Abstract
In this contribution, the influence of Pulsed Electric Fields (PEF) of different intensities (3–9 kV/cm and 0–300 μs) on the extraction of Total Phenols from lemon peel residues by pressing was investigated. According to the cellular disintegration index, the optimum treatment time for the increase in permeability was determined as 30 pulses of 30 μs. It was determined that the effect of PEF was independent of lemon residue size. The effectiveness of pressing-assisted extraction was evaluated by measuring the Total Phenol Content (TPC), the real antioxidant capacity and the concentrations of the main lemon polyphenols, the flavonones hesperidin and eriocitrin. The variables studied in the extraction were time, pressure applied and intensity of the electric fields. This study concludes that electric field intensity of 7 kV/cm increased the efficiency of polyphenol extraction by 300%, giving maximum values of 84 mg of hesperidin in 100 g FW and 176 mg of eriocitrin in 100 g FW. Thus, it was concluded that PEF provides a new methodology to improve polyphenol extraction with a non-thermal, environment-friendly technology, and this represents a method for increasing economic benefits of industrial processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The worldwide production of lemons in 2010 was 4,200,000 tons. Approximately half of that production was destined to be industrially processed, especially in the juice industry [1]. This industry thus generates a large amount of agro-food waste material, which is mostly used as a source of cattle feed, or as fertilizer. Current studies have found other uses for this abundant agro-food waste, for example using it as a source of biomass to produce bioethanol [2]. However, a most valuable option would be to obtain “low volume high price” products such as polyphenols [3].
Polyphenols are described as natural compounds with antioxidant activity due to their free radical scavenging activity [4]. They are widely studied as natural antioxidants with possible uses as additives in the food industry, or as substitutes for synthetic ones [1, 5]. The world market for polyphenols is significant. For example, Leatherhead Food Research (2009) estimated that the current market is worth approximately $200 million. The majority of polyphenols are extracted for sale as nutraceuticals or for use in functional foods [6].
Traditionally, polyphenol extraction was completed by solvent (solid–liquid) extraction commonly assisted by pressing. Widely used solvents in the extraction procedures were hexane, ether, chloroform, acetonitrile, benzene and ethanol. All of them, excluding ethanol, are toxic for humans and dangerous for the environment. Nowadays, other extraction systems that produce food-grade extracts with water or other Generally Recognized as Safe (GRAS) solvents are used [7]. Some of the most popular extraction methods are pressure liquid extraction, supercritical extraction [8], microwave-assisted extraction [9], ultrasonic-assisted extraction [10] and one of the latest innovative technology used for this purpose, namely Pulsed Electric Field-assisted extraction (PEF) [11].
PEF is an emerging technology based on the application of microsecond pulses of high-strength electric fields that induce a non-reversible electroporation in cell membranes [12, 13]. Key advantages of PEF-assisted extraction are that is a non-thermal treatment that does not affect the quality of the extracted products and the treatment might be able to be applied in continuous flow [14], in pilot plants or even at an industrial scale [15].
Based on the electroporation process of biological membranes, different applications of PEF for the food industry have been investigated. The area to which most efforts have been initially dedicated is inactivation [16] of microorganisms for enhancing food safety and stability, since it is a technique that doesn’t affect the nutritional and sensorial characteristics of foods [17]. Several studies have explored the positive effect of samples pre-treated with PEF in the improvement of industrial extraction processes such as juice production [18, 19], olive oil extraction [20] or in the improvement of the extraction of valuable compound in winery production [14, 21]. Other publications are focused on the extraction of phenolic compounds directly form food source [22] but, despite of these studies there are only a reduced number of publications focused on the use of PEF in re-valorization of agro-food waste to extract phenolic compounds [23,24,25].
The main aim of this contribution is to evaluate the potential benefits of the application of a PEF treatment on lemon waste with the purpose of obtaining an extract which is rich in polyphenols using an environment-friendly pressing liquid extraction.
Materials and Methods
Raw Materials
The lemon waste residues were obtained from fresh lemons (Citrus limon) from fresh crops grown in Murcia, Spain purchased at a local supermarket and stored at 4 °C until needed. The citrus pulp was removed from residues and only albedo and flavelo were used. Residues were chopped with a cork borer into pieces of 1, 2 and 3 cm size diameter.
