Abstract
Bottom ash from municipal solid waste incineration is an underutilized secondary resource, which currently gains large attention due to increased landfill costs and the push towards a circular economy. Due to the high concentrations and mobility of pollutants, bottom ash cannot readily replace virgin construction materials. Over the last decade, many research efforts have addressed these issues in view of newly developed engineering applications. However, the required quality of bottom ash varies for each application. In this review we focus on the ternary relationship between engineering applications, chemical barriers/limitations and treatment technologies for municipal solid waste incinerator bottom ash. For each intended engineering application [loose (bulk) construction aggregates; sand, aggregate or cement replacement in concrete; raw material for cement or ceramics] the appropriate treatment technologies are selected to overcome identified chemical barriers. This allows future top-down design decisions, starting from the most promising engineering application of bottom ash. The main chemical barrier for bottom ash recycling as loose construction aggregates is the leaching of heavy metals and/or metalloids. This can be overcome by size separation, carbonation, mild heat treatment or by using mineral additives. In structured concrete, the presence of metallic aluminum or zinc causes early cracking and a high chloride concentration causes corrosion of reinforcement steel. Therefore, recent developments in wet/semi-dry separations facilitated enhanced eddy current separation to remove non-ferrous metals. The washing of bottom ash to remove chloride, is to date the sole technology to prepare bottom ash as raw material for cement kilns. Finally, when bottom ash is used as feedstock for ceramics production, recent knowledge was generated to allow for selecting thermal process parameters in such a way that leaching of both heavy metals and metalloids is minimized.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In Europe to date 455 waste-to-energy (WtE) plants are in operation mainly for municipal solid waste incineration (MSWI) [1]. In the EU 28, about 239 Mt of MSW was produced in 2014, of which 64 Mt is incinerated [2]. Upon incineration, one tonne of MSW yields about 250 kg of bottom ash, so in total 12–15 Mt/year of MSWI bottom ash is produced in the EU 28. Table 1 shows the average concentrations of the main matrix elements of bottom ash.
In view of creating a circular economy in Europe, resource recovery from, and recycling of MSWI bottom ash constitute an important challenge, and are a prerequisite for the survival of MSWI in competition with emerging technologies. In a life cycle environmental impact and cost perspective, incentives for recycling of bottom ash are:
-
Conservation of natural resources e.g. ferrous and non-ferrous metals and minerals, such as sand, gravel, limestone needed for cement production, and avoidance of the environmental impact of their production from virgin raw materials.
-
Economic benefits: landfill costs, taxes, and costs for mining or quarrying of virgin raw materials are avoided.
-
Avoiding new landfills, and reducing the environmental impact of bottom ash.
The growing interest in this topic is demonstrated by changes in legislation [4], and by organization of workshops and special sessions at conferences [5].
Significant progress made in the field of bottom ash recycling in the last decade justifies a critical review of the literature, relating potential engineering applications with novel treatment technologies and the chemical barriers the latter aim to overcome. Vice versa, this paper also presents which treatment technologies are best suited to overcome the chemical barrier(s) e.g. excessive leaching of one or multiple elements with respect to an intended application. Regarding the prospect engineering applications, this paper mainly focuses on the use of the mineral fractions, representing the major bottom ash fraction, in construction applications. To a lesser extent, the recovery of (non-oxidized) metals from bottom ash, probably the major economic incentive, is also addressed.
Engineering Applications and Main Barriers
MSWI bottom ash can fairly easily be separated in fractions in view of recycling (see “Size separation” section). After separation, it can be recycled in roughly four types of engineering applications: (1) as loose construction aggregates; (2) as replacement for sand, gravel or cement in construction material; (3) as raw material in cement production; (4) as feedstock for ceramic materials production. The desired properties of bottom ash are specific for each application type: for some, leaching of heavy metals or chlorides is the major limitation (barrier), for others, the presence of non-oxidized metals has a negative influence on the obtained product quality. Besides these four recycling options, bottom ash can also be used on landfills e.g. as landfill cover or for the construction of roads on the landfill site. This application will not be discussed further in this paper: it does not require an upgrade of the bottom ash quality, and the disposal of waste on landfills will decrease in the future in Europe, significantly reducing the demand.
Use in or as Construction Material
Loose Construction Aggregates
Some bottom ash fractions can be used directly, i.e. without significant treatment, as loose construction aggregates. Until some years ago, the main application was the use as loose aggregates for e.g. base layers for road construction, the construction of large sound and noise barriers, embankments or the construction of artificial slopes. Many laboratory investigations analyzed the mobility of pollutants with respect to the related environmental regulations, and aimed at complying with them [6–11]. In general, the mobility and leaching of the heavy metals Cu, Pb, Zn and Ni, and of the metalloids Sb, Cr and Mo is the most problematic, and in most cases protective measures such as liners are required to prevent leaching of these metals and metalloids from the bottom ash into the surrounding soil.
The performance of bottom ash (fractions) as bulk/loose construction material was evaluated by constructing large scale test sites. Hjelmar et al. [6] constructed six such test sites with bottom ash as sub-layer for road construction, covered with asphalt or pebbles. The test sites were subjected to ambient weather conditions for more than 2 years and the leachate compositions were monitored. The results showed that the leaching did not comply with the local (Danish) legislation, according to which the use is permitted, yet restricted by a maximal thickness of the applied layer, and by requirements concerning infiltration-reducing properties of the top cover.
De Windt et al. [7] and Dabo et al. [8] monitored the 10-year leaching from pilot-scale roads constructed with MSWI bottom ash as base layer and focused on the leaching of major polluting elements. The leaching of these elements from the bottom ash dropped quickly over the first 2 years, and asymptotically reached minimum values after about 10 years. The latter were comparable to those of a reference road built with natural calcareous aggregates. A similar experiment was performed by Izquierdo et al. [9], using MSWI bottom ash as granular base layer for a pavement made with limestone aggregates. Trace pollutants like Pb, Zn and Cd complied with the regulatory limit values, whereas Cu and some oxyanion forming elements (mainly Mo and Sb) exceeded these values and therefore constitute a threat to the environment.
Birgisdottir et al. [10] assessed the life cycle environmental impact of roads constructed with and without MSWI bottom ash, the bottom ash being used as a sub-base layer to replace gravel beneath the lanes. The results indicated that the environmental impact of both scenarios was comparable, mainly because the burden of the bottom ash leaching in road construction is evened out by the environmental benefit of offsetting its landfilling and associated impact. Although using bottom ash can affect groundwater quality, the pollution potential of spreading de-icing salt is an order-of-magnitude higher. Another environmental assessment by Toller et al. [11] also showed that MSWI bottom ash is suitable for replacing natural resources in base layers for road construction or as drainage material in landfills. The most important differences with the reference (‘business as usual’) scenario were reduced use of natural resources and energy, but increased leaching of heavy metals when using bottom ash.
The aforementioned studies show that the use of MSWI bottom ash as loose construction aggregates is technically feasible. Life cycle environmental impact assessments showed that, although landfill impacts were avoided, increased leaching of heavy metals when using bottom ash causes a higher direct toxicity impact than the reference scenario. This was confirmed by field studies, in which elevated leaching of heavy metals and/or chlorides and sulfates were observed. Therefore, protective measures to prevent water from reaching the bottom ash layer and/or to collect and treat leachates are required. Furthermore, aftercare of such construction sites is required, so that they would in fact resemble controlled landfills.
In March 2012, the Dutch Waste Management Association (‘Vereniging Afvalbedrijven’), representing the operators of WTE plants in the Netherlands, together with the government signed the ‘Green Deal on Sustainable Recovery of WTE Bottom Ash’ (‘Green Deal Verduurzaming Nuttige Toepassing AEC-Bodemas’) [4]. With this agreement, the WTE plant operators commit themselves to improve the quality of the bottom ashes and extend the application opportunities in which they are used. The main parts of the agreement are:
-
(a)
By January 1, 2017, 50 % of the bottom ash should be applied in applications without restrictions or protective measures. By January 1, 2020, no more bottom ash can be recycled in restricted/protected applications. This means that the current IBC technology (‘Isoleren, Controleren en Beheersen’—‘Isolate, Control and Monitor’), for which complicated construction, extensive aftercare and monitoring are needed, comes to an end. This also implies that a quality improvement of the bottom ash should be obtained, as one of the other articles of the agreement states that landfilling of bottom ash should be limited to 15 % of the total mass.
