Abstract
This study was performed to produce lipids from the isolated oleaginous yeast Rhodotorula glutinis SO28 using loquat kernel extract (LKE) as substrate. LKE was prepared using acid hydrolysis and alkaline neutralization steps. Lipid production was performed in shaking flaks culture. Even if LKE was used as a sole source of nutritional substances, it could support cell growth and lipid synthesis in the yeast. Additional carbon, nitrogen and phosphorus sources were found to significantly alter the lipid accumulation potential of the yeast. Optimal concentrations of additional carbon (glucose) and nitrogen (ammonium sulphate) sources for lipid accumulation were determined as 15 and 0.5 g/L, respectively. On the other hand, all the concentrations of additional phosphorus source were found to significantly reduce the lipid accumulation. Optimal incubation time was determined as 132 h. Under the optimized culture conditions, the lipid concentration and lipid content of the yeast were determined as 7.82 g/L and 62 %, respectively. Fatty acid methyl ester analysis exhibited that this yeast strain could produce high proportions of C16:0 and C18 fatty acids, which are ideal for biodiesel production. This is the first report on the use of waste loquat kernels as substrate for microbial lipid production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biodiesel is a non-toxic, biodegradable and renewable fuel [1]. In contrast to petroleum diesel, biodiesel does not contain polycyclic aromatic hydrocarbons, and emits very little sulfur dioxide, carbon monoxide, carbon dioxide, and particulates [2]. Therefore, use of biodiesel as an alternative fuel greatly reduces health risks and environmental problems. Production of this fuel is currently performed from vegetable oils, animal fats or waste cooking oil by the transesterification of triglycerides and alcohol in the presence of a catalyst [2, 3]. However, investigators have reported that lipids of oleaginous microorganisms can be also used as promising potential feedstock for biodiesel production, owing to their similar fatty acid compositions to that of vegetable oils [4, 5].
Oleaginous microorganisms are defined as microbes that can accumulate lipids to a level corresponding to more than 20 % of their biomass [6, 7]. Yeasts and algae are the most studied oleaginous microorganisms at the moment due to their high cellular lipid contents [4, 8–10]. Oleaginous yeasts are reported to be generally present in genera such as Candida, Cryptococcus, Rhodotorula, Rhizopus, Trichosporon, Lipomyces, and Yarrowia. These yeasts are capable of accumulating lipids to levels corresponding to 40 % of their biomass. Also, they may accumulate lipids to levels exceeding 70 % of their biomass under nutrient-limited conditions [7]. The fatty acid composition of oleaginous yeasts resembles to those of plants. Hence, yeast lipids are accepted as a good alternative feedstock for biodiesel production [11]. Furthermore, oleaginous yeasts do not require big areas for cultivation and they can grow in low-cost fermentation media containing waste agricultural materials and some industrial byproducts [12]. For example, organic materials such as raw glycerol, lignocellulosic residues, cheese whey, molasses, palm oil and wheat straw are extensively used as cheap growth substrates in cultures of oleaginous yeasts for microbial lipid production [13–18].
Loquat (Eriobotrya japonica L.) is a subtropical evergreen fruit tree. After consumption of fruit flesh, the remaining parts including kernels are currently discarded. Therefore, 225 kg of loquat kernels are generated as waste from 1000 kg of loquat fruits (by dry weight). Loquat kernels are reported to have high carbohydrate (71.2 %) content. The kernels are also rich in minerals, specially potassium, calcium and magnesium [19]. Due to rich nutritional composition, powdered loquat kernels or hydrolysate (extract) prepared from these kernels are used as substrate for exopolysaccharide, enzyme, organic acid and carotenoid production [20]. However, powdered and/or hydrolysate (extract) form of loquat kernels has not been investigated as substrate for production of other microbial products such as ethanol, glutathione, single cell protein and lipids. Therefore, utilization of loquat kernels as substrate in microbial cultivations may provide a new approach for not only reduction of cost of cultivation media but also solution of waste problem.
Hence, the present study was performed to elucidate whether the loquat kernel extract can be used or not as substrate for lipid production in submerged culture of the isolated oleaginous yeast R. glutinis SO28.