PEF Treatment Conditions
The PEF equipment used (Modulator PG, Scandinova, Uppsala, Sweden) generates square waveform pulses of a width of 3 µs, with a frequency of up to 200 Hz. The maximum output used was 30 kV and the maximum current was 200 A. The equipment operation and constituents were described in detail in [24]. A parallel plate treatment chamber with an electrode gap of 2 cm and a diameter of 5 cm was used. The chamber works at non-pressurized conditions and at room temperature and allows treatment of 30 ± 0.5 g of lemon residues. The real voltage and current intensity applied in the treatment chamber were measured with a 100 MHz digital oscilloscope (TDS 3012, Tektronix, Oregon, USA). For signal conditioning a 75 MHz high voltage (P6015A, Tektronix, Wilsonville, OR) and a 100 MHz current probe (Stangenes Industries, Inc., Palo Alto, California, USA) connected to an amplifier system were used. PEF treatments were tested in a range of 0–100 pulses of 3 μs (0–300 μs of total treatment time) and were set also at electric field strengths ranging from 1 to 9 kV/cm. The specific energy of these treatments ranged from 0 to 7.6 kJ/kg. A pulse frequency of 1 Hz was used. All assays were achieved at room temperature conditions.
Cell Disintegration Index
Cell disintegration index (Zp) was used to identify the appropriate PEF treatment conditions for the PEF pre-treatment of the lemon peels before the extraction of polyphenols by pressing. This index characterizes the proportion of permeabilized cells based on the frequency dependence of conductivity of intact and permeabilized plant tissue [26]. The measuring cell was the same treatment chamber used for application of the PEF treatments with the electrodes separated at a distance of 2 cm. 30 ± 0.5 g of chopped lemon peels were introduced into the cell for Zp determination. Zp analysis was carried out using impedance measurement equipment (DIL, Quakenbrück, Germany) and calculated by the Eq. 1, previously described by [24].
where K1 and K1′ are the electrical conductivity of untreated and treated samples respectively, at a low frequency field and Kh and Kh′ are the electrical conductivities of untreated and treated material, respectively at a high-frequency field.
Polyphenol Pressing Assisted Extraction
A laboratory press designed and assembling in Zaragoza University laboratory with press chamber volume of 400 mL and 7.5 cm diameter piston activated by compressed air was used. Two pressure treatments were applied: 2.5 and 5 bars. Cycles of 5 min of pressure were applied. After each pressing cycle, samples of 1.5 mL were removed.
Determination of the Total Polyphenols Content (TPC)
TPC in each extract was quantified by means of the Folin–Ciocalteu method according to [27]. Samples were incubated for 2 h in darkness. The absorbance at 725 nm was read with a spectrophotometer (Unicam, Cambridge, UK) against blank containing bidistilled water. Results were calculated through a calibration curve and were expressed as mg of Gallic Acid Equivalents (GAE) in 100 g Fresh Weight (FW).
Determination of the Main Polyphenol by HPLC
The main polyphenols contained on lemon residues were characterized by HPLC. Alliance Waters equipment (Barcelona, Spain) with a C18 reverse phase column (Kinetex Phoenix, USA), diode-array detector (Waters, Barcelona, Spain) was used. The method described by [1] with modifications was applied. The eluents used, in a constant flow rate of 0.5 mL/min, were A: Acidified bidistillated water (0.1% of glacial acetic acid) and B: Acidified acetonitrile (0.1% of glacial acetic acid). Initial conditions were A: 90% and, then, a gradient starting at minute 2 and continuing for 10 min with a decrease to 70% A. Afterwards, a return to the initial conditions continued from minute 12 to minute 17.
Oxygen Radical Absorbance Capacity (ORAC)
The ORAC method was adapted from [28]. The assay was performed with an automated plate reader of 96-wells at 37 °C and the method was reported in detail in [5].
Kinetics of Total Phenol Extractions
The experimental data were fitted to the following equation commonly used to describe solid–liquid extraction of different intracellular compounds [29].
where Yt is the polyphenols extraction efficiency at time t (min), Ymax is the extraction efficiency at equilibrium (t = ∞), and k is the rate constant depending on the extraction parameters (min−1). k takes into account the diffusion coefficient of the extracted Phenols, the total surface area, the volume of solvent and the size and geometry of solid particles. Since the total surface area, the volume of solvent and the size and geometry of solid particles are constants, the k value easily enables determination of the influence of each treatment condition on the extraction efficiency.
