Abstract
Beef tallow is a promising alternative as a non-edible raw material for biodiesel production, due to its lower price compared to vegetable oils such as soybean oil. The problem of using beef tallow as a raw material for biodiesel is its high acidity level, found as a consequence of hydrolysis and oxidation reactions. These degradation processes are significant in the presence of high levels of humidity and temperature, which are usually found in the storage conditions. In this study, the influence of synthetic and natural antioxidants on the oxidation stability of beef tallow was evaluated using Rancimat tests and by monitoring their acid and peroxide values over 148 days of storage in an oven. The studied synthetic and natural (cashew nut shell liquid, CNSL) antioxidants were effective to prevent oxidation of beef tallow on storage conditions. Biodiesel samples were produced from samples of beef tallow with and without antioxidants. The biodiesel samples produced from beef tallow containing BHT presented the best induction period values. The biodiesel samples produced from beef tallow containing technical CNSL (0.5 wt%) met the requirement of oxidation stability at 110 °C determined by the Brazilian specification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biodiesel production in Brazil has been increasing steadily over the last decade [1]. About 95% of the biodiesel production plants in Brazil use edible vegetable oils as raw material [2, 3]. Beef tallow is a promising alternative as a non-edible raw material for biodiesel production, due to its lower price compared to vegetable oils such as soybean oil, preventing the improper disposal of animal waste on the environment [4, 5].
Beef tallow is normally stored in industries at temperatures around 60 °C, since at lower temperatures, this feedstock is in solid phase, which hinders its handling in the biodiesel production. The problem of using beef tallow as a raw material for biodiesel production is its high acidity level, found as a consequence of hydrolysis and oxidation reactions. These degradation processes are significant in the presence of high levels of humidity and temperature, which are usually found in the storage condition of beef tallow. High acidity levels affect the production yield of biodiesel, mainly due to high consumption of basic catalysts (more commonly used) and the saponification of the free fatty acids [6].
After an increase of acidity levels on beef tallow stored in a biodiesel industry, it is necessary to separate the excess acids contained in beef tallow by vacuum distillation or neutralization. In both processes, free fatty acid and soapstock are generated as waste. Due to the increase of the acid values on stored beef tallow, the fatty acid and the soapstock wastes are cheaper than the purchase price of beef tallow, which represents a financial loss to the biodiesel industry.
One way to try to retard the oxidation and prevent re-processing of beef tallow is through the use of natural or synthetic antioxidants before biodiesel production. Normally, antioxidants act to inhibit the oxidation process and are well-established for its use in the control of biodiesel oxidation. Biodiesel stability in the presence of antioxidants has been studied by several researchers [7,8,9,10]. Yang et al. [11] studied the effect of antioxidants on the biodiesel samples prepared from tallow oil, soybean oil and canola oil. It was found in their studies that pyrogallol was the best in enhancing induction period with a concentration of less than 3000 ppm, however, tert-butylhydroquinone (TBHQ) was the best after 3000 ppm.
Biodiesel fuels derived from fats, having significant amounts of saturated fatty compounds, normally show higher cold filter plugging points (CFPPs), because these compounds have higher melting points, and in a mixture, they crystallize at higher temperatures than the unsaturated fatty compounds. Although it is not possible to use 100% biodiesel produced from beef tallow (CFPP = + 10 °C), pork lard (CFPP = + 5 °C), and chicken fat (CFPP = + 3 °C), blends of 20% biodiesel (v/v) produced from these animal fats mixed with petroleum diesel are viable with some advantages, such as the improved cold-flow properties (CFPP below − 6 °C), lower kinematic viscosity (from 3.10 to 3.28 mm2/s), and higher heating value of the mixture (about 44.6 MJ/kg) [12]. On another study, the evaluation of the influence of 11 different synthetic phenolic antioxidants on critical biodiesel fuel parameters showed no negative impacts on viscosities, densities, carbon residues, CFPP, and sulphated ash contents of biodiesel samples prepared from rapeseed oil, recycled cooking oil, distilled recycled cooking oil and tallow [13].