Materials and Methods
Preparation of Loquat Kernel Extract
Loquat kernel extract (LKE) was re-prepared by applying acid hydrolysis and neutralization procedures according to the previous method [19]. HCl and NaOH were used for the hydrolysis and neutralization steps, respectively. The loquat kernels were dried at 80 °C to the constant weight then ground with a grinder. In this way, loquat kernel flour (LKF) with particle size 1 mm was prepared. Afterwards, 100 g of LKF was mixed with 500 mL of 2 N HCl solution in an autoclavable bottle and the total volume was adjusted to 1 L. After the bottle was introduced into an autoclave, the hydrolysis process of LKF was started. The hydrolysis experiments were carried out at 121oC under pressure of 1.2 atm. After 60 min, the obtained mixture was cooled to room temperature, and its pH was adjusted to 7.0 with 10 N NaOH. The liquid and solid fractions of the mixture were separated from each other by filtration using Whatman no. 1 filter paper. The liquid fraction was termed as loquat kernel hydrolysate. It was dried at 80 °C until the weight became constant and then powdered. The dried and powdered soluble material was termed as loquat kernel extract (LKE). In this way, 98 g of LKE could be prepared. Total sugar content of LKE was analyzed by phenol–sulfuric acid method [21]. Nitrogen content was determined using a micro-Kjeldahl apparatus (Labconco Corporation, Kansas City, MO, USA), and crude protein was estimated by multiplying the nitrogen content by 6.25. Crude fat was determined by ether extraction in a Soxhlet apparatus. Ash was determined by combusting dry sample in a muffle furnace.
Isolation and Screening of Yeast Strains for Lipid Production
Soil samples taken from different localities of Erzurum city (Turkey) were used as isolation source. Isolation of lipid producing yeast strains was performed on loquat kernel agar (LKEA) medium. This isolation medium composed of only 10 g/L LKE and 20 g/L agar (pH 6.0). For the isolation, soil samples serially diluted with sterile saline water were spread on petri dishes containing LKEA medium. After 48-h cultivation, yeast colonies developing on the medium were selected and purified. The isolated strains were then screened in order to find out the most potent lipid producing strain. The screening experiments were performed in 250 mL flaks containing 100 mL of loquat kernel broth (LKEB) medium. This medium contained only 10 g/L LKE (pH 6.0). For the screening experiments, the cultures inoculated with seed cultures were incubated at 30 °C in a shaking incubator at 150 rpm. After 48 h, the isolate SO28 was found to posses the highest lipid concentration. This isolate was then used for the subsequent experiments.
Identification of the Best Lipid Producing Yeast Strain
The identification of the strain S028 was performed by sequencing a fragment of genome. The primers used for the polymerase chain reaction (PCR) were ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) [22]. Amplification reactions were carried out in a 50-µl reaction volume under the following PCR cycling conditions: one cycle of denaturation at 94 °C for 2 min, followed by 30 cycles of denaturation at 95 °C for 45 s, annealing at 55 °C for 1 min, and elongation at 72 °C for 1 min, with a final extension step of 72 °C for 10 min. PCR products were analyzed in 1 % (w/v) agarose gels by horizontal gel electrophoresis. DNAs were visualized by UV excitation after staining with ethidium bromide. The products were purified following the protocols of the PureLink® PCR Purification Kit (Life Technologies, Carlsbad, CA, USA). After purification, ITS rDNA gene was sequenced in both directions with ITS 1 and ITS 4 primers at Medsantek Co., Ltd., Turkey. Sequences chromatograms were assembled into one complete sequence using Bioedit Sequence Alignment Editor version 7.2.5 [23] and the sequence was compared to all known sequences in the Genbank by use of BLASTN 2.2.26 + program [24] and deposited with the GenBank database under the accession number KX017572.
Optimization of Culture Conditions for Lipid Production
The experiments for lipid production were performed in 250-mL flasks containing 100 mL of sterilized LKEB medium (pH 6.0). The preliminary experiments were undertaken to determine the optimal concentration of LKE (10–80 g/L) for lipid production. In this stage, the medium contained only LKE. Then, different concentrations of glucose (0–20 g/L), ammonium sulphate (0–1.5 g/L) and potassium dihydrogen phosphate (0–1.5 g/L) as additional carbon, nitrogen and phosphorus sources were tested for maximum lipid production in LKEB medium, respectively. Effect of incubation time on lipid production was tested for 144 h. The final experiments were performed to determine the composition of fatty acids of the yeast that was grown under optimal culture conditions.
Preparation of Yeast Seed Culture
During the screening and optimization experiments, the yeast seed cultures were prepared in malt extract broth medium (MEB) at 30 °C in a shaking incubator (ZHWY-200B, Zhicheng Analytical Co., Shanghai, China) at 150 rpm. At the end of 48-cultivation period, absorbance of yeast starter cultures was adjusted to 2.0 (at 600 nm) using sterile saline-water [25, 26]. Then, 2 mL of yeast seed culture were used for inoculation of LKEB medium.
