Abstract
Serpentinite tailings from La Nationale chrysotile mine in Thetford Mines (Quebec, Canada) were studied to extract the Mg contained therein. The study began with an initial chemical characterization of the residue to determine the Mg concentration in the different grain size fractions. The resulting Mg-containing fractions were leached under a variety of parameters such as type of acid, acid concentration, treatment time, and temperature, and the obtained solution was neutralized with NaOH for the selective recovery of the metals. The results of this study were used to design a process to obtain Mg as a marketable chemical. The tested process consists of a leaching step using an H2SO4 solution followed by the purification of the leachates using NaOH at pH 8 and Mg recovery with NaOH at pH 10. A final product composed of mirabilite (Na2SO4) and brucite (Mg(OH)2) was obtained. The procedure was tested, and its economic viability was discussed. The approach proved to be technically feasible, promoting clean production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Asbestos is a commercial term designating a group of fibrous metamorphic minerals composed of double-chain silicates and characterized by a number of properties, such as high tensile strength, flexibility, low electrical conductance, and resistance to thermal and chemical degradation [24]. Asbestos exists within two main mineralogical groups: serpentines, which includes chrysotile (white asbestos); and amphibole, which includes amosite (brown asbestos), crocidolite (blue asbestos).
Chrysotile is the most common form of asbestos [33] and one of the three types of serpentine mineral, along with antigorite and lizardite. It is a hydrous Mg-rich silicate [Mg3Si2O5(OH)4] with an average composition of approximately 39.8% MgO, 38.8% SiO2, 3.1% Al2O3, 1.6% Fe2O3, and minor amounts of Ca, K, Na, Cr, and Ni in Canadian samples [34].
Chrysotile mining and processing was for a long time common in the mining industry. Chrysotile fibers were used for their fire retardant proprieties in construction materials, brakes pad, or insulations materials. Nowadays, chrysotile is registered as a banned material resulting in the decline and the closing of the mining facilities. Nevertheless, over 100 years of mining exploitations left large volumes of mining waste. Because of the remaining chrysotile fibers, the tailings can pollute the air, seriously harming human health and the environment [37].
To answer the residues problematic, several applications have been suggested to valorizate serpentinite residues: including use as aggregate (railway ballast, road construction, filling of low land for the construction of parking lots, asphalt production) without great economic success [1, 29]; concrete foam insulation; mineral wool; structural lightweight aggregate; refractory products [1, 29]; CO2 capture and sequestration media [20, 27]; and as raw material for Mg metal production.
Although the usage of Mg metal has been limited by its high production and energy cost, it has been extensively used as an alloying element and in die casting and steel desulfurization, as well as many other uses in industry [17]. Current sources for Mg extraction include seawater, dolomite, brucite, carnallite and, more recently, asbestos tailings [15]. There are two principal routes for producing Mg [13]: electrolytical and pyrometallurgical. In the electrolytic route, which is the more common, includes the Dow, Australian Magnesium (AM), and IG Farben processes, Mg is obtained from fused MgCl2. In the pyrometallurgic route, which includes the silicothermic, carbothermic, Magnetherm, aluminothermic, and Mintek processes, Mg is obtained by treating its ores at high temperatures in the presence of catalysts [40].
Asbestos mine tailings, mostly composed of serpentinite, have been positively treated by the electrolytical (Magnola) and the pyrometallurgical (MAGRAM) routes. The Magnola electrolytic process, which has superior performance to the MAGRAM process, is conducted by leaching serpentinite with warm HCl to obtain MgCl2 brine, which is subsequently subjected to electrolysis to obtain Mg [6]. However, this process has several environmental problems related to the emission of sulfur hexafluoride, dioxins, furans, and chlorinated hydrocarbons; high electric power consumption; and the generation of silica-iron by-products [19]. All these methods are not in use at the current prices of Mg; thus, further research is needed to explore alternatives for the valorization of serpentinite tailings generated from chrysotile mining.
In this line, serpentinite solubilization has been previously discussed for several leaching agents [3, 9, 11, 12, 14, 25, 26, 35, 36, 42]. However, the scientific literature still lacks information about the following steps for an entire hydrometallurgical process for the leachates obtained by any of these reagents. This fact is particularly relevant when the proposed leaching agent is H2SO4 and the treated material is serpentinite tailings. Despite its higher cost with respect to other similar precipitants such as limes [18] and ammonia [14] which have been previously described in similar process in literature, NaOH neutralization was tested in this study because it generates a higher quality effluent while considerably reducing reaction time and the amount of sludge solids generated (e.g., [28]).
The first step in the design of a full-scale hydrometallurgical alternative is a viability analysis [32]. Research in this field should provide innovative approaches that minimize the use of external energy and reagents, thus promoting techno-economic recovery. Within this framework, we introduce a novel process scheme on Mg(OH)2 production from serpentine residues. Thus, this work presents a comprehensive study covering the entirety of the hydrometallurgical process, from the reaction efficiency, including aspects of dissolution, purification, and recovery, to the economic feasibility. Such comprehensive aims to provide another point of view over the potential reuse of asbestos mining residues.