Statistical Analysis
All the results reported are the average of at least two measurements found in two independent assays. To determine the Ymax and k-values from Eq. 2, the least-squares criterion by the Solver function of the Excel 5.0 package (Microsoft, Seattle, Washington, USA) and the GraphPad PRISM (GraphPad Software, Inc., San Diego, California, USA) was used. To describe the relationships between Ymax and k-values versus field strength and pressure, a multiple regression starting from a second-order polynomial model using the software Statgraphics Plus 5.1. (Statistical Graphics Corporation, USA). The response surface representation was determined with the Software Minitab version 16 (Minitab Inc., USA). Figures and graphics were drawn with Microsoft Excel (Microsoft Corporation, USA). A backward regression was used to determine the parameters of the model. The effect of associations which were not significantly associated (P > 0.05) with the response was automatically removed by this procedure.
Results and Discussion
Adjustment of PEF Conditions
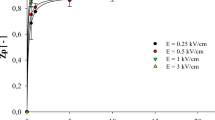
Zp was used to determine the appropriate PEF conditions to permeabilize the lemon peels. Figure 1 shows the influence of PEF treatment time and Zp at electric field strength of 3, 5, 7 and 9 kV/cm. The increase in electric field strength and treatment time resulted in an increase of the Zp values to a highest value of 0.55 for the most intense treatment conditions tested. Independently of the applied electric field strength, the Zp values increased significantly with treatment time (30 pulses of 3 µs). Above these values, an increment in the treatment time barely affected the Zp value. Results show two general trends of the influence of electric field strength and the treatment time on the Zp values observed. On one hand, in samples treated at 5 kV/cm or lower, nearly no cellular damage was caused (Zp < 0.2).In samples treated at 7 kV/cm or higher, Zp values around 0.5 were observed. No statistically significant differences between treatments of 7 and 9 kV/cm were detected.
Zp values observed are significantly higher than values obtained by [24] in orange peels at equal treatment time and 7 kV/cm, where Zp values reached over only 0.3. In contrast, in soft tissues such as potato slices [30], the values of Zp reached 0.57 under treatment with lower electric field strength (≤ 3 kV/cm) and could increase to 0.75 in very soft tissues such as tomato pulp waste [25].
After characterization of the cell damage, it is possible to fix the optimal PEF working conditions at 30 pulses of 3 µs with a maximum electric field of 7 kV/cm. The application of more pulses or a higher electric field does not produce significantly higher Zp values.
TPC extracted from lemon peels cut into different sizes of 1, 2 and 3 cm-diameter was evaluated. Samples were pre-treated with PEF for 30 pulses of 7 kV/cm, and then were subjected to a pressurized water solvent extraction (fixed at 5 bar) process for 30 min. Afterwards, TPC was analyzed and compared with samples not treated with PEF (control samples). Results at 45 min of extraction (Fig. 2) showed remarkable differences between control and PEF treated samples with control samples showing an effect of size (1 cm: 98.63 ± 5.32; 2 cm: 75.84 ± 2.5, 3 cm: 64.56 ± 10.33 mg GAE/100 g FW). This effect in control samples can be explained by differences in mass transfer caused by different size material [31, 32].
However, no statistically significant differences between samples cut into different sizes in PEF treated samples were detected (1 cm: 163.89 ± 4.38; 2 cm: 163.56 ± 3.91; 3 cm: 161.46 ± 2.82 mg GAE/100 g FW). Based on these results, the highest size tested, 3 cm-diameter materials were selected as optimal. This size show the lower values of Gallic Acid in control samples due to allows the detection of differences between treatments with maximum efficacy. In addition, these tests showed that in PEF-assisted extraction the different size materials have equally positive outcomes in polyphenol extraction suggesting that the use of PEF effectiveness is independent of lemon residue size could avoid the use of blending or cutting procedures in industrial processes of residues re-valorization.