Analytical Methods
The sugar consumption in the culture was analyzed by phenol–sulfuric acid method [21]. Yeast biomass concentration was determined by cell dry weight. To do this, 10 mL of culture broth was centrifuged at 3, 857×g for 5 min. The pellet was washed twice with 5 mL of distilled water and dried at 80 °C to constant weight. 24) For the determination of lipid concentration, the extraction of total lipids was performed with chloroform–methanol (2:1, v/v) mixture. For this purpose, dried yeast cells were powdered and transferred into a tube of 50 mL. Then, 5 mL of chloroform–methanol mixture were added on dried cells in this tube. The mixture was mixed for 5 min a using vortex. Finally, the mixture was centrifuged for 5 min and the supernatant was transferred into another tube. Chloroform–methanol extraction was applied four cycles to the yeast cells inside the tube. After extraction, cells were re-dried at 80 °C to constant weight. The decrease in total biomass was expressed as lipid concentration (g/L). The lipid content was determined as fallows: Lipid content (%) = [Lipid yield (g/L)/Biomass concentration (g/L)] × 100. Biomass yield was expressed as g of cell biomass/g of LKE. Lipid yield was expressed as g of total lipid concentration/g of LKE.
For determination of fatty acid composition, the organic extracts (collected chloroform–methanol extracts) were washed with 0.88 % KCl (w/v), dried over anhydrous Na2SO4, and evaporated. Then, the dried lipid residue was re-dissolved in 500 µl of 10 % BF3-methanol (FLUKA, 15716) and incubated in a sealed vial in a 95 °C heater for 20 min. FAMEs were extracted with 300 µl n-hexane after the addition of 300 µl saturated NaCl in water. Analysis of fatty acids composition of lipids was performed by GC–MS using an Agilent Technologies 7890A-5975C GC–MS system equipped with a HP-88 capillary column (60 m × 0.25 mm × 0.20 µm). Fatty acid methyl esters (FAMEs) mix C8–C24 (SUPELCO, USA) was used as a standard. The FAME peaks were identified and quantitated by comparing the unknown responses to those generated from known standards.
Statistical Analysis
Each experiment was repeated at least three times in two replicates. The analysis of variance was conducted using one-way ANOVA test using SPSS 13.0 for Microsoft Windows, and means were compared by Duncan test at the 0.05 level of confidence.
Results and Discussions
Determination of Chemical Composition of Loquat Kernel Extract
The performed experiments demonstrated that 98 g of loquat kernel extract (LKE) could be prepared from loquat kernels using hydrolysis, neutralization, filtration and drying procedures. The chemical analyses demonstrated that 100 g of LKE contained 64.9 g total sugar. High sugar content of LKE may be an important advantage for lipid production, since excessive concentrations of carbon source improve lipid accumulation in oleaginous yeasts [11, 12]. The nitrogen and protein contents of LKE were determined as 1.1 % and 6.8 g/100 g, respectively. It was also determined that LKE had high ash content (24.9 g/100 g) but a low lipid content (1.2 g/100 g). This is an expected result, since chemical hydrolysis methods lead to high ash contents in the final product [19, 27].
Isolation and Screening of Hyper Lipid Producing Yeast Strains
It is well known that yeasts are good lipid producer microorganisms and their lipids can be used as biodiesel feedstock [2–5]. So, the present isolation experiments mainly focused on isolating yeasts rather than filamentous fungi and bacteria. During the isolation studies, loquat kernel extract (LKE) was used as sole source of all nutritional components without additional carbon, nitrogen and mineral sources. In this way, we could isolate the yeasts whose nutritional requirement is simple. The experiments demonstrated that total 37 yeast strains could be isolated on loquat extract agar (LKEA) medium using different soil samples as isolation source. However, growth performances of 30 strains on LKEA were too low. Hence only seven isolates having high growth performance were screened for their lipid production potential in loquat kernel extract broth (LKEB) medium. The screening experiments exhibited that although the isolate SO11 produced maximum cell biomass, the maximum lipid concentration (1.88 g/L) and content (40 %) were achieved for the isolate SO28 (Table 1). Therefore, the isolate SO28 was selected for the subsequent steps and identified as Rhodotorula glutinis.
Optimization of Culture Parameters for Lipid Production in LKE Medium
The initial optimization experiments were performed to determine the optimal concentrations of LKE. In this stage, the medium (LKEB) contained only LKE in the case of the screening experiments.