All things considered, the specific objectives of the current study are the following:
-
To develop a leaching technique for chrysotile residues that can extract over 80% of the Mg. To this end, the following leaching parameters were considered: (1) type of acid (HCl or H2SO4), (2) H2SO4 concentration, (3) solid concentration, (4) temperature, and (5) leaching duration.
-
To improve the leachate purification system for the selective removal of impurities (Fe, Mn, Ni, Al, Cr, etc.) while retaining the Mg in solution. For this purpose, neutralization with NaOH has been studied.
-
Finally, to perform a feasibility study including material balance and economic analysis on the obtaining Mg as a marketable chemical (brucite has an increasing market potential given its applications as environmentally friendly flame-retardant and in environment rehabilitation) while minimizing the formation of secondary wastes.
Materials and Methods
Site Characterization
Serpentinite residues were collected at La Nationale mining site in Thetford Mines (QC, Canada). The location was one of the most important production centers for chrysotile fibers in the West, with numerous underground, open-pit mines, and mineral dressing industries in operation for over 125 years, that were finally closed down in 2011 [7]. This extractive and industrial activity has generated large amounts of tailings from which the chrysotile fibers have been extracted. These tailings are mainly composed of serpentinite, a rock mainly composed of serpentine minerals chrysotile (Mg3Si2O5(OH)4), antigorite ((Mg,Fe)3Si2O5(OH)4), and lizardite (Mg3Si2O5(OH)4), and contain small amounts of magnetite (Fe3O4) and awaruite (Ni3Fe3) [7, 31]. These tailings are located inside the city, and can be affected by mechanical dispersion.
The studied residues are derived from the chrysotile fiber extraction method used by LAB Chrysotile Inc [1]. In this respect, increasing chrysotile dissolution scientific literature from this location is particularly interesting given the significant amount of research being conducted for this residue reuse by both private sector and public bodies. Thus, current research is mostly focused in chrysotile H2SO4 dissolution as a pretreatment for enhanced CO2 sequestration via aqueous carbonation (e.g., [27]).
The samples were collected from diverse locations in the dumping area and mixed to obtain a representative composite sample that was deposited into two polyethylene containers (20 L capacity), which were subsequently stored at room temperature.
Sample Preparation
Samples were oven dried at 105 °C for 24 h and then sieved into the following particle size fractions: >3.18, 3.18–1.7, 1.7–0.85, 0.85–0.3, 0.3–0.15, and <0.15 mm. Three representative samples of each size were subjected to chemical analyses by inductively coupled plasma-optical emission spectrometry (ICP-OES) to determine the mass distribution of Mg in the different particle size fractions. Subsequently, large fragments were ground using a jaw crusher (Sturtevant Mill Co., Hanover, MA) and, finally, the particle size fraction <1 mm was used for the leaching tests.
Leaching Tests
The leaching tests were conducted in stirred tank reactors comprising a 1-L glass Pyrex beaker. The agitation was carried out using magnetic stirrers, whereas the leaching solution was heated using a hot plate stirrer. Leaching tests were performed by adding 40 g of residue to 200 mL of acid leaching solution.
The leaching solutions were obtained with 18M H2SO4 or alternatively 12M HCl, and amounts varying between 120 and 200 mL of distilled water. To test the possible enhanced dissolution rates created by NaCl, this salt was occasionally added to some samples. Tests labeled A-1 to A-6 were conceived to study the performance of HCl and H2SO4 with and without NaCl or external heat supply. A summary of the parameters tested is shown in Table 1.
Additional leaching tests were conducted using 40 g of residues treated between 0 and 360 min, for some of the conditions described in the previous section (test A-3, A-4, A-5), and for different concentrations of 18M H2SO4 (test A-7, A-8, A-9, A-10) according to the parameters shown in Table 2. A control test (residue stirred with 200 mL of water but without the addition of acid) was also performed.
Precipitation Tests
A bulk sample of leachate solution obtained after leaching the serpentinite tailings in batches of 2000 mL in analogous conditions to those of test A-5 (Table 1) was used for the precipitation tests. Precipitation tests were performed in aliquots of this sample using a concentrated solution of 20M NaOH (97% ACS reagent, ACP Chemical, Montreal, QC, Canada) to determine the optimum pH for the removal of impurities (e.g., Mn, Fe, Ni) while retaining the Mg in solution. Finally, pH values ranging from 2.0 to 10.0 were tested in 2-L Pyrex beakers containing 500 mL of leachate.
Magnesium Recovery Process
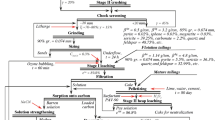
Mg can be recovered from the solution in the form of Mg(OH)2 (brucite) by increasing the pH above the Mg(OH)2 saturation point (pH 8.9). Based on the results obtained in the previous section, the following hydrometallurgical procedure (comprising leaching, purification, and recovery phases) was designed and tested (Fig. 1). The equipment used for these tests is analogous to that used in the previous sections.