Total Phenolic Content, Kinetic Analysis
To evaluate the effect of PEF in the extraction of polyphenols from lemon peels, residues cut at 3 cm-diameter were used, specimens were treated at 0, 2.5 and 5 bars of pressure with a PEF pre-treatment of 30 pulses of 0, 3.5 and 7 kV/cm. TPC was determined every 5 min during all extraction time period (45 min). With the aim to have a global vision of the electric fields effect applied on the extraction procedure, some of the findings obtained are plotted on Fig. 3. The effect of electric field is illustrate on Fig. 3a and the effect of the pressure on Fig. 3b. Total phenolic content was improved significantly by increasing the pressing time and electric field strength. For instance, after 45 min of extraction (the highest time applied) the TPC extracted from samples pre-treated with PEF 7 and 3.5 kV/cm (Fig. 3a) rose respectively to 292 and to 144% in comparison with control samples.
a Kinetic analysis of TPC obtained with pressing assisted extraction at 2.5 bars and with different PEF pretreatments: (filled square) no PEF pre-treated sample (control), (filled diamond) 3.5 kV/cm and (filled triangle) 7 kV/cm. b Kinetic analysis of TPC obtained with a pre-treatment of 3.5 kV/cm and different pressure assisted extractions. (filled square) no pressure extraction, (filled diamond) 2.5 bars (filled triangle) 5 bars
Furthermore, the extraction curves obtained, Fig. 3b, show an increment of TPC liberation with increase in the pressure applied. TPC obtained as a result of applying different pressure conditions (2.5 and 5 bar) showed an increase of 156 and 247%, respectively in extraction in comparison with samples extracted without pressing. The same tendency (not represented) was also observed with the other pulse electric field treatments tested.
These results show the increment of total polyphenol extraction when the pressure and energy applied for extraction are higher [24] found comparable results: the TPC increased with the intensity of the electric fields. In the literature report, polyphenols increased linearly and with independence of the pressing time. The positive outcomes obtained by the application of PEF before pressing extraction agree with the results obtained by [33], where polyphenol extraction from mushrooms increased by nearly 100%.
In order to analyze if the extraction model matches with the exponential extraction model reported in [29], (Eq. 2) Ymax and k-values were calculated with their corresponding confidence limit intervals (Table 1). In addition, the fitting to the equation was also calculated. In general, R2 obtained show a good adjustment to the equation in all conditions tested. The highest extraction values (Ymax) were produced in cases where stronger pulse electric fields were applied. These results agree with those of other works, such as [34, 35].
The accurate analysis of Ymax values obtained with different pressing conditions show that in samples without pressing treatment, there are no significant differences between Ymax values and samples treated with different pulse electric field strength (6.2, 8.5 and 9 in samples treated with 0, 3.5 and 7 kV/cm, respectively). This shows the necessity of using a combined treatment of PEF simultaneously with pressing extraction in order to maximize the effective extraction of bioactive compounds from lemon waste material.
At 2.5 bars of pressure a linear relationship between Ymax (obtained in the adjustment) and the intensity of the pulse electric field applied was observed. The highest values were obtained at 7 kV/cm. In samples treated at 5 bars of pressure the influence of electric field became non-linear, with 3.5 kV/cm, the extraction process at equilibrium (Ymax) produces the maximum value suggesting this treatment as the most effective.
Furthermore, an accurate analysis of the relationship of the electric field and k-values shows that there are no significant differences in any pressure treatments with PEF pre-treatments of 3.5 and 0 kV/cm. In control samples (0 bars) and in samples extracted with 2.5 bars, k-values showed significant differences from values obtained by pre-treatment with 7 kV/cm. These results entails that the application of 7 kV/cm significantly increases the extraction velocity.
Correlation Between TPC and Real Scavenging Activity
Oxygen Radical Absorbance Capacity (ORAC) allows the evaluation of the real scavenging activity of the extracts. The ORAC analysis can also be used as a tool to determine if there is any decay in the antioxidant activity of the sample due to the PEF treatment [36] suggested that some possible changes in molecules can occur when substances are under PEF treatment.
The results obtained suggest that no oxidant effect due to PEF was produced. Samples with higher phenol content rate showed higher antioxidant activity. The antioxidant activity of the samples followed a good correlation (Fig. 4) with ORAC (expressed as mg TE/100 g FW) and the TPC [37, 38] found similar results. Furthermore, the statistical analysis conducted showed that there are no statistically significant differences in any of the treatments tested for ORAC and phenol analysis.
In addition, the good correlation between TPC and ORAC is consistent with the hypothesis that most of the antioxidant compounds in the extracts are phenols; thus, the antioxidant effect produced by other compounds such as ascorbic acid or citric acid can be dismissed.