Among the natural antioxidants are the tocopherols, which are monophenolic antioxidants that help stabilize most vegetable oils [14, 15]. The fruit of the cashew tree, Anacardium occidentale L., is commonly known as cashew nut, which has plenty of a dark liquid, almost black, caustic and flammable, named cashew nut shell liquid (CNSL) [16]. The CNSL represents approximately 25% of the cashew nut weight and is considered a by-product of the cashew agribusiness, with very low value [15, 16]. It is one of the richest sources of naturally occurring non-isoprenoid phenolic lipids [15, 17].
The inhibition of degradation of beef tallow with the use of natural antioxidants from CNSL and synthetic antioxidants was not yet investigated in details. In this study, samples of beef tallow with and without antioxidants were tested using the Rancimat method and by monitoring their acid and peroxide values over 148 days of storage in an oven. The performance of natural antioxidants from CNSL was compared with traditional synthetic antioxidants on the oxidation stability of beef tallow before biodiesel production. The biodiesel samples produced were evaluated to obtain the performance of antioxidants and their effects on biodiesel properties.
Experimental Section
Materials
Samples of beef tallow, obtained directly from a butcher shop in Fortaleza (Brazil), were heated up to 120 °C in order to make beef tallow go from solid to liquid state. The beef tallow in liquid state was then filtered and separated from the rest of the fat material which remained in solid phase. The filtered beef tallow was stored in a freezer at − 6 °C until the moment to be used in the experiments.
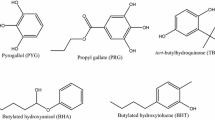
3,5-Di-tert-4-butylhydroxytoluene (BHT, ≥ 99 wt%) was from Dinâmica (Brazil); tert-Butylhydroquinone (TBHQ, 97 wt%) was from Sigma–Aldrich (USA); mixture of 86% of 2,6-Di-tert-butylphenol and 14% of 2,4,6-tri-tert-butylphenol (IONOL, > 99 wt %) was from Sigma-Aldrich (USA).
A typically solvent-extracted CNSL is composed mainly by: anacardic acid (60–70%), cardol (15–20%), cardanol (10%), and traces of 2-methylcardol. When obtained as a residue from the industrial roasting shell process, which employs elevated temperatures, anacardic acid suffers a decarboxylation reaction and CNSL is then considered as Technical CNSL, which contains mainly cardanol (60–70%), cardol (15–20%), polymeric material (10%), and traces of 2-methylcardol [16, 18]. The decarboxylation process of the anacardic acid can be seen in Fig. 1 [16, 19,20,21,22,23].
The standard used for the chromatographic analysis (F.A.M.E. Mix C8-C24, 100 mg, Supelco) was purchased from Sigma-Aldrich (USA). All chemicals used for beef tallow characterization were of pro analysis grade.
Beef Tallow Characterization
Beef tallow was characterized considering: acid value and percentage of free fatty acids as reported in AOCS methods Cd 3d-63 and Ca 5a-40, respectively, with modifications suggested by Moretto and Fett [24, 25]; iodine value as in ABNT NBR 9231:2012 [26]; peroxide value, as in AOCS method Cd 8-53 [27]; saponification value as in ABNT NBR 10448:2012 [26]; kinematic viscosity at 100 °C as in ASTM D44515a [28]; and, oxidation stability at 110 °C, according to EN 14112:2003 (using 873 Biodiesel Rancimat® from Metrohm, Switzerland) [29]. For biodiesel, density at 20 °C and kinematic viscosity at 40 °C were determined according to ASTM D-7042-16e1 [30].
The beef tallow composition was obtained from the methyl ester profile evaluated by GC analysis (Varian 450 Gas Chromatograph, USA) according to 056/IV—Method 3 from Instituto Adolfo Lutz [31].