The first phase leaching was performed after a preliminary grinding of the serpentinite residue (Ro) to obtain a material (G) with a particle size <1 mm. This material was leached in water (W0) to which 18M H2SO4 solution (A1) was, for a reaction time of 10 min, and at a pulp density of approximately 16.7% w/w (200 g residue/L). Once leaching was completed, the slurry (L1) was subjected to filtration for 35 min at 110 °C, producing a SiO2-rich solid fraction (R1) that was rinsed using distilled water (W1), and Mg-rich liquid fraction (L2) containing some undesirable elements, such as Fe and Mn.
Thereafter, the fraction L2 was collected and purified by increasing the pH of the solution to between 8.0 and 8.5 using 20M NaOH solution (S1), which selectively precipitates the undesirable elements while retaining Mg in solution. The obtained slurry (L3), which had a temperature of approximately 85 °C, was vacuum filtered for 25 min and rinsed (W2), generating a Fe-rich solid fraction (R2) and a liquid fraction (L4) with a high concentration of Mg. This filtrate, which had an initial temperature of approximately 80 °C, was cooled down (to decrease the solubility of the metals in solution) to approximately 23 °C (L5) and cold filtered to yield L6 and the residue R3 rich in Na2SO4. The pH of the filtrate (L6) was adjusted to approximately 8.5 using 20M NaOH solution (S2) to reduce the residual concentration of Fe and Mn in solution, producing L7 and theoretically promoting a residue rich in Fe and Mn (R4) after filtration. To maximize the separation of Na and Mg, the solution (L7) was heated to approximately 65 °C, which ensured the maximum solubility of Na2SO4, and then sent to filtration and neutralized with 20M NaOH solution (S3) to pH 10, obtaining a solid product (R5aq + Paq) and a final effluent (L8). The solid was placed on a sieve and stored dried in an oven at 60 °C for a period ranging from 24 to 36 h. This technique optimizes the separation of Na and Mg as a precipitate (R5) rich in Mg (Mg(OH)2) retained on a screen, whereas the final product rich in thenardite/mirabilite (P) is formed after the evaporation of the water.
Analytical Procedures
Characterization of the Solids
The concentration of major, minor, and trace elements (e.g., Mg, Mn, Fe, Al, Ni, Na, Cr) determined after near-total digestion in a nitric acid, hydrofluoric acid, and perchloric acid (method 3030I; [2]). Approximately 0.5 g of the final residue was ground at 400 rpm for 40 s using a vibratory disk mill (RS 100 Retsch) to a particle size <125 µm, digested, and then analyzed by ICP-OES.
When necessary, mineralogical analyses were performed by X-ray diffractometry (Siemens D5000 (Cobalt) at the Centre for Microscopy, University Laval, Québec, QC, Canada).
The toxicity characteristic leaching procedure (TCLP) test was used to evaluate the toxicity of the by-products generated during the process (Method 1311; [38].
Characterization of the Leachates
Samples ranging between 1 and 5 mL were collected throughout the different experiments and vacuum filtered on Whatman 934-AH membranes. They were then acidified and the metal concentrations were measured by inductively coupled plasma—optical emission spectrometry (Vista AX CCO Simultaneous ICP-OES, Varian, Mississauga, ON, Canada). The analytical quality controls were performed with a certified standard solution (PlasmaCal Multielement Standard 900-Q30-100, SCP Science, Baie d’Urfé, QC, Canada).
ORP and pH were measured using a pH meter (Accumet Research AR25 Dual Channel pH/Ion meter, Fischer Scientific Ltd., Nepean, ON, Canada) equipped with a double-junction Cole-Parmer electrode with a Ag/AgCl reference cell (daily pH calibration between 2 and 10). ORP was measured with a platinum electrode.
Economic Study
Only the chemical consumption, sludge disposal, and energy consumption were considered for the economic study. Leaching tests of the inorganic components of chrysotile residue were performed by the addition of H2SO4 or HCl. The costs of reagents consumed during treatment are given in United States dollars (US$). Prices of 86 US$/t 100% H2SO4 (96% w/w, d20: 1.83 kg/L) and 438 US$/t 100% HCl (36.5% w/w, d20: 1.19 kg/L), and 500 US$/t NaOH were used for the cost assessment. The neutralization of the leachate, the precipitation of the inorganic elements, and the recovery were achieved using NaOH. The sale price of the brucite, according to its purity and value as industrial reactive was fixed at 1.21 US$/kg.
Results and Discussion
Particle Size Fractioning
The granulometric analyses of the bulk residues showed a preponderance of gravel and coarse sand, over the silt and clay grain size fractions (<1.84% w/w). In fact, particles >3.18 mm were the highest mass percentage (53.1% w/w), particles between 3.18 and 1.7 mm represented 16.0% w/w, particles between 1.7 and 0.85 mm stood for 14.4% w/w, grains in the interval from 0.85 to 0.3 mm represented a proportion of the 11.7% w/w, whereas those in the range 0.3–0.15 mm accounted for only 3.0% w/w, finally, the grain size fraction minor than 0.15 mm comprised less than 1.84% w/w of the total mass.
