such a desorption has already been observed in soil as the equilibrium was perturbed [31].
The molecular analysis was performed by THM-GC/MS. The same molecules were detected all along the process with stanols, sterols and fatty acids as main compounds (Table 3). An increase of respectively 27 and 73% for acids and steroids is observed for LF-85 followed by a decrease of respectively 29 and 50% for acids and steroids for LF-120. As observed by Gobé et al. (2000), it is highly probable that polycyclic alcohols (stanols and sterols) and fatty acids were linked to the macromolecular network of polar lipids by ether and ester bonds [30]. In the first stage of thermal drying (LF-85) these bonds could have been broken leading to an increase in stanols, sterols and fatty acids. These released compounds were degraded in the second stage of drying (LF-120).
The branched (iso+anteiso) to linear fatty acids ratio (Table 4) decreased along the process demonstrating that the drying process has an inhibiting effect on bacterial activity [32]. Moreover the relative increase in stanols versus sterol traduces reducing conditions which are not favourable to bacterial activity.
E-beam Irradiation
Considering the siccity of the fresh sludge (5%) water makes up the greater part of the irradiated system and a large fraction of the absorbed radiation energy will induce water radiolysis (reaction 1). Thus the radiation is expected to have mainly an indirect effect on the organic material of sludges i.e. the chemical changes will come from the reactions with e− aq, the HO• and H• radicals, and secondary radicals like O2 •− formed from the high reactivity of the hydrated electrons e− aq with oxygen (reaction 2). As a consequence, oxidation (from HO• and O2 •−; ref) but also reduction reactions (from e− aq, H• and O2 •−; ref) can occur in the process. Among these short lived species, e− aq (under oxygen limiting conditions) and HO• are the most reactive. The direct effect of the radiation is expected to play a small part forming radicals and other reactive species from the organic material (reaction 3).
The raw floated sludge pellet exhibited a pH equals to 8.0 while its supernatant was equal to 6.6. The pH of the 1.25 kGy irradiated sludge (B-1) is 6.4 whereas the pH of the supernatant is 7.3. The irradiation of the organic material, as described in reactions 1 and 2 therefore induced an acidification. The pH of the sludge irradiated to 50 kGy (B-50) is 6.0 and that of the supernatant is 6.7. The acidification is related to the increase in concentration of the ion [H3O+] produced by radiolysis of water increased with the irradiation dose.
The decrease in pH which is higher in the supernatant than in the pellet confirms that irradiation has a greater impact on water (radiolysis) than on the organic matter [33].
The evolution of the weight loss associated with exotherm 2 to exotherm 1 ratio (RTGA), is presented in Table 5. The irradiation at low dose (B-1) induces an increase in the RTGA ratio associated with a stable OM content which demonstrates a complexification of the OM. The irradiation at 50 kGy (B-50) causes a slight decrease in the RTGA ratio compared to the LF-0. This decrease is significant in comparison to the RTGA ratio observed for B-1. Thus contrary to the first dose, 50 kGy irradiation induced a weakening of OM.
The stable organic matter content correlates with the total organic carbon measurements (TOC) measured on secondary effluents before and after irradiation [33].
The intensities of bands associated to aromatic C=C (1620 cm−1) increased relatively to the intensities of the band associated with C–H (2925 cm−1) as a result of radiation (Table 5, RIR1). This traduces an aromatization phenomenon that can be correlated to the complexification of organic matter demonstrated by thermal analysis. The relative intensity of the signal associated to the C–O bonds (1030 cm−1) to the signal associated with C–H (Table 5, RIR2) which reflects the oxidation of organic matter also increases for both radiation doses received by the samples. However the oxidation does not induce a change in C–O to C=O bonds (respectively 1030 and 1540 cm−1) ratio (Table 5, RIR3).
Independently of the irradiation dose, the humin fraction does not vary significantly (Table 2). For a dose of 1.25 kGy, the relative amounts of humic acid increase while those of lipids and fulvic acids were reduced. This means that the relative amount of the less complex organic matter decreased in favor of the most complex organic matter.