Biodiesel Synthesis
Firstly, beef tallow neutralization was carried out as reported in AOCS method Ca 9b-52 with the required adaptation for beef tallow [24]. The transesterification of biodiesel was carried out by homogeneous basic catalysis using potassium hydroxide by the methylic route. The mixtures were submitted to stirring with heating under reflux at 60 °C for 1 h. The biodiesel was separated from glycerol by decantation, then washed five times with distilled water (7 mL each time) and finally dehumidified using an evaporator at 80 °C for 50 min.
Oxidation Stability Measurements
Samples of beef tallow without antioxidant and with the antioxidants BHT, TBHQ, IONOL, Natural CNSL and Technical CNSL were prepared in duplicate and were stored in an oven at a temperature of 70 °C during 148 days. Measurements of acid value and peroxide value were taken in order to evaluate the storage stability of the samples. Rancimat (EN 14112) measurements were conducted in duplicate for each sample of beef tallow and biodiesel.
Fourier Transformed Infrared Spectroscopy (FTIR)
An IRAffinity-1 Shimadzu spectrophotometer, equipped with a DLATGS detector, with temperature control system and Ge coated on KBr beam splitter, was used. Wavelengths were scanned in the range 400–4000 cm−1, with a resolution of 8.0 cm−1. The samples (ca. 500 mg) were heated until molten and then mixed with 25 mL of heptane P.A. using a vortex and analyzed in a standard cell (KBr) with an optical path of 0.05 mm. The transmittance spectral band of C=O of saturated aliphatic esters occurs between 1750 and 1735 cm−1 [32]. This spectral band is characteristic of the axial deformation of the carbonyl group, which is present in most oxidation products [33, 34]. The oxidation of the samples was quantified by calculating the area of the transmittance spectral band corresponding to the vibration of the carbonyl radicals around 1750 cm−1, measured from 1620 to 1860 cm−1.
Results and Discussion
The physicochemical properties of beef tallow are presented in Table 1. Beef tallow was evaluated using the Rancimat test showing induction period (IP) of approximately 10 h.
The fatty acid composition of beef tallow might be expressed by the fatty acid methyl ester (FAME) profile obtained by gas chromatography, as shown in Table 2.
Beef tallow consists predominantly of saturated fatty acids, mostly palmitic and stearic fatty acids, which represent ca. 60 wt% of the fat, similar to values normally reported by other authors [35, 36].
The results obtained with the Rancimat tests are presented in Fig. 2. The orders of effectiveness of the antioxidants were: TBHQ > BHT ≈ IONOL ≈ Natural CNSL ≈ Technical CNSL, for the concentration of 1000 mg/kg; TBHQ > Natural CNSL > Technical CNSL > IONOL > BHT, for the concentration of 5000 mg/kg.
Based on their electronegativity (defined as the tendency of the hydroxyl functional group to attract a pair of bonding electrons), the antioxidants can be ranked according to their efficiency as BHT < TBHQ [37,38,39]. IONOL and BHT both have substituents at the ortho and para positions in the phenolic ring, which is the reason for their similarity, as observed in the results of Rancimat tests and thermogravimetric analysis in other report [15].
As presented in Fig. 3, the acid values of the fresh sample (without antioxidant) over storage in an oven increased more than those of the samples with the synthetic and natural antioxidants. Only the sample with Technical CNSL at concentration of 1000 mg/kg presented higher acid values than those of the fresh sample. Rodrigues et al. [40] have proposed that the oligomerization or polymerization of CNSL, cardanol and derivatives could have influence over their antioxidant effect. Low molecular weight antioxidants are easily lost from polymer through migration, evaporation and extraction. The presence of polymerized material could decrease the migration of the antioxidant to the surface, reduce its volatilization and retain the antioxidant activity during heating [41].
Acid values for fresh sample (open circle) and for samples with the antioxidants BHT (filled triangle), TBHQ (filled circle), IONOL (filled square), Natural CNSL (filled diamond) and Technical CNSL (filled star) over 148 days of storage in an oven in different concentrations. a Concentration of 1000 mg/kg, mean standard error = 1. b Concentration of 5000 mg/kg, mean standard error = 0.33. The results presented are the mean values of the acid values of the duplicate samples
Samples containing the synthetic antioxidants BHT, TBHQ and IONOL at the concentration of 5000 mg/kg presented lower acid values than the samples containing the same synthetic antioxidants at the concentration of 1000 mg/kg. Among the synthetic and natural antioxidants, the BHT was the most effective on increasing the oxidation stability of beef tallow stored in an oven for 148 days according to the results of the acid values.