-
(b)
The percentage of non-ferrous metals to be recovered from the bottom ash fraction >6 mm should be increased to >75 % by January 1, 2017. A goal for the fraction <6 mm is not yet been set.
For the Netherlands, this Green Deal implies that after January 1, 2020, bottom ash can no longer be applied as loose construction aggregates as currently done in many countries i.e. with extensive measures to control and monitor run-offs from the construction site. This Green Deal is an incentive to improve bottom ash quality and to find new and better technologies and applications for bottom ash recycling. It is unclear whether other countries will follow the example of the Netherlands, but it is clear that this is a step forward to more sustainable application of bottom ash. In other countries, e.g. Belgium, such regulations are not (yet) in place.
Use as Sand/Gravel Replacement in Structured Materials
Bottom ash can also be used in structured materials, e.g. as a replacement for natural aggregates in concrete. The two most important conditions to be met by the final product are sufficient compressive strength, depending of the application, and leaching of heavy metals below the environmental limit values. Meyer [12] was among the first to show the detrimental effect of metallic species (e.g. non-oxidized aluminum or zinc) present in MSWI BA on the compressive strength of concrete due to the formation of pop-outs. These pop-outs occur when metallic species react with alkali salts present in the cement to form aluminum (hydr)oxides, ettringite, hydrogen gas and hydrocalumite. Near the concrete surface, the expansion caused by the formation of these compounds may rupture the surface [13]. Pera et al. [14] attempted to tackle this problem, by suggesting to produce concrete with pretreated bottom ash as aggregates. They used the 4–20 mm fraction of MSWI bottom ash to produce 2 types of concrete with 50 and 100 % replacement of the natural gravel, resp. When bottom ash was directly introduced in the concrete, swelling and cracking of the concrete species was observed due to the formation of hydrogen gas after oxidation of the metallic aluminum present in the samples, confirming the findings of Meyer [12]. The bottom ash was therefore, before use, immersed in a sodium hydroxide solution for 15 days to oxidize all of the metallic aluminum present. Concrete with this pretreated bottom ash as aggregates did no longer show the same swelling and cracking. Nevertheless, regardless of this pretreatment, the 28-day compressive strength decreased with the percentage of bottom ash, and both the 50 and 100 % gravel replacement had lower compressive strengths than concrete without bottom ash, but in both cases the required 28-day compressive strength of 25 MPa was reached. Since these first trials, many other researchers have investigated the possibilities to use bottom ash in concrete.
Keppert et al. [15] produced concrete replacing 10 % of the used sand by a 0–4 mm fraction of MSWI bottom ash. This had no significant effect on the short term strength development (after 28 days), but the longer-time behavior (90 days) differed from the reference mixture. This was probably due to the presence of metallic aluminum causing the formation of hydrogen bubbles, although this was not visually observed. A similar negative effect on the concrete strength was observed by Muller and Rübner [16], who also attributed this to the reaction of aluminum with the cement paste to form hydrogen gas. Besides this reaction, also an alkali-silica reaction (ASR) was observed between glass compounds in the bottom ash and the alkaline cement paste. This leads to the formation of a calcium-silicate-hydrate (CSH) gel that swells in contact with water and causes expansion and cracks. The damage related to ASR was however less severe than that due to hydrogen gas formation. Other studies also acknowledged the detrimental effect of metallic aluminum and other metals on the compressive strength of concrete. Nielsen et al. [13] established a maximum content of metallic aluminum of 1 % in the 2–6 mm bottom ash fraction as criterion to avoid a detrimental effect on the compressive strength of the concrete. Complying with that criterion, no pop-outs were observed after 2.5 years of testing.
Saikia et al. [17] observed cracks in concrete mortars made with the sand fraction (0.1–2 mm) of MSWI bottom ash, but were one of the few to test the leaching behavior of the concrete produced with bottom ash. The loose bottom ash sand fraction as such showed leaching concentrations for Cd, Cr, Cu, Mo and Sb above the limit values for recycling of waste as construction material. When incorporated in the cement mortar, leaching of most elements was reduced due to various chemical processes: formation of calcium metallates, metal hydroxides or incorporation in hydration products. Only the concentrations of Cu and Pb exceeded the limit values; this was attributed to the dissolution of these amphoteric elements at the high pH of the mortar, which was 12.5, compared to 10.5 for bottom ash.
The aforementioned studies [12–17] show that the main barrier to use MSWI bottom ash in structured materials as replacement for sand and/or gravel is the presence of metallic species like aluminum and zinc. These species are easily oxidized in alkaline environments, leading to the formation of hydrogen gas and expansive minerals, causing cracks and pop-outs in the concrete structure reducing its compressive strength. Reducing the amount of metallic Al and Zn in the bottom ash will constitute the main challenge in order to increase the amount of bottom ash that can be used as sand/gravel replacement, and will also bring along economic benefits. The leaching of several toxic elements is drastically reduced by incorporating bottom ash in structured materials, although for some elements leachate concentrations are detected that are above the regulatory limit value.
Use as Cement Replacement in Structured Materials
Bottom ash can not only be used as aggregate in concrete, it can also (partially) replace cement. By partially replacing cement, CO2 and other pollutant emissions that would otherwise be generated in the cement production process, are avoided. Due to their high amounts of mainly (amorphous) silica and to a lesser extent calcium oxide (Table 1), bottom ash can have pozzolanic or cementitious properties [18–20], but, just like cement, the bottom ash has to be finely ground to have a high specific surface area, and thus a high reactivity.
Many researchers performed studies on replacing ordinary portland cement (OPC) by bottom ash. Aiming at highly reactive materials, the bottom ash is always milled to increase its specific surface area. Whittaker et al. [21] produced concrete in which 10 and 40 % of OPC was replaced by MSWI bottom ash. Replacing 10 % of OPC did not influence the structural properties of the concrete, but 40 % was detrimental to concrete strength. Krammart and Tangtermsirikul [22] replaced 5 and 10 % of cement in their concrete by bottom ash. Again, the compressive strength decreased with higher bottom ash percentages, which was attributed to the lower amount of CaO/Ca(OH)2 in the bottom ash, resulting in the formation of less tricalcium silicate in the concrete and thus in a lower compressive strength. Li et al. [23] replaced between 10 and 50 % OPC in blended cement by MSWI bottom ash by. The mechanical properties of the blended cement prepared with MSWI bottom ash gradually decreased with increasing amounts of bottom ash and it was advised to limit the bottom ash content to 30 %. The heavy metals leaching from the mortars complied with the relevant (Chinese) legislation, but it must be noted that the leachate concentrations would be above the European limit values. Collepardi et al. [20] performed tests on the pozzolanic activity of ground bottom ash: they prepared concrete samples in which they replaced 20 % (OPC) by ground bottom ash (mean particle size of 1.7, 3 or 5 µm), or other pozzolanic materials i.e. fumed silica or coal fly ash. The finest ground bottom ash performed as good as fumed silica on the tested parameters, which were compressive strength, water permeability, chloride diffusion and CO2 penetration, and was comparable to or better than the control mixture with only Portland cement. The leaching of contaminants from the concrete matrix was below the European limit values.
Because the aforementioned studies showed that ground bottom ash acts as fine aggregates, rather than as a cementitious material, attempts were made to improve the compressive strengths of mortars produced with MSWI bottom ash through physical or chemical activation. Physical activation relies on intensive milling to increase the surface area available for reaction [24]. Chemical activation is based on the addition of chemical agents capable of breaking down the structure of alumino-silicate minerals, releasing silicate and aluminate ions, which can thereafter be transformed into mechanically resistant phases. Onori et al. [24] tried to activate bottom ash chemically by treating it with NaOH, KOH, CaCl2 or CaSO4. CaCl2 and to some extent CaSO4 showed the most positive effect on the development of the mechanical properties of the blended mortars. Mixtures with 20 % of bottom ash with CaCl2 activated OPC showed a higher compressive strength than the control mixture (containing no bottom ash); the compressive strength with 40 % replacement was only slightly below that of the control mixture. CaCl2 is known to promote the onset of pozzolanic reactions in cement mortars. The dissolved Ca can react with the amorphous, reactive silicates in the bottom ash to form the hydrated phases that are responsible for the compressive strength.