The irradiation at 50 kGy causes an evolution of the different fractions of OM. Indeed, the relative amounts of lipids and humic acids decreased while the relative amount of fulvic acids increased. The relative amount of humin does not vary (Table 2), which means that the relative amount of less complex organic matter decreases without changing that of humin. These results tend to demonstrate a slight complexification of organic matter with high irradiation dose.
Anaerobic Digestion
In order to observe the evolution of organic matter in the digestate, the study was conducted in batch mode, without the addition of sludge in the pilot during methanation. There is a cessation of gas production in the 3rd week (D3), the study was stopped after 5 weeks. In order to validate the study and to confirm the production of methane, the produced gas was analysed by gas chromatography and methane accounts for 42% of the produced gas.
According to elemental analysis (Table 1), there is no change induced by anaerobic digestion on bulk parameters. In addition, analysis done directly after sludge and innoculum mixing show no change in elemental analysis (data not shown). The C/N ratio of 5.4 is much lower than recommended in several studies, which can explain the amount of produced gas. Ash content (Table 2) slightly increased during anaerobic digestion, from 22 to 32% traducing a loss in total OM.
The lipid content (Table 2) increased by 30% compared to raw sludge (LF-0) after the first week of methanation (D1) and then declined for the next three weeks (D-1 to D3). The final lipids amount consists of 62% of the initial concentration. The lipid fraction is the biodegradable fraction that is digested by methanogens bacteria. The increase in lipid content in a first phase, can be attributed to hydrolysis. Then acetogenesis and methanogenesis cannot be distinguished during the second phase where lipids content decrease.
The characterization of the digestate was performed by THM-GC–MS. Fatty acids and steroids were the main identified products from the first shot (Table 3). The amount of fatty acids decreases during the digestion from from 54.9 to 36.5%. On the contrary, the amount of steroids oscillating over the digestion period (Table 3).
Compared to LF-0, the amount of fatty acids (Table 3) increased after 1 week (D-1) and then decreased until the end of the experiment, as lipid content do (Table 2). At the contrary, a decrease in steroids amount (Table 3). Methanogenic bacteria stop the process before the total consumption of nutrients (fatty acids and steroids). The branched to linear fatty acids ratio increased after 1 week methanisation (D-1) when compared to LF-0 (Table 4), which was characteristic of a high bacterial activity and then fall from the 2nd week of methanation. This indication of bacterial activity correlates with the strong production of biogas in the 1st week and a stop of this production at the 5th week. Moreover the high stanol/sterol ratio observed after 1 week digestion (D-1) traduced the anaerobic environment.
Conclusions
The impact of three different handling processes on the physico-chemical characteristics of wastewater sludge has been studied. Thermally dried, digested and irradiated sludge were compared with the initial floated sludge. The OM was characterised at the global and molecular scales using elemental analysis, infra-red spectroscopy and thermochemolysis.
The e-beam irradiation induced an acidification and the digested sludge showed a loss in OM content but globally after the investigated handling processes, the three studied WWS presented similar characteristics. However the OM chemical fractionation emphasises the strong influence of thermal drying processes on OM. Indeed, the pelletisation step at 120 °C of the thermal drying process induced a weakening of OM which is traduced by an increase in lipids concomitant with a decrease in “humin”. At low doses, the e-beam irradiation resulted in a decrease in the lipidic fraction and distribution in the various humic fractions in line with a complexification. At the contrary, a higher dose induced a weakening of the organic matter. Thermal analysis corroborated these results while infrared spectroscopy demonstrated an oxydation and an aromatisation of OM with increasing irradiation dose.
At the molecular level, thermal drying resulted in a decrease in branched to linear fatty acids ratio and an increase in stanols to sterols ratio while e-beam irradiation resulted in a decrease in all the identified compounds. During the 5 weeks of digestion, the amount of fatty acids decreased while that of steroids oscillated.