HPLC Analysis of the Main Polyphenols Contained in the Extracts
The previous analysis obtained determined that extracts obtained are a rich source of phenols, between all phenolic compounds; polyphenols have a special industrial interest. The main polyphenols contained in lemons are the flavonones eriocitrin and hesperidin [39]. Both flavonones were analyzed by HPLC in lemon waste extracts obtained from PEF pressing-assisted extraction (Table 2) and conditions required to obtain the maximum extraction were studied. The highest eriocitrin and hesperidin contents were found in samples extracted with the highest electric field energy applied, obtaining an increment of nearly 300% giving maximum experimental values of 84 mg of hesperidin in 100 g of FW and 176 mg of eriocitrin in 100 g of FW.
The experimental data obtained were analyzed by a multiple regression ANOVA (Table 3), resulting in a second order polynomial equation for each polyphenol analyzed Eq. 3 for Hesperidin and Eq. 4 for eriocitrin after removing statistically insignificant terms (P > 0.05).
where t is the extraction time, E is the electric field strength and P is the pressure applied.
In eriocitrin analysis, a coefficient of determination (R2) of 0.89 was obtained. Model significance was assessed by R2, the lack of fit (p > 0.05) and the F value (17.42) which indicate that the model was significant. Therefore, it can be used to predict the response. In hesperidin analysis, R2 obtained was 0.865 and the F value 23.73, which implied that the model is significant. There is only 0.01% chance that a Model F Value this large could occur due to noise. All values for p value were less than 0.005, p < 0.05. These results show that the terms of the model are significant. According to the F values for the model, pressing time and electric field strength were the most significant parameters.
In Fig. 5 the response surface obtained in the extraction of eriocitrin and hesperidin at the maximum pressing extraction time (45 min) is graphically represented. The extraction is shown with respect to the electric field and pressure applied. Results showed an increasing concentration of both polyphenolic compounds in relation to the pressure and electric field. These results show a different behavior from that of total phenol extraction (Fig. 3). The total polyphenol extraction rate at 5 bars of pressure did not increase when the electric strength increased from 5 to 7 kV/cm. In addition, with the maximum electric field applied, 7 kV/cm, TPC had similar values at 2.5 and 5 bars of pressure. However, in the case of the concentration of these two target compounds, the concentration extracted increased with the electric field and pressure. These results suggest that the low increment in concentration of these two compounds did not have a significant impact on the increment of the antioxidant capacity of the extracts.
Conclusions
From the conducted experiments it can be concluded that according with cellular disintegration index (Zp), and different extractions assessed with residues chopped at different size, the adequate PEF treatment time is defined in 90 µs with independence of residue size. Results achieved with different PEF and pressing treatments (3.5–7 kV/cm and 0–5 bars) determine that the TPC improves with a combined treatment of pressing and PEF at 7 kV/cm. The extraction kinetic analysis concludes that despite of maximum k-values and Ymax values are obtained at 7 kV/cm, the most efficient extraction is determined at 5 bars and 3.5 kV/cm. In addition it is demonstrated that in all extracts obtained there are a high correlation between TPC and ORAC. The analysis of the main polyphenols contained in lemon residues, hesperidin and eriocitrin reveals a an increasing concentration of both polyphenolic compounds in relation to the pressure and electric field obtaining an increment of nearly 300% giving maximum experimental values of 84 mg of hesperidin in 100 g of FW and 176 mg of eriocitrin in 100 g of FW.