Bertolini et al. [19] replaced 30 % of OPC by ground bottom ash. They also compared dry and wet ground bottom ash, and observed higher compressive strengths for wet ground bottom ash than for dry ground bottom ash. This was due to hydrogen bubbles formed in the concrete made with dry ground bottom ash, due to the aforementioned oxidation of metallic aluminum (and other non-ferrous metals) at alkaline pH. Hydrogen gas formation initiates during wet grinding of bottom ash, and is completed to a large extent before the wet bottom ash is introduced to the concrete mix. Nonetheless, a large variability was observed with respect to the time required to terminate the hydrogen gas formation, and thus to use the ground bottom ash appropriately as cement replacement.
When MSWI bottom ash is used as cement replacement in structured materials, the same problem arises as when it is used as sand/gravel replacement: the presence of metallic species causes formation of hydrogen gas, which leads to pop-outs and cracks in the structure. Furthermore, the replacement of cement by bottom ash often leads to materials with a lower compressive strength. This is attributed to the lower reactivity of the bottom ash due to a lower specific surface area and/or due to the lower concentration of hydrating minerals. The main challenges for using bottom ash as cement replacement are thus: (1) limiting the amount of metallic species and (2) activating the material for an increased formation of hydrated species. The low activity of bottom ash had already been described extensively in literature and solutions like physical and chemical activation are well-established. Therefore, the main focus of the treatment technologies described in “Treatment technologies” section will be on the removal of metallic species. In practice, MSWI bottom ash is only sporadically used in reinforced concrete because the presence of high chloride concentrations in the bottom ash increases steel corrosion.
Use as Raw Material in Cement Production
The high concentrations of minerals of Si, Ca and Al (Table 1) make bottom ash suitable for replacing raw materials in the production of Portland cement clinker. CaO-bearing materials, like MSWI bottom ash, can reduce CO2 emissions from typical OPC production by reducing the use of limestone [25]. Indeed, the heating of limestone releases CO2 in two ways: directly by converting CaCO3 into CaO and CO2, and indirectly by the CO2 emitted by the combustion of fossil fuel to heat the limestone. Typically, around 800 kg CO2 eq. is emitted during the production of 1 tonne of cement [26].
The mixture that is used for clinker production needs to fulfill several requirements e.g. the ratio of silica to alumina and iron(III) oxide (silica ratio), the ratio of alumina to iron(III) oxide (alumina ratio), lime saturation, to meet the standards of the cement industry. Lam et al. [25] mixed the components that usually make up the clinker mixture i.e. limestone, sand, copper slag and pulverized-fuel ash with of bottom ash. They used ash percentages between 2 and 8 %, and adapted the amounts of the other components accordingly to meet the industry standards. X-ray fluorescence (XRF) and diffraction (XRD) showed that the phase compositions with percentages up to 6 % of the bottom were comparable with those of OPC. The leaching behavior was also tested; all clinkers incorporated with bottom ash complied with the regulatory limits due to stabilization of the toxic elements in the clinker matrix.
In similar experiments, Pan et al. [27] produced OPC with and without bottom ash. Due to the chloride content in the bottom ash, the added percentage had to be limited to 3.5 %, as the chloride concentration of the raw clinker mixture should not exceed 100 ppm on a mass base. The presence of chlorides during the high temperature clinker manufacturing process can cause severe corrosion in the cement kiln. XRF analysis showed that the chemical composition of the clinker produced with bottom ash was identical to that of the clinker produced without ash. However, the addition of bottom ash lengthened the setting time by approximately 5–15 %. The compressive strengths of all concrete samples produced with bottom ash were greater than the standard required values for Portland cement (a compressive strength higher than 281 kg/cm2 after 28 days of curing) and did not differ significantly from the samples produced without ash.
The aforementioned studies [25, 27] show that bottom ash can serve as a suitable replacement for a part of the raw materials in cement production. The main limitation for using large amounts of MSWI bottom ash in cement kilns is the presence of chlorides that can cause severe corrosion of the cement kiln and reduce the compressive strength of concrete prepared with this cement. However, using even low amounts of MSWI bottom ash (e.g. 5 %) to replace raw materials in cement production can be sufficient to reuse all of the bottom ash produced.
Ceramics
MSWI bottom ash can be used to produce ceramic materials that, in their turn, can be used for e.g. building applications. Cheeseman et al. [28] produced lightweight aggregates by ‘rapid sintering’ of MSWI bottom ash. Sintering at temperatures between 1000 and 1050 °C provided aggregates with good technical characteristics such as density, water absorption, compressive strength. The leaching of toxic elements from the sintered products was low, although the leaching of Cr, Zn and Cd was slightly higher than from the untreated bottom ash. The leaching of these elements could be further reduced by sintering at even higher temperatures (up to 1100 °C), but this also changed other product properties, like the density and water absorption of the aggregates [29]. Bourtsalas et al. [30] also produced ceramics from the fine (<4 mm) fraction of MSWI bottom ash by calcining it at temperatures between 600 and 1100 °C. Calcining at 1080 °C provided the best structural properties, and the pH dependent leaching of ceramics produced at this temperature was compared with the pH dependent leaching of untreated bottom ash. In the entire pH region tested (pH 1–11), leaching of Cu, Pb and Zn was lower for the calcined samples than for the untreated samples, due to incorporation and encapsulation of the metals into newly formed glassy and crystalline phases, like diopside, clinoenstatite and andradite.
Other researchers have produced ceramics made from MSWI bottom ash by vitrifying the ash at temperatures between 1100 and 1400 °C [31–33]. The obtained products are hard, dense and amorphous, embedding the toxic elements in the amorphous matrix, so that leaching of these elements is reduced. Ceramic materials like tiles and stoneware are subject to some aesthetic requirements, and it is uncertain whether these prerequisites can be met by using bottom ash alone. Therefore, instead of producing ceramics from bottom ash alone, only part of the raw material (5–10 %) used to make ceramic materials (natural clays) may be replaced with bottom ash, as done by [32]. This did not influence the technical properties of the produced ceramics, and slightly lowered the required firing temperature.
Attempts were also made to use vitrified bottom ash as replacement for filler, sand or aggregate in concrete mixtures [34, 35]. The conclusions were that vitrified bottom ash ground to the appropriate sizes can replace up to 20 % of filler material and up to 75 % of gravel in concrete products. Replacement of sand was not possible, as the strength of the end product was negatively influenced. Although the use of vitrified bottom ash can save large amounts of natural materials, and reduces the landfilling of MSWI bottom ash, it can be questioned whether the advantages outweigh the cost and the environmental impact of the energy intensive vitrification process.
From an economic and environmental point of view, the most advantageous way to recycle bottom ash in ceramics appears to only use the smallest sizes of MSWI bottom ash for this purpose, as this fraction usually presents most problems with regard to leaching of toxic elements. The best option seems to use the small size fractions of bottom ash to partially replace natural raw materials in the production of ceramics, as technical and aesthetical properties are more easily met than with bottom ash alone.
Overview of the Main Limitations
From the overview of the current MSWI bottom ash application areas given in “Use in or as construction material to Ceramics” section, it appeared that each of them has its specific limitations. For using bottom ash as loose construction aggregates, the main limitation is the leaching of heavy metals, so that leachate collection is required. Regarding emerging new regulations on bottom ash recycling, this procedure might no longer be acceptable, and improvement of the bottom ash quality is necessary. When using bottom ash as sand/gravel or cement replacement in structured materials, the main problem is the presence of metallic aluminum and/or zinc. These metallic species form hydrogen gas from water upon oxidation in alkaline environments, which can be detrimental to the compressive strength of the produced concrete. As cement replacement, untreated bottom ash often shows too low hydraulic activity.