Finally our results suggested that drying which reduces the volume of WWS thus lowering its cost of management, but change in OM characteristics can have an impact on the choice of WWS disposal. Indeed, the weakened OM of thermally dried sludge aim to be used as fertilizer while the complex OM of sludge irradiated with a low dose should be used as amendment.
References
Uggetti, E., Ferrer, I., Llorens, E., García, J.: Sludge treatment wetlands: a review on the state of the art. Bioresour. Technol. 101, 2905–2912 (2010)
Neyens, E., Baeyens, J., Dewil, R., Deheyder, B.: Advanced sludge treatment affects extracellular polymeric substances to improve activated sludge dewatering. J. Hazard. Mater. 106B, 83–92 (2004)
Urbain, V., Block, J.C., Manem, J.: Bioflocculation in activated-sludge—an analytic approach. Water Res. 27(5), 829–838 (1993)
Flemming, H.-C., Wingender, J.: Relevance of microbial extracellular polymeric substances (EPSs). Part I: Structural and ecological aspects. Water Sci. Technol. 43(6), 1–8 (2001)
Eriksson, L., Alm, B.: Study of flocculation mechanisms by observing effects of complexing agent on activated sludge properties. Water Sci. Technol. 24(7), 21–28 (1991)
Kang, S.M., Kishimoto, M., Shioya, S., Yoshida, T., Suga, K.I.H., Taguchi, H.: Dewatering characteristics of activated sludges and effect of extracellular polymer. J. Ferment. Bioeng. 68(2),117–122, (1989)
Dignac, M.F., Urbain, V., Ryabacki, D., Bruchet, A., Snidaro, D., Scribe, P.: Chemical description of extracellular polymers: implication on activated sludge floc structure. Water Sci. Technol. 38(8–9), 45–53 (1998)
Neyens, E.: The Development of Advanced Techniques for Reducing Sludge Quantities and Improving Sludge Dewaterability. PhD Thesis, Katholieke Universiteit Leuven, 230 (2003)
Yin, G., Huang Liao, P., Lo, K.V.: An ozone/hydrogen peroxide/microwave-enhanced advanced oxidation process for sewage sludge treatment. J. Environ. Sci. Health Part A 42, 8 (2007)
Wang, J., Wang, J.: Application of radiation technology to sewage sludge processing: a review. J. Hazard. Mater. 143(1–2), 2–7 (2007)
Borrely, S.I., Cruz, A.C., Del Mastro, N.L., Sampa M.H.O., Somessari, E.S.: Radiation processing of sewage and sludge. A review. Prog. Nucl. Energy. 33(1–2), 3–21 (1998)
Changqing, C., Min, W.: Treatment of municipal sewage sludge by electron beam irradiation. Nucl. Sci. Techn. 23, 29–33, (2012)
Ernst and Young, Etude du marché de la méthanisation et de la valorisation du biogaz. Ademe and GrDF, France (2008)
Arnaiz, C., Guitierrez, J.C., Lebrato, J.: Biomass stabilization in the anaerobic digestion of wastewater sludges. Bioresour. Technol. 97(10), 1179–1184 (2006)
Liu, Y., Li, X., Kang, X.: Effect of volume ratio on anaerobic co-digestion of thermal hydrolysis of food waste with activated sludge. CESE-2014—Chall. Environ. Sci. Eng. Ser. Conf. 102, 154–158 (2015)
Li, X., Dai, X., Takahashi, J., Li, N., Jin, J., Dai, L., Dong, B.: New insight into chemical changes of dissolved organic matter during anaerobic digestion of dewatered sewage sludge using EEM-PARAFAC and two-dimensional FTIR correlation spectroscopy. Bioresour. Technol. 159, 412–420 (2014)
Zonta, Ž., Alves, M.M., Flotats, X., Palatsi, J.: Modelling inhibitory effects of long chain fatty acids in the anaerobic digestion process. Water Res. 47(3), 1369-1380 (2013)
Kim, M., Ahn, Y.-H., Speece, R.: Comparative process stability and efficiency of anaerobic digestion; mesophilic vs. thermophilic. Water Res. 36(17), 4369-4385 (2002)