ICP-OES analyses determined that major elements were Mg (ranging between 20 and 27% w/w), Ni (2010 and 2610 mg/kg), Cr (1070 and 1670 mg/kg), and Mn (680 and 879 mg/kg) were evenly distributed in the different particle size fractions. On the contrary, a clear relationship between decreasing grain size and element enrichment (normalizer elements) was observed for the Fe (from 4.1% up to 10.9% w/w), and Al (from 2130 up to 10,400 mg/kg).
According to this finding, and since there is no preferential concentration of Mg in any particular grain size, all fractions can be derived to the leaching step after grinding to <1 mm.
Determination of Optimum Leaching Conditions
Several of the leaching parameters were studied in this section, namely, the type of acid, acid concentration, reaction time, addition of an external source of heat, and the addition of NaCl.
First, a set of tests were executed to study the performance of both H2SO4 and HCl in the leaching process. When these tests were conducted without an external heat supply (A-1 and A-5), the addition of NaCl (A-1) appeared to limit the reaction in terms of Mg solubilization (Table 3). In this sense, regarding tests without external heating or addition of NaCl, the A-5 test (only H2SO4 leaching) yielded 77% Mg solubilization, that is to say, 4.5 times more than the A-3 test (only HCl leaching) which solubilized 17% of the Mg. Thus, H2SO4 proved to be the most effective leaching agent to solubilize the Mg present in the serpentinite.
The Mg solubilization results as a function of time for conditions A-3, A-4 and A-5 (Fig. 2) corroborate and expand some of the results previously discussed. In this respect, to compare the different trends, regression using Eq. 1 was performed for each test.
with C Mg the concentration of Mg in the aqueous phase (g/L), k the rate constant (g/L min), and b a constant.
The comparison between the b values obtained for each assay clearly indicates that the Mg dissolution is rapid and instantaneous. An interesting aspect is that both assays with HCl show a similar constant (b = 10), while the regression with H2SO4 shows a significantly higher value (b = 48). This better performance can be explained by the strong exothermic nature of its reaction with the silicates. Indeed, the temperature increased by approximately 120 °C (see the following section) compared to 75 °C for tests realized with a 12M HCl solution. In respect to the constant b, H2SO4 acid reacts almost instantaneously, making it unnecessary to continue the leaching beyond 90 min; therefore, a maximum processing time of 90 min can be established for the process. On the other hand, it can be stated that the use of an external heat supply strongly enhances Mg leaching when HCl is used (A-4 vs. A-3). Furthermore, the recovery with HCl and an external heat source (A-4) is comparable to that of H2SO4 without any external heat supply (A-5). This fact can be consequence of the higher mobility of reacting ions after the decrease in the viscosity of leaching solution caused by heating. In addition, serpentinite dissolution is a surface controlled process associated with the formation of a silica passivation layer due to the incongruent dissolution process [22]. The diffusion through the passivation is thus increased at higher temperature giving higher dissolution rates.
These global high reaction rates could be the consequence of the existence of important amounts of Al and Fe in serpentine as well as to the probable presence of brucite in the bulk sample as reported by [4]. This is so, because Si-Al bonds are stronger than those between Al, Fe, and O, thus, resulting in an increase in the vulnerability towards acid attack [21]. Another possible explanation could be fibril morphology in Quebec´s samples which are more reactive than the cylindrical ones as described by Veblen and Wylie [39].
In conclusion, the conditions of the A-5 test would be optimal for the Mg dissolution in terms of: (a) efficiency, based on the relatively high Mg concentration after leaching; (b) energy efficiency, given that it does not involve an external heat supply, which could increase the operating costs; (c) minor reagent consumption; unitary price, given that H2SO4 (86 US$/t) is five times more economical than HCl (440 US$/t); and, finally, (d) the total cost of reagents consumed, 0.30 US$/kgDR in the case of H2SO4 and 0.38 US$/kgDR in the case of HCl.
Moreover, the content of 18M H2SO4 in the leaching solution also affects the leaching process. The effect of the variation of acid concentration on the concentration of dissolved Mg in the leachate as well as the reaction temperature has also been studied. According to Fig. 3, 40, 35, and 30% v/v of 18M H2SO4 behaved similarly after 10 min of the reaction, although significant differences in the Mg dissolution were detected in the first 10 min of the reaction. These differences are more obvious for lower concentrations of H2SO4, and it can be concluded that concentrations below 35% v/v of 18M H2SO4 performed poorly, yielding lower Mg concentrations in the leachate (Table 4). This is in accordance with the results obtained by [10, 36, 41] for similar serpentinites and other acids. However, further increase of H2SO4 concentration may led to a free acids increase in the leaching solution, thus resulting in higher neutralizing agent consumption during the precipitation step.