References
González-Molina, E., Domínguez-Perles, R., Moreno, D., García-Viguera, C.: Natural bioactive compounds of Citrus limon for food and health. J. Pharm. Biomed. Anal. 51, 327–345 (2010)
Boluda-Aguilar, M., López-Gómez, A.: Production of bioethanol by fermentation of lemon (Citrus limon L.) peel wastes pretreated with steam explosion. Ind. Crops Prod. 41, 188–197 (2013)
Mahro, B., Timm, M.: Potential of biowaste from the food industry as a biomass resource. Eng. Life Sci. 7, 457–468 (2007)
Alamed, J., Chaiyasit, W., McClements, D.J., Decker, E.: Relationships between free radical scavenging and antioxidant activity in foods. J. Agric. Food Chem. 57, 2969–2976 (2009)
Skowyra, M., Falguera, V., Gallego, G., Peiró, S., Almajano, M.P.: Antioxidant properties of aqueous and ethanolic extracts of tara (Caesalpinia spinosa) pods in vitro and in model food emulsions. J. Sci. Food Agric. 94, 911–918 (2014)
Wijngaard, H., Hossain, M.B., Rai, D.K., Brunton, N.: Techniques to extract bioactive compounds from food by-products of plant origin. Food Res. Int. 46, 505–513 (2012)
Gil-Chávez, J., Villa, J., Ferando Ayala-Zavala, J., Basilio Heredia, J., Sepulveda, D., Yahia, E.M., González-Aguilar, G.: Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: An overview. Compr. Rev. Food Sci. Food Saf. 12, 5–23 (2013)
Plaza, M., Amigo-Benavent, M., del Castillo, M.D., Ibáñez, E., Herrero, M.: Neoformation of antioxidants in glycation model systems treated under subcritical water extraction conditions. Food Res. Int. 43, 1123–1129 (2010)
Al Bittar, S., Périno-Issartier, S., Dangles, O., Chemat, F.: An innovative grape juice enriched in polyphenols by microwave-assisted extraction. Food Chem. 141, 3268–3272 (2013)
Şahin, S., Şamlı, R.: Optimization of olive leaf extract obtained by ultrasound-assisted extraction with response surface methodology. Ultrason. Sonochem. 20, 595–602 (2013)
Zderic, A., Zondervan, E., Meuldijk, J.: Breakage of cellular tissue by pulsed electric field: extraction of polyphenols from fresh tea leaves. Chem. Eng. Trans. 32, 1795–1800 (2013)
Luengo, E., Martínez, J.M., Coustets, M., Álvarez, I., Teissié, J., Rols, M.-P., Raso, J.: A comparative study on the effects of millisecond- and microsecond-pulsed electric field treatments on the permeabilization and extraction of pigments from Chlorella vulgaris. J. Membr. Biol. 248, 883–891 (2015)
Puértolas, E., Cregenzán, O., Luengo, E., Álvarez, I., Raso, J.: Pulsed-electric-field-assisted extraction of anthocyanins from purple-fleshed potato. Food Chem. 136, 1330–1336 (2013)
Luengo, E., Franco, E., Ballesteros, F., Álvarez, I., Raso, J.: Winery trial on application of pulsed electric fields for improving vinification of Garnacha Grapes. Food Bioprocess Technol. 7, 1457–1464 (2014)
Puértolas, E., Saldaña, G., Álvarez, I., Raso, J.: Effect of pulsed electric field processing of red grapes on wine chromatic and phenolic characteristics during aging in oak barrels. J. Agric. Food Chem. 58, 2351–2357 (2010)
Puértolas, E., López, N., Condón, S., Raso, J., Alvarez, I.: Pulsed electric fields inactivation of wine spoilage yeast and bacteria. Int. J. Food Microbiol. 130, 49–55 (2009)
Monfort, S., Saldaña, G., Condón, S., Raso, J., Álvarez, I.: Inactivation of Salmonella spp. in liquid whole egg using pulsed electric fields, heat, and additives. Food Microbiol. 30, 393–399 (2012)
Jaeger, H., Schulz, M., Lu, P., Knorr, D.: Adjustment of milling, mash electroporation and pressing for the development of a PEF assisted juice production in industrial scale. Innov. Food Sci. Emerg. Technol. 14, 46–60 (2012)
Moussa-Ayoub, T.E., Jaeger, H., Youssef, K., Knorr, D., El-Samahy, S., Kroh, L.W., Rohn, S.: Technological characteristics and selected bioactive compounds of Opuntia dillenii cactus fruit juice following the impact of pulsed electric field pre-treatment. Food Chem. 210, 249–261 (2016)
Abenoza, M., Benito, M., Saldaña, G., Álvarez, I., Raso, J., Sánchez-Gimeno, C.: Effects of pulsed electric field on yield extraction and quality of olive oil. Food Bioprocess Technol. 6, 1367–1373 (2012)
Saldaña, G., Cebrián, G., Abenoza, M., Sánchez-Gimeno, C., Álvarez, I., Raso, J.: Assessing the efficacy of PEF treatments for improving polyphenol extraction during red wine vinifications. Innov. Food Sci. Emerg. Technol. 39, 179–187 (2017)