MSWI bottom ash can be used to replace raw materials in the cement production, the main barrier for this application being the presence of chlorides, which may cause enhanced corrosion in the cement kilns and are detrimental for cement quality. The major barrier for using bottom ash in the production of ceramic materials is the high cost of sintering or vitrifying the material, and the uncertainty about the aesthetic properties. Table 2 presents an overview of the main limitations for each engineering application of MSWI bottom ash, together with the corresponding treatment technologies that will be discussed in “Treatment technologies” section.
A general, less evident challenge, is that nowadays the limit values for the recycling of MSWI bottom ash differ largely between countries, even within the EU 28. A subdivision into categories based on the application, could be a helpful tool for the harmonization of the limit values.
Finally, besides technical and environmental limitations, public acceptance of materials produced with bottom ash, a waste material, may constitute an additional barrier [3, 36]. Therefore, the implementation of the European end-of-waste criteria for MSWI bottom ash (and other waste-derived materials) could be of key importance. A waste material that achieves end-of-waste status is no longer subject to waste legislation, but becomes a product. At the moment, in contrast to e.g. scrap metals, no end-of-waste criteria are set for MSWI bottom ash, but at least the methodologies to assess limit values for pollutants in MSWI bottom ash and similar materials are under investigation [37]. The authors think that when the end-of-waste criteria come into force (maybe even subdivided into criteria per application category), this will boost the recycling of MSWI bottom ash, as unambiguous goals are set for the (leaching) limit values that have to be reached for MSWI bottom ash to be no longer considered a waste. By reaching the end-of-waste status, it will also become easier to convince the public of the benefits of recycling MSWI bottom ash and other similar materials.
Treatment Technologies
In the next paragraphs, treatment technologies to tackle the limitations mentioned above will be discussed. First, technologies for size separation of MSWI bottom ash and for the removal of metallic ferrous and non-ferrous metals (Cu, Al, Zn, Pb,…) will be reviewed. Many bottom ash applications preferably use only a limited size range of bottom ash, rather than the entire size range. The importance of removing metallic species for some applications was already demonstrated earlier, and moreover, metal recovery is highly beneficial for economic reasons. Afterwards, technologies for the removal of chlorides, and more recent developed technologies for reducing the leaching of Cu and of oxyanion forming elements, such as Sb, Mo and Cr, will be discussed.
Size Separation
Traditionally, wet or semi-dry (also sometimes called moist or dry) techniques are used for size separation and metal removal. Wet techniques usually yield a higher purity of the remaining metal and mineral fractions, because the adsorbed impurities are dissolved in the process, but have the disadvantage of requiring subsequent waste water treatment. A typical system for wet washing of bottom ash consists of water addition, sieving and screening, and magnets and eddy current separators to separate ferrous and non-ferrous metals. The following mineral ash fractions are typically obtained: >6 mm (coarse granulates), 2–6 mm (fine granulates), <2 mm (sand fraction) and sludge, which is filtered to obtain filter cakes [38]. In addition to this treatment, the bottom ash fractions are typically aged for 3 months. During this period carbonation reduces the pH and the leaching of many metals. Typically, the two granulate fractions are the least contaminated, and can be used as such in bulk applications [38]. The sand fraction, typically 20–25 % of the mass of the bottom ash, suffers high leaching of heavy metals, e.g. Cu. In the semi-dry techniques no water is added to the bottom ash, but the material is moist, because of the prior quenching of the bottom ashes.
Removal of Metallic Aluminum and/or Zinc
The effect of the presence of metallic Al or Zn particles can be decreased by immersing the bottom ash in a sodium hydroxide solution, as is described in “Use in or as construction material” section for lab experiments. The practicality of this is debatable on a larger scale, and this section therefore focuses on other processes to remove the metallic species. A drawback of wet or semi-dry separation processes is that, due to the presence of water, fine bottom ash particles adhere to each other, or to coarse material, impeding the magnetic or eddy current separation of adhered ferrous and non-ferrous metals. To mitigate this problem, new MSWI bottom ash processing technologies have been developed recently.
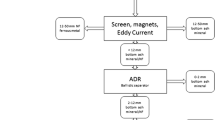
An example of such new technology is the patented ‘advanced dry recovery’ (ADR), as offered by Inashco, a Dutch company, and developed by the Technical University of Delft [39]. The ADR process adds no water to size-separate MSWI bottom ash, but the bottom ash itself is moist, as it originates from the wet ash extractor (quencher) at the end of the incineration plant. This makes the bottom ash clumpy and difficult to separate. The ADR technology uses kinetic energy to break the bonds that are formed by moisture and fine particles, and the fines are then separated from the coarse fraction ballistic ally, i.e. the lighter (fine) fraction travels a shorter distance than the heavier (coarse) fraction. The metals are afterwards separated from the mineral fraction by magnets and Eddy current separators, resulting in a good overall metal recovery. Further separation of the mineral fraction can be done by using screens and wind sifters [39].
In several installations in Japan and in 2 waste incinerators in Switzerland (KEZO, Hinwil and SATOM, Monthey) real dry bottom ash discharge systems were integrated in the waste incineration plant [40]. Dry discharge offers some significant advantages: not only are the recoverable metals of a better quality (i.e. less oxidized after wet quenching), the fine fraction can also be separated more effectively from the coarse fraction and be recycled separately, and less water is consumed in the process. The Martin dry discharge system in Monthey consists of (1) a ram-type discharger, operated without water; (2) an air-separator, where bottom ash is transported to by gravity and vibration; (3) a dust removal system (cyclone) to extract the bottom ash dust and fine fraction; (4) an air system to maintain a negative pressure inside the discharge system [41]. The bottom ash is divided into 3 product streams: (1) a coarse fraction with recyclable metal content, which can be recycled after metal removal, e.g. in road construction; (2) a fine fraction (<1 (or 5) mm) which is recycled or landfilled and contains almost no metal; (3) bottom ash dust supplied to the combustion process [40]. It is claimed that non-ferrous metal recovery is 90 % higher and ferrous metal recovery is 100 % higher with a dry ash discharge system than with a traditional wet discharge system [42]. The KEZO, Hinwil dry-discharge technology is somewhat different from that of Martin, but the discharged bottom ash is kept at high temperatures while ‘tertiary air’ is being introduced, which promotes burnout [40, 43]. Due to the better burnout, the obtained granulates have a lower loss on ignition, a lower TOC than with wet or semi-dry treatment, as well as lower heavy metal (Pb, Cd, Cu) leaching [44]. The latter can be attributed to destruction of organic components, including humic and fulvic acids that are responsible for increased leaching concentrations of Cu and other heavy metals [45–47].
To further increase the recycling of dry discharged bottom ash, Bourtsalas et al. [48] produced ceramics starting from the fine (<1 mm) fraction of this bottom ash. When the fine fraction of the dry discharged bottom ash was calcined at 1080 °C, the pH dependent leaching of Cu, Zn ad Pb was strongly decreased compared to the pH dependent leaching of the untreated material.
Obviously, the enhanced recovery of ferrous and non-ferrous metals after a dry ash discharge or ADR process has economic and environmental benefits, as more revenues can be obtained from the sales of the metals (the major source of income from bottom ash recycling) and less water is consumed. On the other hand, chlorides and other soluble ions are not removed from the bottom ash, as this can only be done by washing. The mineral fractions obtained after the ADR process have a higher chloride, sulfate and alkali content than the same fraction would have after a wet process [49]. The higher chloride concentration may exclude the use of the obtained granulates for production of reinforced concrete, as the high chloride content can lead to increased corrosion rates, or as raw material for cement production.