As presented in Fig. 4, the peroxide values showed a periodic behavior along storage time up to 148 days. This result might be related with the explanation of Pullen and Saeed [42] that during the oxidative process the hydroperoxides accumulate and then later decompose to form aldehydes, alcohols, short chain carboxylic acids and higher molecular weight oligomers. The antioxidant intercepts the peroxyl radical in order to prevent it from creating another radical through the autoxidation mechanism.
Peroxide values for fresh sample ( ) and for samples with the antioxidants BHT (
) and for samples with the antioxidants BHT ( ), TBHQ (
), TBHQ ( ), IONOL (
), IONOL ( ), Natural CNSL (
), Natural CNSL ( ) and Technical CNSL (
) and Technical CNSL ( ) on the concentration of 5000 mg/kg over 148 days of storage in an oven. The results presented are the mean values of the peroxide values of the duplicate samples
) on the concentration of 5000 mg/kg over 148 days of storage in an oven. The results presented are the mean values of the peroxide values of the duplicate samples
It was also observed that peroxide values were higher for the fresh sample than for the samples with synthetic and natural antioxidants. According to the results of the peroxide values, the antioxidant BHT was the most effective on increasing the oxidation stability of beef tallow stored in an oven for 148 days among the synthetic and natural antioxidants.
As presented in Fig. 5, for samples after 148 days of oxidation in an oven, the mean area values of the carbonyl transmittance spectral bands were lower for most of the samples with synthetic and natural antioxidants than for the fresh sample. Only the sample with Technical CNSL at concentration of 1000 mg/kg had a higher mean area value of the carbonyl transmittance spectral bands than the fresh sample, which again might be related with the explanation proposed by Rodrigues et al. [40] that the oligomerization or polymerization of CNSL could have influence over their antioxidant effect [41]. In comparison with the fresh sample, the percentage reduction of the mean area values of the carbonyl band for the samples with the antioxidants at the concentration of 5000 mg/kg was 74.6, 64.5, 63.6, 55.8 and 39.1% for Technical CNSL, IONOL, BHT, TBHQ and Natural CNSL, respectively. This result confirms that the antioxidants indeed inhibited the formation of oxidation products on beef tallow stored in an oven for 148 days.
Mean area values of the carbonyl transmittance spectral bands for fresh sample ( ) and for samples with the antioxidants BHT (
) and for samples with the antioxidants BHT ( ), TBHQ (
), TBHQ ( ), IONOL (
), IONOL ( ), Natural CNSL (
), Natural CNSL ( ) and Technical CNSL (
) and Technical CNSL ( ) in different concentrations after 148 days of storage in an oven. The mean area values of the carbonyl transmittance spectral bands were calculated based on the four replicates which were obtained for each sample
) in different concentrations after 148 days of storage in an oven. The mean area values of the carbonyl transmittance spectral bands were calculated based on the four replicates which were obtained for each sample
The antioxidants Natural CNSL, technical CNSL, BHT and TBHQ were added to samples of refined beef tallow and the main properties of the biodiesel samples obtained are summarized in Table 3. The concentration of free fatty acids of the neutralized beef tallow was 0.1 wt%.
As it is shown in Table 3, the oxidation stability at 110 °C of the biodiesel samples produced from beef tallow containing Technical CNSL, BHT and TBHQ at concentration of 5000 mg/kg were higher than the 8 h required by the Brazilian specification [43]. The antioxidant BHT has an extremely low water solubility (1.1 mg/L at 20 °C), while TBHQ is slightly soluble in water [30,31,32]. Thus, TBHQ might have left the biodiesel samples during the washing steps with distilled water. This might explain why the biodiesel samples produced from refined beef tallow containing BHT presented the best induction period values (> 85 h, for BHT).