Carrère, H., Dumas, C., Battimelli, A., Batstone, D.J., Delgenès, J.P., Steyer, J.P., Ferrer, I.: Pretreatment methods to improve sludge anaerobic degradability: a review. J. Hazard. Mater. 183(1–3), 1–15 (2010)
McLeod, J.D., Othman, M.Z., Beale, D.J., Joshi, D.: The use of laboratory scale reactors to predict sensitivity to changes in operating conditions for full-scale anaerobic digestion treating municipal sewage sludge. Bioresour. Technol. 189, 384–390 (2015)
Maspolim, Y., Zhou, Y., Guo, C., Xiao, K., Ng, W.J.: Comparison of single-stage and two-phase anaerobic sludge digestion systems—performance and microbial community dynamics. Wastewater-Energy Nexus Sustain. Wastewater Reclam. 140, 54–62 (2015)
Collard, M., Teychené, B., Lemée, L.: Improved quantitative analysis of molecular constituents of wastewater sludge pellets using double-shot thermochemolysis-GCMS. J. Anal. Appl. Pyrolysis. 114, 265–272 (2015)
Tandy, S., Healey, J.R., Nason, M.A., Williamson, J.C., Jones, D.L., Thain, S.C.: FT-IR as an alternative method for measuring chemical properties during composting. Bioresour. Technol. 101, 5431–5436 (2010)
Smidt, E., Meissl, K.: The applicability of Fourier transform infrared (FT-IR) spectroscopy in waste management. Waste Manag. 27, 268–276 (2007)
Grube, M., Lin, J.G., Lee, P.H., Kokorevicha, S.: Evaluation of sewage sludge-based compost by FT-IR spectroscopy. Geoderma. 130, 324–333 (2006)
Réveillé, V., Mansuy, L., Jardé, É., Garnier-Sillam, É.: Characterisation of sewage sludge-derived organic matter: lipids and humic acids. Org. Geochem. 34, 615–627 (2003)
Otero, M., Calvo, L.F., Estrada, B., García, A.I., Morán, A.: Thermogravimetry as a technique for establishing the stabilization progress of sludge from wastewater treatment plants. Thermochim. Acta 389, 121–132 (2002)
Calderoni, G., Schnitzer, M.: Effects of age on the chemical structure of paleosol humic acids and fulvic acids. Geochim. Cosmochim. Acta 48, 2045–2051 (1984)
Som, M.-P., Lemée, L., Amblès, A.: Stability and maturity of a green waste and biowaste compost assessed on the basis of a molecular study using spectroscopy, thermal analysis, thermodesorption and thermochemolysis. Bioresour. Technol. 100, 4404–4416 (2009)
Gobé, V., Lemée, L., Amblès, A.: Structure elucidation of soil macromolecular lipids by preparative pyrolysis and thermochemolysis. Org. Geochem. 31, 409–419 (2000)
Ambles, A., Lemee, L., Jambu, P., Mayoungou-Vembet, P.: Equilibrium between free and bound lipids in a rendzina soil in natural conditions and with laboratory disturbances. Agrochimica. 41, 196–208 (1997)
Guignard, C., Lemée, L., Amblès, A.: Lipid constituents of peat humic acids and humin. Distinction from directly extractable bitumen components using TMAH and TEAAc thermochemolysis. Org. Geochem. 36, 287–297 (2005)
Meng, M., Pellizzari, F., Boukari, S.O.B., Vel Leitner, N.K., Teychene, B.: Impact of e-beam irradiation of municipal secondary effluent on MF and RO membranes performances. J. Membr. Sci. 471, 1–8 (2014)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lemée, L., Collard, M., Vel Leitner, N.K. et al. Changes in Wastewater Sludge Characteristics Submitted to Thermal Drying, E-beam Irradiation or Anaerobic Digestion. Waste Biomass Valor 8, 1771–1780 (2017). https://doi.org/10.1007/s12649-017-9946-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-017-9946-5