The recovery of ferrous and of non-ferrous metals is the major source of income from bottom ash recycling. However, as metals correspond typically only to 5–10 % of the MSWI bottom ash [40], and as bottom ash corresponds to around 25 % of the original MSW, it is very clear that only recycling the metals is far from a complete solution. Chlorides are not removed in the process, and leaching of heavy metals from the mineral fractions does, in general, not originate from the metals present, but mainly from salts adsorbed to the surface or contained in the minerals. It is therefore not a sustainable solution to only recycle the metals, if this implies that the remaining mineral fraction of the bottom ash, containing chlorides and leachable heavy metals, has to be send to a landfill, rather than recycling it.
Removal of Chlorides
To our knowledge, no proven methods to chemically or physically stabilize chlorides currently exist, hence washing is commonly applied to remove chlorides from combustion residues. Typically, water is sprayed on MSWI bottom ash that is transported on a perforated belt, after which the rinsed bottom ash is passed through some screens and sieves to separate it into several fractions. This typically yields four fractions: a coarse and a fine gravel fraction, a sand fraction, and sludge. During the washing and size separation, the added water leaches out a large part of the chlorides. Simultaneously, magnets and eddy current separators separate ferrous and non-ferrous metals from the bottom ash.
The smallest sized fractions, i.e. the sand and sludge fraction, typically contain more chlorides [50] and oxidized ferrous and non-ferrous metals, and show higher leaching concentrations of cation forming heavy metals e.g. Cu, and of oxyanion forming elements e.g. Sb, which can be attributed to their higher specific surface area. Therefore, after the washing process, the coarse and fine gravel fractions will be the leanest in chloride, and can be recycled both in concrete applications and as loose aggregates. The washing process alone is thus not sufficient to treat all MSWI bottom ash, as the sand fraction still contains significant amounts of chlorides, but it can be a first step in upgrading the bottom ash.
Immobilization of Cu, Sb, Mo and Cr
Due to the toxicity of the heavy metals and oxyanion forming elements Cu, Sb, Mo and Cr, and their leaching potential from several engineering applications, and because their mobility depends strongly on the kind of metal, pH and temperature of the process, some treatment technologies are discussed in this section, that take into account all of these variables.
The leaching of heavy metals and oxyanion forming elements can be reduced by carbonation, washing, mild thermal treatment or by using additives. Carbonation of the bottom ash is especially useful for amphoteric heavy metals, as it lowers the pH to values for which the leaching is generally low i.e. pH values between 8 and 10, and it alters the mineralogy, so that the heavy metals can precipitate to minerals with low solubility. Washing is a well-established technique, and was already discussed before. The next paragraphs, covering technologies reducing the leaching of problematic elements from bottom ash, mainly focusses on more recently developed technologies: (mild) heat treatment and addition of mineral additives to bottom ash. Carbonation will be discussed briefly as a way to assess the long term leaching behavior.
Sb Leaching
The mobility of antimony in bottom ash may be influenced by 4 different mechanisms: (a) the formation of calcium antimonates, (b) adsorption to iron (hydr)oxides, (c) the formation of iron antimonate, and (d) incorporation in or adsorption of Sb on ettringite [51]. The degree of occurrence of either of these 4 mechanisms depends on the pH of the bottom ash matrix. The adsorption of Sb(V)-compounds, which is the main prevalent Sb oxidation state in bottom ash leachates [52], to iron (hydr)oxides occurs at pH values below 7, and is thus not relevant for bottom ash, as the pH of bottom ash ranges between 8.5 (weathered bottom ash) and 12.5 (fresh bottom ash). Incorporation in ettringite was believed to be the controlling mechanism for the leaching of Sb in the alkaline pH region for many years, but recent findings [53] suggest that the formation of calcium antimonates (romeites) explains the observed leaching behavior of Sb from bottom ashes more accurately. The formation of iron antimonates was suggested by Okkenhaug et al. [54], but in their experiments this appeared a slow process, taking up to 260 days and more.
Based on the research done by Cornelis et al. and Okkenhaug et al. [53, 54], Van Caneghem et al. [51] selected several Ca- and Fe-based additives i.e. CaO, CaCl2, CaCO3, Fe2(SO4)3 and/or FeCl3 and tested their effect on Sb leaching from MSWI bottom ash. The compounds were added in w/w% between 1 and 5 %, and were able to reduce the Sb leaching from bottom ash from around 0.70 mg/kg to values between 0.08 and 0.29 mg/kg. Also the addition of activated carbon was shown to reduce Sb leaching. In recent lab experiments by Verbinnen et al. [55], addition of 5 w/w% activated carbon to bottom ash decreased the Sb leaching from 0.70 to 0.22 mg/kg.
The influence of carbonation on Sb leaching reported in literature is not straightforward, and it is difficult to draw unambiguous conclusions from these studies. Some papers report increased Sb leaching after carbonation [52, 56], others report a decrease [57, 58]. Verbinnen et al. [55] carbonated MSWI bottom ash, with an initial moisture content of 20 %, in a CO2 chamber at 30 °C with a CO2 partial pressure of 0.2 atm. The samples were carbonated for 7 days, and rewetted every 2 days to keep the moisture content as close as possible to 20 %. Their results (Fig. 1) show that Sb leaching decreased after carbonation. After 7 days of carbonation, the pH of the leachate had dropped from about 11.5–9 and the Sb concentration in the leachate was about 4 times lower than in untreated bottom ash at the same pH value (pH 9). It was hypothesized that—comparable to Cu–Sb was complexed with organic acids, and that after carbonation, Sb (or the organic complex) is adsorbed onto newly formed Fe/Al hydroxides, favored by the decreased pH [59].Cu and other heavy metals.
pH Dependent leaching and leaching after carbonation (0, 2, 4, 7 days) of Sb from MSWI bottom ash, intrinsic pH 11.5 [54]
The leaching of Cu and other heavy metals from bottom ash is strongly influenced by the presence of organic matter, mainly humic and fulvic acids [46, 47]. These acids form highly soluble organo-metallic complexes with cations of heavy metals such as Cu, Ni, Pb and Zn at alkaline pH values, preventing them from precipitating as hydroxides. The stability of the Cu-humic acid complex is higher than that of other heavy metal–humic acid complexes [60], so that Cu has generally the highest leaching concentrations of all heavy metals in the presence of these organic acids. Removal, destruction or immobilization of organic matter can therefore decrease the leaching of Cu from bottom ash. Several technologies have been developed for this purpose: heat treatment, carbonation, and addition of activated carbon.
Upon heating MSWI bottom ash at temperatures above 1000 °C e.g. for the production of ceramic structured materials, organic matter is destructed, and hence the Cu leaching is decreased [28]. Even by heating the bottom ash at lower temperatures, e.g. 400 °C, the organic material is destructed. Arickx et al. [45] showed that the Cu leaching can accordingly be decreased by mildly heating MSWI bottom ash: after 30 min heating at 400 °C, Cu leaching was reduced from 59.3 mg/kg to below the 0.5 mg/kg limit value for recycling in or as construction material in Flanders, Belgium [61]. At this temperature and residence time, the leaching of the other measured heavy metals (Pb, Zn) and dissolved organic carbon (DOC) also decreased. Hyks et al. [62] performed similar tests on a larger scale: they reheated MSWI bottom ash in a rotary kiln operating at two temperatures: 930 and 1080 °C and for 40 and 70 min. After treatment, the leaching of DOC was reduced, and this directly influenced the leaching of Cu, which was decreased by one order of magnitude. Due to evaporation, the leaching of chlorides was also reduced. On the other hand, the leaching of Cr and Mo was increased due to the treatment [62], as discussed and explained by Verbinnen et al. [63]. Santos et al. [64] compared ageing, heat treatment and accelerated carbonation for the stabilization of heavy metals in bottom ash. The leaching of the heavy metals Pb, Ni, Cu and Zn was reduced the most effectively by heat treatment.
Verbinnen et al. [55] showed that implementing heat treatment should be done with caution, as there is a high risk of increased leaching of e.g. Cr. In Fig. 2, the leaching of Cu and Cr from bottom ash is shown as a function of the heating temperature (heating for 1 h). After heating at 400 °C for 1 h, the leaching of Cu was reduced to values below 0.1 mg/kg as a result of the destruction of organic acids in the ash. However, at higher temperatures, which are needed to give a structured product, Cr leaching starts to increase.