Conclusions
The effectiveness of synthetic and natural antioxidants was studied using different oxidation stability measurements. Samples of beef tallow with TBHQ had the best induction period values on the Rancimat tests. Samples of beef tallow with TBHQ and BHT stored in an oven had the lowest acid and peroxide values during storage time, which suggested that TBHQ and BHT were the most effective antioxidants to prevent beef tallow oxidation in storage conditions.
Biodiesel samples produced from refined beef tallow containing Technical CNSL, BHT and TBHQ met the minimum of 8 h for the oxidation stability at 110 °C. These results suggested that besides having increased the oxidation stability of beef tallow, the antioxidants partially remained on the biodiesel samples even after the transesterification reaction and the washing steps with distilled water. The use of antioxidants before biodiesel production increased from 9 to 150 times the induction period of biodiesel from beef tallow.
References
Brasil: Boletim mensal dos combustíveis renováveis. Ministério de Minas e Energia. http://www.mme.gov.br (2017). Accessed 6 April 2017
Gui, M.M., Lee, K.T., Bhatia, S.: Feasibility of edible oil vs. non-edible oil vs. waste edible oil as biodiesel feedstock. Energy. 33, 1646–1653 (2008). doi:10.1016/j.energy.2008.06.002
Sulistyo, H., Almeida, M.F., Dias, J.M.: Influence of synthetic antioxidants on the oxidation stability of biodiesel produced from acid raw Jatropha curcas oil. Fuel Process. Technol. 132, 133–138 (2015). doi:10.1016/j.fuproc.2014.12.003
Imahara, H., Minami, E., Saka, S.: Thermodynamic study on cloud point of biodiesel with its fatty acid composition. Fuel. 85, 1666–1670 (2006). doi:10.1016/j.fuel.2006.03.003
Santos, A.G.D.: Avaliação da estabilidade térmica e oxidativa do biodiesel de algodão, girassol, dendê e sebo bovino. Universidade Federal do Rio Grande do Norte, Natal (2010)
Rincón, L.E., Jaramillo, J.J., Cardona, C.A.: Comparison of feedstocks and technologies for biodiesel production: an environmental and techno-economic evaluation. Renew. Energy. 69, 479–487 (2014). doi:10.1016/j.renene.2014.03.058
Jakeria, M.R., Fazal, M.A., Haseeb, A.S.M.A.: Influence of different factors on the stability of biodiesel: a review. Renew. Sustain. Energy Rev. 30, 154–163 (2014). doi:10.1016/j.rser.2013.09.024
Knothe, G.: Some aspects of biodiesel oxidative stability. Fuel Process. Technol. 88, 669–677 (2007). doi:10.1016/j.fuproc.2007.01.005
McCormick, R.L., Ratcliff, M., Moens, L., Lawrence, R.: Several factors affecting the stability of biodiesel in standard accelerated tests. Fuel Process. Technol. 88, 651–657 (2007). doi:10.1016/j.fuproc.2007.01.006
Xin, J., Imahara, H., Saka, S.: Kinetics on the oxidation of biodiesel stabilized with antioxidant. Fuel 88, 282–286 (2009). doi:10.1016/j.fuel.2008.08.018
Yang, Z., Hollebone, B.P., Wang, Z., Yang, C., Landriault, M.: Factors affecting oxidation stability of commercially available biodiesel products. Fuel Process. Technol. 106, 366–375 (2013). doi:10.1016/j.fuproc.2012.09.001
Mata, T.M., Cardoso, N., Ornelas, M., Neves, S., Caetano, N.S.: Evaluation of two purification methods of biodiesel from beef tallow, pork lard, and chicken fat. Energy Fuels 25, 4756–4762 (2011). doi:10.1021/ef2010207