In consequence, these results show the importance of the acid concentration in the development of strong exothermic reactions. Increasing the acid content amplifies the initial thermal shock, thus increasing the Mg contained in the leachate [41]. This effect is accentuated during the first few minutes of reaction. According to this, a concentration of 35% v/v of 18M H2SO4 was considered optimal for the extraction of the most Mg in the least time, because it decreases free acid in the leaching solution (see for example, [10]), reducing the operating costs without affecting the efficiency of the reaction.
Neutralization Tests
The leachate obtained contains Mg as well as several other metals, such as Fe, Al, Cr, Zn, and Ni. Purification is therefore the next step in isolating the Mg solution before recovering Mg in the form of hydroxide. A set of tests with increasing pH using of 20M NaOH solution were performed to the leachate solution obtained according to the optimal conditions described in the previous section, to determine the behavior of the metals in solution. The initial concentrations of Fe, Al, Ni, Cr, Mn, and Zn in the leachate were 5355, 217, 200, 79, 59, and 2.35 mg/L, respectively, whereas the concentration of Mg was 20,000 mg/L.
Based on the initial concentration, the amount of each major element present in solution as function of the pH was expressed (Fig. 4). The patterns of element precipitation are clearly showed. Thus, four different precipitation behaviors can be differentiated.
The first species to precipitate are Al and Cr. Indeed, at a pH of 5.5 both elements disappeared from the liquid phase. The precipitation initiation phase is observed around pH 3.5. A similar initiation is also observed for the other elements expect for Mg and Mn. The precipitation rate for Fe and Zn is then quite similar. Both elements concentration quickly decreased between pH 3.5 and 4.5 to then slightly decrease until pH 8. Otherwise, Ni precipitation is showing a similar trends in terms of pH range but the decrease is mostly constant. All the elements discussed above are precipitated at a pH below 8.
Mg and Mn precipitation did not start until approximately pH 8. Mn is showing a quicker precipitation behavior and is mostly precipitated at pH 9.5. Furthermore, Mg is completely recovered at pH 10. Indeed, the significant quantity of Mn remaining in solution at pH >8, at which point Mg precipitation begins, hamper the production of a pure solution of the latter. These results were coherent with their respective potential-pH equilibrium diagrams [5, 30].
Thus, a compromise between accompanying metal removal and Mg loss by precipitation must be established. Nevertheless, based on these results, pH 8 seems to be optimal for the precipitation of most of the elements present in solution while minimizing the losses of Mg (purification). These results are in clear accordance with hydroxides solubility diagrams [23]. Thus, it can be observed that metal forming solution divalent cations can be precipitated at lower pH than the divalent. In this respect, Mg (alkaline earth metal and therefore divalent) was the most difficult to precipitate. Moreover, it can be clearly appreciated that precipitation lines for Fe (in this case Fe2+ since Fe3+ precipitation is achieved even at lower pH) and Zn are located close to each other and sometimes superposed. This fact can be clearly explained according to the aforementioned diagrams and has been thoroughly reported in the bibliography (e.g., [8]).
According to the results presented in Table 5, the amount of NaOH (20M) required to achieve a given pH is not proportional to the volume of the treated leachate. Therefore, other factors, such as the yields of the leaching and filtration steps, neutralization time, and filtrate temperature, must govern the precipitation. Likewise, a pH between 8.0 and 8.5 virtually eliminates all impurities except for Fe and Mn, which remain in solution in the neutralized filtrate, albeit in very low concentrations. The P-15 test was the most effective in terms of the removal of Fe and Mn versus the amount of residual Mg. Thus, a concentration of 720 g NaOH/L provided a Fe concentration of below 1 mg/L and a Mn content of approximately 30 mg/L after precipitation.
Complete Mg(OH)2 Recovery Process
According to the information gathered in the previous sections, a process for producing Mg(OH)2 from chrysotile residues using NaOH as a precipitating agent has been designed and tested.
Mass Balance
Two separate mass balances were established during the entire production process, covering leaching (phase 1), purification, and recovery (phases 2 and 3, respectively). To verify the accuracy of the obtained data, the output/input ratio (ideally, inputs should equal to the outputs) was used. Overall, the mass balances indicated that the experiment was reliable. For the following considerations, the results corresponding to elements accounting for less than 1% of the total mass of the final residue (e.g., Ca, P, Pb, B, K) are not presented.
According to the water mass balance of the phase 1 (Table 6), out of 242 g of Mg contained in (R0), over 200 g was solved in the leachate solution (L1), and approximately 42 g (approx. 20%) was lost during the leaching process (R1). Iron, which was easily solubilized during leaching, was present in the chrysotile mine tailings (R0) in high concentrations relative to other metals, excluding Mg. As a result, out of the 70 g of Fe initially present in the chrysotile residue, approximately 45 g were solubilized (L1), while 23 g were separated in solid form in R1.