Leaching of Cu and Cr from MSWI bottom ash as a function of heating temperature (400–600 °C, 1 h) [54]
Other studies [28, 65–67] also mention an increase after heating of Cr leaching to above the environmental limit values. Verbinnen et al. [63, 67] showed that this can be attributed to the oxidation of Cr(III) to Cr(VI) compounds in the presence of O2 and alkali and alkaline earth salts. This oxidation can be avoided by heating under inert atmosphere, or adding additives that inhibit the oxidation of Cr(III).
Therefore, it might be more useful to integrate a mild heat treatment in the incineration process itself, by means of dry ash discharge instead of a wet ash discharge. This technology is already applied by Martin GmbH at the KEZO Hinwil facility in Switzerland and shows to be beneficial for the leaching of Cu and other heavy metals [40].
Another alternative to reduce Cu leaching is carbonation [57, 59]. Experiments performed by Verbinnen et al. [55] showed that carbonation in a CO2 chamber at 30 °C with a CO2 partial pressure of 0.2 atm and an initial moisture content of the samples of 20 % for 7 days reduced the leaching of Cu from MSWI bottom ash drastically (Fig. 3). The effect of carbonation surpasses the effect of a reduced pH, evidenced by the leachate concentrations after carbonation (triangles in Fig. 3), which are significantly lower than the initial pH dependent leaching curve. The explanation for this reduction is still debated in literature, but probably either slightly soluble Cu carbonates are formed, or the organic fraction is adsorbed onto newly formed Fe/Al hydroxides, favored by the decreased pH after carbonation [59].
pH Dependent leaching and leaching after carbonation (0, 2, 4, 7 days) of Cu from MSWI bottom ash, intrinsic pH 11.5 [54]
Finally, Verbinnen et al. [55] showed that the addition of activated carbon can reduce Cu leaching. Activated carbon can adsorb Cu in the pH region relevant for bottom ash, but more probably, the organometallic Cu-organic acid complex is adsorbed in its entirety to the activated carbon. In recent lab experiments by the same authors, the addition of 5 w/w% activated carbon to bottom ash decreased Cu leaching from 2.3 to 0.2 mg/kg.
Cr and Mo
As shown previously, to reduce the leaching of heavy metals, some treatment methods (i.e. dry ash discharge, mild heat treatment, production of ceramics) rely on the heating of bottom ash to destroy organic acids. However, the leaching of Cr and/or Mo often increases after this heat treatment [62, 65, 66]. This was explained by Verbinnen et al. [63, 67]: upon heating of slightly mobile Cr(III)- and Mo(IV)-compounds in the presence of oxygen (and for Cr also in the presence of alkali and alkaline earth metals salts), mobile Cr(VI)- and Mo(VI)-compounds were formed. Concentrations of the toxic Cr(VI) in the leachate as high as 22 mg/kg were observed (heating at 500 °C for 6 h, Fig. 4a), significantly above the limit value (0.5 mg/kg) for the use in or as secondary raw materials in construction applications in Flanders, Belgium [61] and other European countries. The leaching of Mo also increases after heating, but the increase is less pronounced (Fig. 4b). The leaching of both Cr and Mo decreases again with increasing temperature after having reached a maximum at around 500–600 °C. Several explanations were suggested in literature to explain this decrease: (a) the presence of reducing agents in the samples (e.g. Al0), (b) the formation of a solid solution with ettringite, (c) the formation of a glassy phase encapsulating of Cr(VI) and/or Mo(IV) and (d) more reducing conditions at higher temperatures [68–71]. Explanations (a) and (b) have been refuted by Verbinnen et al. [63, 67] and most likely, a combination of (c) and (d) explains the decreased leaching at higher temperatures. Verbinnen et al. [63, 67] hypothesized that in the case of Cr, newly formed Na and K chromates can form a binary liquid phase with SiO2, resulting in the formation of an amorphous phase after cooling, preventing Cr from leaching by encapsulation. Explanation (d) is supported by a recent study of Kavouras et al. [72] who found that at temperatures above 800 °C, part of the chromates were reduced to Cr(III), in the form of MgCr2O4. However, 70 % of the total Cr content was still present under the form of Cr(VI) at 1200 °C. The explanation for the decreased leaching observed at higher temperatures is thus probably a combination of explanations (c) and (d).
Leaching of Cr (a) and Mo (b) from MSWI bottom ash heated at 400–700 °C for 0.5–6 h [54]
Overview of the Main Treatment Technologies
Every barrier to a specific application can be resolved by applying the appropriate treatment technology. Table 2 (“Overview of the main limitations” section) gives an overview of the different treatment technologies for each barrier. The identified barriers do not necessarily apply to all types of application as shown in the table: mostly only one, or at most two problems apply per type of application. Therefore, it is essential to differentiate between the treatment technologies per type of final bottom ash application. Heavy metal leaching, the main barrier for applying bottom ash as loose construction aggregates, can be remediated by applying size separation, (mild) heat treatment, carbonation, or with the aid of mineral additives. During the production of ceramics, heavy metal leaching can be avoided by carefully selecting the heating conditions. When using bottom ash as raw material for cement production, the presence of chlorides is the main problem, but this can be overcome by washing processes. The use of bottom ash in structured materials is hindered by the presence of metallic Al or Zn, and this can be resolved by enhanced dry or wet separation processes. Cl content and heavy metal leaching can also be a problem; the treatment technologies suggested for the other applications also apply here. The relationship between engineering applications, chemical barriers/limitations and treatment technologies for municipal solid waste incinerator bottom ash is illustrated in Fig. 5.
Conclusion
The recycling of MSWI bottom ash is subject to a tangle of limit values and restrictions imposed by public authorities, which may differ largely between countries. This paper presented a framework for top-down design of treatment technologies, starting from newly developed engineering applications for bottom ash. Each application type, use of bottom ash as loose/bulk construction aggregates, in concrete, or as raw material for cement or ceramics, is associated with specific problem(s) on the nature of untreated bottom ash such as heavy metal leaching, the presence of metallic Al or Zn, or a high chloride concentration. It is important to understand though, that those problems do not necessarily apply to all application types. Therefore, it is essential to differentiate between the treatment technologies per type of final bottom ash application. The range of technologies reviewed in this paper allows to overcome each barrier, and ultimately allows tailored design of bottom ash secondary resources.