Schober, S., Mittelbach, M.: The impact of antioxidants on biodiesel oxidation stability. Eur. J. Lipid Sci. Technol. 106, 382–389 (2004). doi:10.1002/ejlt.200400954
Palozza, P., Rossella, S., Picci, N., Buzzoni, L., Ciliberti, N., Natangelo, A., Manfredini, S., Vertuani, S.: Design, synthesis, and antioxidant potency of novel α-tocopherol analogues in isolated membranes and intact cells. Free Radic. Biol. Med. 44, 1452–1464 (2008). doi:10.1016/j.freeradbiomed.2008.01.001
Santos, F.F.P.: Avaliação de antioxidantes aplicados à produção de biodiesel (2013)
Mazzetto, S.E., Lomonaco, D., Mele, G.: Óleo da castanha de caju: oportunidades e desafios no contexto do desenvolvimento e sustentabilidade industrial. Quim. Nova. 32, 732–741 (2009). doi:10.1590/S0100-40422009000300017
Trevisan, M.T.S., Pfundstein, B., Haubner, R., Würtele, G., Spiegelhalder, B., Bartsch, H., Owen, R.W.: Characterization of alkyl phenols in cashew (Anacardium occidentale) products and assay of their antioxidant capacity. Food Chem. Toxicol. 44, 188–197 (2006). doi:10.1016/j.fct.2005.06.012
Lomonaco, D., Maia, F.J.N., Clemente, C.S., Mota, J.P.F., Junior, A.E.C., Mazzetto, S.E.: Thermal studies of new biodiesel antioxidants synthesized from a natural occurring phenolic lipid. Fuel 97, 552–559 (2012). doi:10.1016/j.fuel.2012.01.059
Gedam, P.H., Sampathkumaran, P.S.: Cashew nut shell liquid: extraction, chemistry and applications. Prog. Org. Coat. 14, 115–157 (1986). doi:10.1016/0033-0655(86)80009-7
Attanasi, O., Filippone, P., Grossi, M.: Synthesis of some phosphorus derivatives of cardanol. Phosphorus Sulfur Relat. Elem. 35, 63–65 (1988). doi:10.1080/03086648808079365
Rios, M.A.S.: Síntese e Aplicabilidade de Antioxidantes derivados do Cardanol Hidrogenado. Universidade Federal do Ceará, Ceará (2008)
Lopes, A.A.S.: Síntese de um aditivo tiofosforado a partir do líquido da casca da castanha de caju (Anacardium occidentale Lin) (2005)
Mele, G., Vasapollo, G.: Fine chemicals and new hybrid materials from cardanol. Mini Rev. Org. Chem. 5, 243–253 (2008)
American Oil Chemists’ Society: Official Methods and Recommended Practices of the AOCS. AOCS, Urbana (2009)
Moretto, E., Fett, R.: Tecnologia de óleos e gorduras vegetais na indústria de alimentos. Varela Editora e Livraria Ltda, São Paulo (1998)
Associação Brasileira de Normas Técnicas: ABNT NBR 13573, Amostragem de insumos químicos para curtimento e acabamento de couros. ABNT, Rio de Janeiro (2012)
American Oil Chemists’ Society: Official methods and recommended practices of the American Oil Chemists’ Society. AOCS Official method Cd 8-53. AOCS, Champaign (1990)
ASTM International: Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity). ASTM International, West Conshohocken (2016)
BSI British Standards: Fat and oil derivatives. Fatty acid methyl esters (FAME). In: Determination of oxidation stability (accelerated oxidation test). BSI, London (2003)
ASTM International: ASTM D7042-16e1. Standard Test Method for Dynamic Viscosity and Density of Liquids by Stabinger Viscometer (and the Calculation of Kinematic Viscosity). ASTM International, West Conshohocken (2016)
Instituto Adolfo Lutz: Normas Analíticas do Instituto Adolfo Lutz. Métodos Físico-Químicos para Análise de Alimentos. Ministério da Saúde, Agência Nacional de Vigilância Sanitária, Brasília (2005)