Concerning the metal mass balance for the second part of the process (phases 2 and 3), out of the approximately 204 g of Mg that were initially contained in the leachate (L2), around 152 g (75%) were recovered in the final product (P). Purification by the addition of the 20M NaOH solution (S1), followed by filtration caused a loss of approximately 38 g of the Mg initially contained in the solution in the form of the residue R2. In addition, Mg lost during cold filtration (performed at low temperature to decrease the solubility of the metals in solution) was slightly more than 13 g (approx. 20%) in the form of residue R3, whereas all the Mg contained in the filtrate L6 was recovered by precipitation with NaOH (S2) in the leachate L7, because no residue (R4) was produced in this stage. Finally, only 0.36 g of the Mg initially contained in the leachate L7 was lost in the final effluent L8 during the recovery.
Iron was almost eliminated during the purification phase, thus, out of an initial concentration of approximately 48 g in the leachate L2, no Fe was measured in the leachate L6 after the cold filtration. On the other hand, Mn was the element that caused most of the problems during the purification with NaOH and 23 g still remained in solution after the purification. Thus, although 50% of the Mn (from 56 in L2 to 26 g/L in L6) was eliminated during the neutralization at pH 8 and subsequent filtration, the remaining concentration was very persistent in solution despite the addition of NaOH in subsequent steps.
In this way, Ni, Cr, and Al showed no problems during the purification with NaOH, in fact, they were fully precipitated at pH 8, disappearing from the leachate (L4) and resulting in a final solid product (P) rich in Mg(OH)2.
Regarding the performance of the overall process (phases 1 and 2), approximately 152 g (60%) of the Mg initially contained in the serpentinite tailings (R0) was recovered in the final product in the form of Mg(OH)2. These results prove the efficiency of the procedure.
Characterization of the Final Product and Residues
During the purification process, four main residues are formed. XRD mineralogical characterization of these residues indicates the prevalence of the following mineral phases.
-
A leaching residue rich in silica.
The H2SO4 leaching residue was composed mainly of antigorite ((Mg,Fe)3Si2O5(OH)4), lizardite (Mg3Si2O5(OH)4), chrysotile (Mg3Si2O5(OH)4), and quartz (SiO2).
-
A purification residue rich in Fe.
The residue obtained after the purification of the leachate by the addition of NaOH at a pH between 8.0 and 8.5 is essentially composed of hematite and some thenardite (Na2SO4), cristobalite (SiO2) and chromium oxide (Cr2O3). In addition, it should be noted that an important amorphous signal was observed indicating the presence of non-crystalline phases.
-
A residue rich in sodium sulfate.
This residue primarily consists of thenardite (Na2SO4) and also contains a small amount of epsomite (MgSO4(H2O)7). However, this finding seems to be inconsistent with studies by Habashi [15, 16], which indicated that these solutions at 30 °C should promote the formation of mirabilite (Na2SO4·10H2O) rather than thenardite (Na2SO4). Temperature filtration of these residues and oven drying may partially explain this discrepancy. When the process was optimized using brucite as a precipitating agent, this residue was not produced. Thenardite has applications in wood pulp production as well as in glass industry.
-
A final product rich in brucite.
According to the XRD analyses, Mg is mostly present as Mg(OH)2·nH2O, whereas Na is present as thenardite (Na2SO4) and mirabilite (Na2SO4·10H2O). Chemical analyses of the product by XRF corroborated the composition shown in Table 6, indicating, a part from Mg, traces of Mn, and Fe, but with no (or negligible) concentrations of Ni, Cr, or Al. Further analysis by the phenolphthalein titration of OH ions with the SO4 −2 in the residue established a brucite concentration of 22.8%.
In accordance with the TCLP performed for all the residue (data not shown), it is reasonable to assume that none of them are a real threat to the environment.
Economic Analysis of the Process
The economic viability of the method was studied for the three phases: leaching, purification, and recovery. The study was performed to provide an economic balance per kilogram of the initial residue (kgDR). Results are shown in Table 7.
In this regard, the consumption of reactive was 3.17 kg of H2SO4 per kgDR for the lixiviation; 2.34 kg of NaOH per kgDR, for the purification; and 0.57 kg of NaOH per kgDR for the recovery. The total cost associated with these reactives was 0.27 US$/kgDR for the H2SO4 and 1.46 US$/kgDR for the NaOH.
Over all, the process is a technical and ecological success; however, the excessive NaOH consumption, particularly during the purification phase, hinders the achievement of a positive economic balance, causing an economic loss of 1.29 US$/kgDR.
Despite this, leaching and purification phases are economically feasible; the viability of the overall process depends on the costs of NaOH. Thus, the reduction of the amount needed or its substitution is desirable for the recovery phase in order to obtain a positive economic balance.
Conclusion
This study has focused on the serpentinite mining tailings of La Nationale, aiming to recover the Mg contained therein using a hydrometallurgical route. The experiments revealed that most of the Mg was evenly distributed among all grain size fractions, suggesting that all these fractions are appropriate leaching feed after grinding and sieving.