References
CEWEP, Environmentally sound use of bottom ash. www.cewep.eu
Eurostat: Municpal Waste Statistics. http://ec.europa.eu/eurostat/statistics-explained/index.php/Municipal_waste_statistics (2016). Accessed 18 Mar 2016
Crillesen, K., Skaarup, J.: Management of bottom ash from WTE plants. ISWA Working Group Thermal Treatment. http://www.iswa.org/uploads/tx_iswaknowledgebase/Bottom_ash_from_WTE_2006_01.pdf (2006)
Dutch Ministry of Infrastructure and Environment: Green deal 076: Sustainable useful application of WtE bottom ash (in Dutch). http://www.greendeals.nl/gd076-verduurzaming-nuttige-toepassing-aec-bodemassen/ (2012)
Vandecasteele, C., Heynen, J., Goumans, H.: Materials recycling in construction: a review of the last 20 decades illustrated by the Wascon conferences. Waste Biomass Valoriz. 4, 695–701 (2013)
Hjelmar, O., Holm, J., Crillesen, K.: Utilisation of MSWI bottom ash as sub-base in road construction: first results from a large-scale test site. J. Hazard. Mater. 139, 471–480 (2007)
De Windt, L., Dabo, D., Lidelöw, S., Badreddine, R., Lagerkvist, A.: MSWI bottom ash used as basement at two pilot-scale roads: comparison of leachate chemistry and reactive transport modeling. Waste Manag. 31, 267–280 (2011)
Dabo, D., Badreddine, R., De Windt, L., Drouadaine, I.: Ten-year chemical evolution of leachate and municipal solid waste incineration bottom ash used in a test road site. J. Hazard. Mater. 172, 904–913 (2009)
Izquierdo, M., Querol, X., Josa, A., Vazquez, E., López-Soler, A.: Comparison between laboratory and field leachability of MSWI bottom ash as a road material. Sci. Total Environ. 389, 10–19 (2008)
Birgisdóttir, H., Pihl, K.A., Bhander, G., Hauschild, M.Z., Christensen, T.H.: Environmental assessment of roads constructed with and without bottom ash from municipal solid waste incineration. Transp. Res. Part D Transp. Environ. 11, 358–368 (2006)
Toller, S., Kärrman, E., Gustafsson, J.P., Magnusson, Y.: Environmental assessment of incinerator residue utilisation. Waste Manag. 29, 2071–20777 (2009)
Meyer, B.: Scories d’ordures incinérées pour béton. Bulletin du ciment. 54(7), 1–7 (1986)
Nielsen, P., Quaghebeur, M., Laenen, B., Kumps, R., Van Bommel, P.: The use of MSWI-bottom ash as aggregate in concrete—limitations and possible solutions. In: Mehu, J., Goumans, J.J.J.M., Heynen, J.J.M. (eds.) WASCON 2009 Proceedings (2009)
Pera, J., Coutaz, L., Ambroise, J., Chababbet, M.: Use of incinerator bottom ash in concrete. Cem. Concr. Res. 27, 1–5 (1997)
Keppert, M., Pavlík, Z., Tydlitát, V., Volfová, P., Svarcová, S., Syc, M., Cerny, R.: Properties of municipal solid waste incineration ashes with respect to their separation temperature. Waste Manag. Res. 30, 1041–1048 (2012)
Müller, U., Rübner, K.: The microstructure of concrete made with municipal waste incinerator bottom ash as an aggregate component. Cem. Concr. Res. 36, 1434–1443 (2006)
Saikia, N., Cornelis, G., Mertens, G., Elsen, J., Van Balen, K., Van Gerven, T., Vandecasteele, C.: Assessment of Pb-slag, MSWI bottom ash and boiler and fly ash for using as a fine aggregate in cement mortar. J. Hazard. Mater. 154, 766–777 (2008)
del Valle-Zermeño, R., Formosa, J., Chimenos, J.M., Martínez, M., Fernández, A.I.: Aggregate material formulated with MSWI bottom ash and APC fly ash for use as secondary building material. Waste Manag. 33, 621–627 (2013)
Bertolini, L., Carsana, M., Cassago, D., Quadrio Curzio, A., Collepardi, M.: MSWI ashes as mineral additions in concrete. Cem. Concr. Res. 34, 1899–1906 (2004)
Collepardi, M., Collepardi, S., Ongaro, D., Curzio, A. Q., Sammartino, M.: Concrete with bottom ash from municipal solid wastes incinerators. In: Proceedings of the 2nd International Conference on Sustainable Construction Materials and Technologies, 289–298 (2010)
Whittaker, M., Taylor, R., Li, Q., Li, S., Black, L.: The behaviour of finely ground bottom ash in Portland cement. In: 29th Cement and Concrete Science Conference, pp. 70–73. Leeds, UK (2009)
Krammart, P., Tangtermsirikul, S.: Properties of cement made by partially replacing cement raw materials with municipal solid waste ashes and calcium carbide waste. Constr. Build. Mater. 18, 579–583 (2004)
Li, X.-G., Lv, Y., Ma, B.-G., Chen, Q.-B., Yin, X.-B., Jian, S.-W.: Utilization of municipal solid waste incineration bottom ash in blended cement. J. Clean. Prod. 32, 96–100 (2012)
Onori, R., Polettini, A., Pomi, R.: Mechanical properties and leaching modeling of activated incinerator bottom ash in Portland cement blends. Waste Manag. 31, 298–310 (2011)
Lam, C.H.K., Barford, J.P., McKay, G.: Utilization of incineration waste ash residues in Portland cement clinker. In: Chemical Engineering Transactions, vol. 21, pp. 757–762. Italian Association of Chemical Engineering - AIDIC (2010)
Rehan, R., Nehdi, M.: Carbon dioxide emissions and climate change: policy implications for the cement industry. Environ. Sci. Policy 8, 105–114 (2005)
Pan, J.R., Huang, C., Kuo, J.-J., Lin, S.-H.: Recycling MSWI bottom and fly ash as raw materials for Portland cement. Waste Manag. 28, 1113–1118 (2008)
Cheeseman, C.R., Makinde, A., Bethanis, S.: Properties of lightweight aggregate produced by rapid sintering of incinerator bottom ash. Resour. Conserv. Recycl. 43, 147–162 (2005)
Bethanis, S., Cheeseman, C.R., Sollars, C.J.: Effect of sintering temperature on the properties and leaching of incinerator bottom ash. Waste Manag. Res. 22, 255–264 (2004)
Bourtsalas, A., Vandeperre, L.J., Grimes, S.M., Themelis, N., Cheeseman, C.R.: Production of pyroxene ceramics from the fine fraction of incinerator bottom ash. Waste Manag. 45, 217–225 (2015)
Monteiro, R.C.C., Alendouro, S.J.G., Figueiredo, F.M.L., Ferro, M.C., Fernandes, M.H.V.: Development and properties of a glass made from MSWI bottom ash. J. Non-Cryst. Solids 352, 130–135 (2006)
Rambaldi, E., Esposito, L., Andreola, F., Barbieri, L., Lancellotti, I., Vassura, I.: The recycling of MSWI bottom ash in silicate based ceramic. Ceram. Int. 36, 2469–2476 (2010)
Schabbach, L.M., Bolelli, G., Andreola, F., Lancellotti, I., Barbieri, L.: Valorization of MSWI bottom ash through ceramic glazing process: a new technology. J. Clean. Prod. 23, 147–157 (2012)
Ferraris, M., Salvo, M., Ventrella, A., Buzzi, L., Veglia, M.: Use of vitrified MSWI bottom ashes for concrete production. Waste Manag. 29, 1041–1047 (2009)
Bassani, M., Santagata, E., Baglieri, O., Ferraris, M., Salvo, M., Ventrella, A.: Use of vitrified bottom ashes of municipal solid waste incinerators in bituminous mixtures in substitution of natural sands. Adv. Appl. Ceram. 108, 33–43 (2009)
Lamers, F.J.M., Kokmeijer, E.: Expertvisie Technische Haalbaarheid Kwaliteitsverbetering AEC-Bodemas. Kema Nederland BV, Arnhem (2011)
Saveyn, H., Eder, P.,Garbarino, E, Muchova, L., Hjelmar, O., Van der Sloot, H., Comans, R., Van Zomeren, A. Hyks, J., Oberender, A.: Study on methodological aspects regarding limit values for pollutants in aggregates in the context of the possible development of end-of-waste criteria under the EU waste framework directive. JRC Technical Report. European Commission Joint Research Centre (2014)
Vandecasteele, C., Wauters, G., Arickx, S., Jaspers, M., Van Gerven, T.: Integrated municipal solid waste treatment using a grate furnace incinerator: the indaver case. Waste Manag. 27, 1366–1675 (2007)
De Vries, W, Rem, P., de Keizer, M.: Value creation out of municipal solid waste incinerator (MSWI) bottom ash. In: Arm, M., Vandecasteele, C., Heynen, J.J.M., Suer, P., Lind, B. (eds.) WASCON 2012 Proceedings (2012)