Silverstein, R.M., Webster, F.X., Kiemle, D.J.: Identificação Espectrométrica de Compostos Orgânicos. LTC, Rio de Janeiro (2013)
Silverstein, R.M., Bassler, G.C., Morrill, T.: Spectrometric Identification of Organic Compounds. Wiley, New York (1991)
Araújo, S. V., Rocha, B.S., Luna, F.M.T., Rola, E.M., Azevedo, D.C.S., Cavalcante, C.L.: FTIR assessment of the oxidation process of castor oil FAME submitted to PetroOXY and Rancimat methods. Fuel Process. Technol. 92, 1152–1155 (2011). doi:10.1016/j.fuproc.2010.12.026
Pullen, J., Saeed, K.: Experimental study of the factors affecting the oxidation stability of biodiesel FAME fuels. Fuel Process. Technol. 125, 223–235 (2014). doi:10.1016/j.fuproc.2014.03.032
Cunha, M.E., Krause, L.C., Moraes, M.S.A., Faccini, C.S., Jacques, R.A., Almeida, S.R., Rodrigues, M.R.A., Caramão, E.B.: Beef tallow biodiesel produced in a pilot scale. Fuel Process. Technol. 90, 570–575 (2009). doi:10.1016/j.fuproc.2009.01.001
Tang, H., Wang, A., Salley, S.O., Ng, K.Y.S.: The effect of natural and synthetic antioxidants on the oxidative stability of biodiesel. J. Am. Oil Chem. Soc. 85, 373–382 (2008). doi:10.1007/s11746-008-1208-z
Liang, C., Schwarzer, K.: Comparison of four accelerated stability methods for lard and tallow with and without antioxidants. J. Am. Oil Chem. Soc. 75, 1441–1443 (1998). doi:10.1007/s11746-998-0196-3
Loh, S.-K., Chew, S.-M., Choo, Y.-M.: Oxidative stability and storage behavior of fatty acid methyl esters derived from used palm oil. J. Am. Oil Chem. Soc. 83, 947–952 (2006). doi:10.1007/s11746-006-5051-9
Rodrigues, F.H.A., Feitosa, J.P.A., Ricardo, N.M.P.S., França, F.C.F., Carioca, J.O.B.: Antioxidant activity of cashew nut shell liquid (CNSL) derivatives on the thermal oxidation of synthetic cis-1,4-polyisoprene. J. Braz. Chem. Soc. 17, 265–271 (2006). doi:10.1590/S0103-50532006000200008
Rodrigues, F.H.A., Souza, J.R.R., França, F.C.F., Ricardo, N.M.P.S., Feitosa, J.P.A.: Thermal oligomerisation of cardanol. e-Polymers (2006). doi:10.1515/epoly.2006.6.1.1027
Pullen, J., Saeed, K.: An overview of biodiesel oxidation stability. Renew. Sustain. Energy Rev. 16, 5924–5950 (2012). doi:10.1016/j.rser.2012.06.024
Agência Nacional do Petróleo Gás Natural e Biocombustíveis: Resolução ANP No. 51-25.11.2015-DO 26.11.2015. http://www.anp.gov.br (2017). Accessed 6 April 2017
Acknowledgements
The authors acknowledge financial support from CNPq, CAPES and Federal Institute of Education, Science and Technology of Ceará.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kleinberg, M.N., Rios, M.A.S., Buarque, H.L.B. et al. Influence of Synthetic and Natural Antioxidants on the Oxidation Stability of Beef Tallow Before Biodiesel Production. Waste Biomass Valor 10, 797–803 (2019). https://doi.org/10.1007/s12649-017-0120-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-017-0120-x






 ) and for samples with the antioxidants BHT (
) and for samples with the antioxidants BHT ( ), TBHQ (
), TBHQ ( ), IONOL (
), IONOL ( ), Natural CNSL (
), Natural CNSL ( ) and Technical CNSL (
) and Technical CNSL ( ) in different concentrations, showing Rancimat cell conductivity (µs/cm) with test duration (h)
) in different concentrations, showing Rancimat cell conductivity (µs/cm) with test duration (h)