The development of an adequate leaching procedure included testing several acids. Concentrated H2SO4 demonstrated its effectiveness, dissolving between 80 to 85% of Mg at moderate cost. In contrast, the use of NaOH for neutralization was effective but too costly, limiting the economic viability of the process given the current price for Mg. Despite these limitations, the process provided solid low grade Mg(OH)2 in a relatively clean process. In regard of the increasing demand for Mg(OH)2 in environmental applications, such as flue gas desulfurization, carbon capture and storage by mineral carbonation, or in water treatment area, this study offers valuable information to conduct further research on this topic.
For instance, a cost reduction for the process could be obtained by following the subsequent considerations. Firstly, magnetic separation of the ferro-nickel contained in the waste and the use of a larger volume of washing water and better agitation may increase the efficiency of the process, promoting Mg solubilization. Regarding leaching, slightly less H2SO4 (2–3%) can be used for leaching to promote cost savings while maintaining efficiency. Moreover, the losses observed in the Mg balance during purification can be eliminated by the use of Mg(OH)2 in dissolved form. Finally, residual Mn present in the neutralized leachate may be removed by oxidation before Mg recovery.
References
Aïtcin, P.C., Delvaux, P.: L’amiante Chrysotile. Université de Sherbrooke, Sherbrooke (1978)
American Public Health Association (APHA), American Water Works Association (AWWA), Water Environment Federation (WEF): Standard Methods for the Examination of Water and Wastewater, 20th edn. APHA, Washington, DC (1999)
Apostolidis, C.I., Distin, P.A.: The kinetics of the sulphuric acid leaching of nickel and magnesium from reduction roasted serpentine. Hydrometallurgy 3, 181–196 (1978)
Bates, T.F., Comer, J.J.: Further observations on the morphology of chrysotile and halloysite1. Clays Clay Miner. 6, 237–248 (1957)
Brookins, D.G.: Eh-pH Diagrams for Geochemistry. Springer, Berlin (1988)
Brown, R.E.: Magnola: The Noranda magnesium process. Light Metal Age 56(1/2), 60–63 (1998)
Cecchi, E.:. Revalorisation des résidus d’extraction d’amiante blanc par la production de chlorure de magnésium via la réaction de carbochloruration. Ph.D. Thesis, INRS-ETE, Université du Québec, Québec (2008)
Claassen, J.O., Meyer, E.H.O., Rennie, J., Sandenbergh, R.F.: Iron precipitation from zinc-rich solutions: defining the Zincor Process. Hydrometallurgy 67(1), 87–108 (2002)
Dutrizac, J.E., Chen, T.T., White, C.W.: Fundamentals of serpentine leaching in hydrochloric acid media. In: Kaplan, H.I., Hryn, J.N., Clow, B.B. (eds.) Magnesium Technology. TMS Annual Meeting, The Minerals, Metals and Materials Society, Nashville, pp. 41–51 (2000)
El-Leef, E.S.M.A., Abeidu, A.E.M., Mahdy, A.E.M.: Utilization of serpentine ore for production of magnesium sulphate. World J. Eng. Pure Appl. Sci. 2(2), 31–39 (2012)
Fedoročková, A., Hreus, M., Raschman, P., Sučik, G.: Dissolution of magnesium from calcined serpentinite in hydrochloric acid. Miner. Eng. 32, 1–4 (2012)
Fouda, M.F.R., Amin, R.E.-S., Abd-Elzaher, M.M.: Extraction of magnesia from Egyptian serpentine ore via reaction with different acids. I. Reaction with sulfuric acid. Bull. Chem. Soc. Jpn. 69(7), 1907–1912 (1996)
Friedrich H.E., Mordike B.L. (2006) Magnesium Technology-Metallurgy, Design Data, Applications. Springer, Berlin
Gladikova, L., Teterin, V., Freidlina, R.: Production of magnesium oxide from solutions formed by acid processing of serpentinite. Russ. J. Appl. Chem. 81(5), 889–891 (2008)
Habashi, F.: Textbook of Hydrometallurgy, 2nd edn. Département des mines, métallurgies et génie des matériaux, Université Laval, Sainte-Foy (1999)
Habashi, F.: Magnesium. Handbook of Extractive Metallurgy. Wiley, Weinheim (1997)
Harben, P.W., Smith, C. Jr: Industrial minerals and rocks; commodities, markets and uses. SME, Littleton, pp. 679–683 (2006)
Karidakis, T., Agatzini-Leonardou, S., Neou-Syngouna, P.: Removal of magnesium from nickel laterite leach liquors by chemical precipitation using calcium hydroxide and the potential use of the precipitate as a filler material. Hydrometallurgy 76, 105–114 (2005)