Bourtsalas, A.: Review of WTE ash utilization processes under development in northwest Europe. WTERT UK (2013)
Koralewska, R.: Industrial-scale R&D in challenging times. In: Proceedings of the 18th Annual North American Waste to Energy Conference, 1–6 (2010)
Von Raven, R., Koralewska, R., Schönsteiner, M.: Waste-to-energy as part of urban mining—recovery of metals from bottom ash. ISWA Beacon Conference on Waste-to-Energy. http://www.beacon-wte.net/fileadmin/avfallsverige/Documentation_2013/BLOCK2_4.pdf (2013)
Vehlow, J., Seifert, H.: Management of residues from waste-to-energy processes. http://www.ieabioenergytask36.org/vbulletin/attachment.php?attachmentid=298&d=1362675611 (2012)
Wieduwilt, M., Müller, R., Luzzatto, M., Brison, A.: Advanced urban mining: a summary of the state of the art of metal recovery out of dry bottom ash. http://www.vivis.de/phocadownload/2015_wm/2015_WM_293-304_Mueller_Wieduwilt.pdf (2015)
Arickx, S., Van Gerven, T., Knaepkens, T., Hindrix, K., Evens, R., Vandecasteele, C.: Influence of treatment techniques on Cu leaching and different organic fractions in MSWI bottom ash leachate. Waste Manag. 27, 1422–1427 (2007)
Van Zomeren, A., Comans, R.N.J.: Contribution of natural organic matter to copper leaching from municipal solid waste incinerator bottom ash. Environ. Sci. Technol. 38, 3927–3932 (2004)
Meima, J.A., van Zomeren, A., Comans, R.N.J.: Complexation of Cu with dissolved organic carbon in municipal solid waste incinerator bottom ash leachates. Environ. Sci. Technol. 33, 1424–1429 (1999)
Bourtsalas, A., Vandeperre, L.J., Grimes, S.M., Themelis, N., Koralewska, R., Cheeseman, C.R.: Properties of ceramics prepared using dry discharged waste to energy bottom ash dust. Waste Manag. Res. 33, 794–804 (2015)
Van der Wegen, G., Hofstra, U., Speerstra, J.: Upgraded municipal solid waste incinerator (MSWI) bottom ash as aggregate in concrete. In: Arm, M., Vandecasteele, C., Heynen, J.J.M., Suer, P., Lind, B. (eds.) WASCON 2012 Proceedings (2012)
Chen, H.C., Chiou, I.-J.: Distribution of chloride in MSWI bottom ash and de-chlorination performance. J. Hazard. Mater. 148, 347–352 (2007)
Van Caneghem, J., Verbinnen, B., Cornelis, G., De Wijs, J., Mulder, R., Billen, P., Vandecasteele, C.: Immobilization of antimony in waste-to-energy bottom ash by addition of calcium and iron containing additives. Waste Manag. 54, 162–168 (2016)
Cornelis, G., Van Gerven, T., Vandecasteele, C.: Antimony leaching from uncarbonated and carbonated MSWI bottom ash. J. Hazard. Mater. 137, 1284–1292 (2006)
Cornelis, G., Van Gerven, T., Vandecasteele, C.: Antimony leaching from MSWI bottom ash: modelling of the effect of pH and carbonation. Waste Manag. 32, 278–286 (2012)
Okkenhaug, G., Amstätter, K., Lassen Bue, H., Cornelissen, G., Breedveld, G.D., Henriksen, T., Mulder, J.: Antimony (Sb) contaminated shooting range soil: Sb mobility and immobilization by soil amendments. Environ. Sci. Technol. 47, 6431–6439 (2013)
Verbinnen, B., Billen, P., Van Caneghem, J., Vandecasteele, C.: Novel treatment methods for oxyanion forming elements and heavy metal ions in view of MSWI bottom ash recycling in construction. In: Proceedings of the 9th International Conference on the Environmental and Technical Implications of Construction with Alternative Materials, Santander, Spain, pp. 398–404 (2015)
Arickx, S., Van Gerven, T., Vandecasteele, C.: Accelerated carbonation for treatment of MSWI bottom ash. J. Hazard. Mater. 137, 235–243 (2006)
Van Gerven, T., Van Keer, E., Arickx, S., Jaspers, M., Wauters, G., Vandecasteele, C.: Carbonation of MSWI-bottom ash to decrease heavy metal leaching, in view of recycling. Waste Manag. 25, 291–300 (2005)
Todorovic, J., Ecke, H.: Demobilisation of critical contaminants in four typical waste-to-energy ashes by carbonation. Waste Manag. 26, 430–441 (2006)
Arickx, S., De Borger, V., Van Gerven, T., Vandecasteele, C.: Effect of carbonation on the leaching of organic carbon and of copper from MSWI bottom ash. Waste Manag. 30, 1296–1302 (2010)
Pandey, A.K., Pandey, S.D., Misra, V.: Stability constants of metal-humic acid complexes and its role in environmental detoxification. Ecotoxicol. Environ. Saf. 47, 195–200 (2000)
Flemish decree on the sustainable management of material cycles and waste. https://navigator.emis.vito.be/pdfservlet?woId=41707&woLang=en&version=2015-03-30&compareVersion=2015-03-30&lang=en (2012)
Hyks, J., Nesterov, I., Mogensen, E., Jensen, P.A., Astrup, T.: Leaching from waste incineration bottom ashes treated in a rotary kiln. Waste Manag. Res. 29, 995–1007 (2011)
Verbinnen, B., Billen, P., Van Coninckxloo, M., Vandecasteele, C.: Heating temperature dependence of Cr(III) oxidation in the presence of alkali and alkaline earth salts and subsequent Cr(VI) leaching behavior. Environ. Sci. Technol. 47, 5858–5863 (2013)
Santos, R.M., Mertens, G., Salman, M., Cizer, Ö., Van Gerven, T.: Comparative study of ageing, heat treatment and accelerated carbonation for stabilization of municipal solid waste incineration bottom ash in view of reducing regulated heavy metal/metalloid leaching. J. Environ. Manag. 128, 807–821 (2013)
Van Gerven, T., Chen, X., Evens, R., Hindrix, K., Vandecasteele, C.: Upgrading MSWI bottom ash by extraction, heating or dense medium separation, in view of recycling. In: Proceedings of the Sixth International Conference on the Environmental and Technical Implications of Construction with Alternative Materials, Belgrade, Serbia and Montenegro, pp. 507–518 (2006)
Selinger, A., Schmidt, V., Bergfeldt, B., Vehlow, J., Simon, F.G.: Investigation of sintering processes in bottom ash to promote the reuse in civil construction—Part 1: element balance and leaching. In: Proceedings of the International Conference on the Environmental and Technical Implications of Construction with Alternative Materials, Houthem St. Gerlach, The Netherlands, pp. 41–49 (1997)
Verbinnen, B., Billen, P., Vandecasteele, C.: Thermal treatment of solid waste in view of recycling: chromate and molybdate formation and leaching behaviour. Waste Manag. Res. 32, 536–542 (2014)
Astrup, T., Rosenblad, C., Trapp, S., Christensen, T.H.: Chromium release from waste incineration air-pollution-control residues. Environ. Sci. Technol. 39, 3321–3329 (2005)
Bodénan, F., Guyonnet, D., Piantone, P., Blanc, P.: Mineralogy and pore water chemistry of a boiler ash from a MSW fluidized-bed incinerator. Waste Manag. 30, 1280–1289 (2010)
Sorensen, M., Koch, C.B., Stackpoole, M.M., Bordia, R.K., Benjamin, M.M., Christensen, T.H.: Effects of thermal treatment on mineralogy and heavy metal behavior in iron oxide stabilized air pollution control residues. Environ. Sci. Technol. 34, 4620–4627 (2000)
Wei, Y., Hsieh, H., Yang, Y., Lee, J., Liang, W.: Molecular study of thermal immobilization of Chromium(VI) with clay. J. Air Waste Manag. Assoc. 55, 411–414 (2005)
Kavouras, P., Pantazopoulou, E., Varitis, S., Vourlias, G., Chrissafis, K., Dimitrakopulos, G.P., Mitrakas, M., Zouboulis, A.I., Karakostas, T., Xenidis, A.: Incineration of tannery sludge under oxic and anoxic conditions: study of chromium speciation. J. Hazard. Mater. 283, 672–679 (2015)
Acknowledgments
The authors would like to thank Indaver N.V, Feniks B.V and Afvalzorg N.V for their cooperation and support to this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verbinnen, B., Billen, P., Van Caneghem, J. et al. Recycling of MSWI Bottom Ash: A Review of Chemical Barriers, Engineering Applications and Treatment Technologies. Waste Biomass Valor 8, 1453–1466 (2017). https://doi.org/10.1007/s12649-016-9704-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-016-9704-0