Segovia, F.J., Luengo, E., Corral-Pérez, J.J., Raso, J., Almajano, M.P.: Improvements in the aqueous extraction of polyphenols from borage (Borago officinalis L.) leaves by pulsed electric fields: pulsed electric fields (PEF) applications. Ind. Crops Prod. 65, 390–396 (2015)
Barba, F.J., Zhu, Z., Koubaa, M., Sant’Ana, A.S., Orlien, V.: Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: a review. Trends Food Sci. Technol. 49, 96–109 (2016)
Luengo, E., Álvarez, I., Raso, J.: Improving the pressing extraction of polyphenols of orange peel by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 17, 79–84 (2013)
Luengo, E., Álvarez, I., Raso, J.: Improving carotenoid extraction from tomato waste by pulsed electric fields. Front. Nutr. 1, 12 (2014)
Ade-Omowaye, B.I.O., Angersbach, A., Taiwo, K.A., Knorr, D.: Use of pulsed electric field pre-treatment to improve dehydration characteristics of plant based foods. Trends Food Sci. Technol. 12, 285–295 (2001)
Singleton, V.L., Orthofer, R., Lamuela-Raventos, R.M.: Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 299, 152–178 (1999)
Ou, B., Hampsch-Woodill, M., Prior, R.L.: Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 49, 4619–4626 (2001)
López, N., Puértolas, E., Condón, S., Raso, J., Álvarez, I.: Enhancement of the solid-liquid extraction of sucrose from sugar beet (Beta vulgaris) by pulsed electric fields. LWT—Food Sci. Technol. 42, 1674–1680 (2009)
Ersus, S., Barrett, D.M.: Determination of membrane integrity in onion tissues treated by pulsed electric fields: use of microscopic images and ion leakage measurements. Innov. Food Sci. Emerg. Technol. 11, 598–603 (2010)
Janositz, A., Noack, A.-K., Knorr, D.: Pulsed electric fields and their impact on the diffusion characteristics of potato slices. LWT—Food Sci. Technol. 44, 1939–1945 (2011)
Puértolas, E., Saldaña, G., Condón, S., Álvarez, I., Raso, J.: Evolution of polyphenolic compounds in red wine from Cabernet Sauvignon grapes processed by pulsed electric fields during aging in bottle. Food Chem. 119, 1063–1070 (2010)
Parniakov, O., Lebovka, N.I., Van Hecke, E., Vorobiev, E.: Pulsed electric field assisted pressure extraction and solvent extraction from mushroom (Agaricus Bisporus). Food Bioprocess Technol. 1–10 (2013)
López, N., Puértolas, E., Hernández-Orte, P., Álvarez, I., Raso, J.: Effect of a pulsed electric field treatment on the anthocyanins composition and other quality parameters of Cabernet Sauvignon freshly fermented model wines obtained after different maceration times. LWT—Food Sci. Technol. 42, 1225–1231 (2009)
Spigno, G., Tramelli, L., De Faveri, D.M.: Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 81, 200–208 (2007)
Zhang, Y., Sun, J., Hu, X., Liao, X.: Spectral alteration and degradation of cyanidin-3-glucoside exposed to pulsed electric field. J. Agric. Food Chem. 58, 3524–3531 (2010)
García-Salas, P., Gómez-Caravaca, A.M., Arráez-Román, D., Segura-Carretero, A., Guerra-Hernández, E., García-Villanova, B., Fernández-Gutiérrez, A.: Influence of technological processes on phenolic compounds, organic acids, furanic derivatives, and antioxidant activity of whole-lemon powder. Food Chem. 141, 869–878 (2013)
Rusaczonek, A., Swiderski, F., Waszkiewicz-Robak, B.: Antioxidant properties of tea and herbal infusions—a short report. Polish J. Food Nutr. Sci. 60, 33–35 (2010)
Gironés-Vilaplana, A., Mena, P., García-Viguera, C., Moreno, D.: A novel beverage rich in antioxidant phenolics: maqui berry (Aristotelia chilensis) and lemon juice. LWT—Food Sci. Technol. 47, 279–286 (2012)
Acknowledgements
The author S. Peiró gratefully acknowledge the financial support of “Talent Empresa (TEM-DG) 2010” Program from AGAUR, Generalitat de Catalunya. The authors would like to thank Michael H. Gordon for his valuable revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peiró, S., Luengo, E., Segovia, F. et al. Improving Polyphenol Extraction from Lemon Residues by Pulsed Electric Fields. Waste Biomass Valor 10, 889–897 (2019). https://doi.org/10.1007/s12649-017-0116-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-017-0116-6