Kim, J.: Magnesium Extraction from Asbestos Mine Tailings: A Report. Howard Manosh Vermont Asbestos Group, Vermont Geological Survey, Department of Environmental Conservation, Waterbury (1998)
Lackner, K.S., Wendt, C.H., Butt, D.P., Joyce, B.L., Sharp, D.H.: Carbon dioxide disposal in carbonate minerals. Energy. 20, 1153–1170 (1995)
Levenspiel, O.: Chemical Reaction Engineering. Wiley, New York (1978)
Luce, R. W., Bartlett, R. W., Parks, G. A.: Dissolution kinetics of magnesium silicates. Geochim. Et Cosmochim. Acta. 36(1), 35–50 (1972)
Monhemius, A. J.: Precipitation diagrams for metal hydroxides, sulfates, arsenates and phosphates. T. I. Min. Metall. C 86, C202–C206 (1977)
Mossman, B.T., Bignon, J., Corn, M., Seaton, A., Gee, J.B.L.: Asbestos: scientific developments and implications for public policy. Science 247(4940), 294–301 (1990)
Nagamori, M., Boivin, J.A.: Technico-economic simulation for the HCl-leaching of hybrid serpentine and magnesite feeds. Can. Metall. Q. 40(1), 47–60 (2001)
Nduagu, E., Björklöf, T., Fagerlund, J., Mäkilä, E., Salonen, J., Geerlings, H., Zevenhoven, R.: Production of magnesium hydroxide from magnesium silicate for the purpose of CO2 mineralization—Part 2: Mg extraction modeling and application to different Mg silicate rocks. Miner. Eng. 30, 87–94 (2012). doi:10.1016/j.mineng.2011.12.002
Pasquier, L.-C., Mercier, G., Blais, J.-F., Cecchi, E., Kentish, S.: Reaction mechanism for the aqueous-phase mineral carbonation of heat-activated serpentine at low temperatures and pressures in flue gas conditions. Environ. Sci. Technol. 48, 5163–5170 (2014)
Petrovski, P., Gligoric, M.: Usage of serpentine for MgO and active SiO2 production. In: Vincenzini, P.. (ed.), High Tech Ceramics. Elsevier, Amsterdam, pp. 2267–2278 (1987)
Pigg, R.: Uses of chrysotile asbestos. Ann. Occup. Hyg. 38(4), 453–458 (1994)
Pourbaix, M.: Atlas of Electrochemical Equilibria in Aqueous Solutions. National Association of Corrosion Engineers, Houston (1974)
Ramachandra Rao, S.: Resource Recovery and Recycling from Metallurgical wastes. Waste Management Series, vol. 7. Elsevier, Amsterdam (2006)
Ray, M.S., Sneesby, M.G. Chemical Engineering Design Project: A Case Study Approach, 2nd edn. Gordon and Breach Science Publishers, Amsterdam (1998)
Schrier, H.: Asbestos in the Natural ENVIRONMENT. Studies in Environmental Science. No. 37. Elsevier, Amsterdam (1989)
Skinner, H.C.W., Ross, M., Frondel, C.: Asbestos and Other Fibrous Materials. Oxford University Press, New York (1988)
Taubert, L.: Hydrochloric attack of serpentinites: Mg2+ leaching from serpentinites. Magnes. Res. 13(3), 167–173 (2000)
Teir, S., Revitzer, H., Eloneva, S., Fogelholm, C.J., Zevenhoven, R.: Dissolution of natural serpentinite in mineral and organic acids. Int. J. Miner. Process. 83, 36–46 (2007)
U.S. Department of Health and Human Services: 12th Report on Carcinogens. Public Health Service, National Toxicology Program, Research Triangle Park (2011)
United States Environmental Protection Agency (USEPA): Toxicity characteristic leaching procedure, method 1311. http://www.epa.gov/wastes/hazard/testmethods/SW846/pdfs/1311.pdf (1992)
Veblen, D.R., Wylie, A.G.: Mineralogy of amphiboles and 1:1 layer silicates. In: Guthrie, G.D., Mossman, B.T. (eds.) Health Effects of Mineral Dusts. Reviews in Mineralogy, vol. 28, pp. 61–137. Mineralogical Society of America, Bookcrafters Inc., Chelsea (1993)
Wulandari, W., Brooks, G., Rhamdhani, M., Monaghan, B.: Magnesium: current and alternative production routes. The 40th Chemeca: Australasian Conference on Chemical Engineering, September 26–29, Adelaide (2010)
Yoo, K., Kim, B.S., Kim, M.S., Lee, J.C., Jeong, J.: Dissolution of magnesium from serpentine mineral in sulfuric acid solution. Mater. Trans. 50(5), 1225–1230 (2009)
Zhang, Q., Sugyiama, K., Saito, F.: Enhancement of acid extraction of magnesium and silicon from serpentine by mechanochemical treatment. Hydrometallurgy 45(3), 323–331 (1997)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sierra, C., Chouinard, S., Pasquier, L.C. et al. Feasibility Study on the Utilization of Serpentine Residues for Mg(OH)2 Production. Waste Biomass Valor 9, 1921–1933 (2018). https://doi.org/10.1007/s12649-017-9926-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-017-9926-9