The experiments demonstrated that biomass concentration and lipid concentration as well as lipid content significantly increased with the increase in LKE concentration from 10 to 60 g/L, whereas a further increase in LKE concentration from 60 to 80 g/L decreased them (Table 2). Exactly, the maximum yeast biomass (9.66 g/L) and lipid concentration (4.54 g/L) as well as the maximum lipid content (47 %) were attained at a LKE concentration of 60 g/L. In other words, the increasing concentrations of LKE provided more carbon source to the yeast, thereby increased cell growth and lipid accumulation. Inhibitory effect of LKE concentration above 60 g/L on growth and lipid accumulation might be attributed to the presence in LKE of some toxical compounds. This is because it has been reported that high temperatures produce unwanted byproducts from some sugars such as glucose, maltose and sucrose, and these by products may negatively affect microbial growth [28, 29]. Also, it has been documented that the hydrolysates that are used as fermentation substrate can contain some antimicrobial substances, such as weak acids, furan derivatives, and phenolic compounds generated during the hydrolysis [30]. Therefore, LKE might contain the similar antimicrobial substances that exerted an inhibitory effect on the fungal growth at especially high concentrations of LKE. Furthermore, high salt (especially NaCl) content of LKE might be another reason of inhibitory effect on microbial growth. Because, NaCl increas osmotic pressure and therefore can inhibit or kill microorganisms [31]. On the other hand, the present results confirmed that R. glutinis SO28 had an oleaginous character, since microorganisms that can accommodate lipid contents of more than 20 % of their dry biomass are defined as oleaginous [8].
Second stage experiments were carried out to determine the effect of additional nitrogen and carbon sources on lipid accumulation in R. glutinis S028. In this regard, different concentrations (0–20 g/L) of glucose as additional carbon sources were tested in LKE medium. Similarly, ammonium sulphate was tested as additional nitrogen source at the different concentrations from 0 to 1.5 g/L. As seen from Table 3, additional nitrogen and carbon sources significantly affected yeast growth and lipid accumulation. For example, addition of 0.5 g/L nitrogen source into the medium caused the significant increases in lipid content. Conversely, concentrations of additional nitrogen source above 0.5 g/L gave rise to significant reductions in lipid content at all the concentrations of additional carbon source. For instance, the lipid content of yeast dropped from 52 to 49 % when the concentration of additional nitrogen source was increased from 0.5 to 1.0 g/L in the LKE medium containing 5 g/L glucose (additional carbon source). Similarly, an increase in concentration of additional nitrogen source from 0.5 to 1 g/L dropped the lipid content from 55 to 51 % when the additional carbon source was stable at 10 g/L. The experiments exhibited that the maximum lipid concentration (7.03 g/L) and lipid content (57 %) were attained in LKE medium containing 15 g/L glucose and 0.5 g/L ammonium sulphate as additional carbon and nitrogen sources, respectively. These results could be attributed to the C/N ratio of LKE medium. This is because lipid accumulation in oleaginous yeasts and molds occur more when the nitrogen in the medium becomes limited and the carbon source is present in excess. Namely, high carbon/nitrogen (C/N) in the culture medium increases lipid accumulation in oleaginous species, including R. glutinis strains [11, 12, 32, 33]. The obtained results indicated that additional carbon and nitrogen sources improved lipid accumulation in the yeast. But, it is well known that addition of these substances can increase the production cost. In this point, we consider that LKE alone can be used as a substrate in the medium for lipid production in large-scale. This is because the experiments demonstrated that when LKE was used as a sole source of nutritional substances, it could support yeast growth and lipid synthesis (Tables 1, 2, 3). For example, a lipid concent of 47 % could be attained in the medium containing only 60 g LKE without additional nitrogen and carbon sources (Table 3). This situation could be attributed to the rich nutritional composition of LKE as reported by previous studies [19, 20].
The experiments showed that when KH2PO4 (potassium dihydrogen phosphate) was used as an additional phosphorus source, its all concentrations significantly increased biomass production (Table 4). In contrast to biomass production, all the concentrations of additional phosphorus source (KH2PO4) led to significant decreases in lipid accumulation. For instance, although KH2PO4 concentration of 1.5 g/L provided higher biomass concentration, it caused the lowest lipid concentration (5.91 g/L) and lipid content (43 %). This finding is good in agreement with those of early studies showing that excessive phosphorus exerts a negative effect on lipid accumulation potential of oleaginous yeasts such as Candida 107, Rhodotorula glutinis and Rhodosporidium toruloides [34–36]. That is, phosphorus-limited conditions are accepted as more favorable for lipid accumulation in oleaginous yeasts.
Contrast to the biomass concentration, the maximum lipid concentration (7.03 g/L) and content (57 %) were attained in additional phosphorus source-free medium. Based on the present results, it could be concluded that phosphorus content of LKE alone was enough to support lipid accumulation in the yeast and the addition of external phosphorus source was not necessary. Therefore, the following experiments were carried out in LKE medium containing only additional carbon (15 g/L) and nitrogen (0.5 g/L) sources without additional phosphorus source.
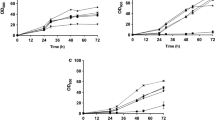
The experiments showed that the cell growth and sugar consumption in the medium were rapid within the first 48 h of fermentation, especially between 24 and 48 h. Conversely, less cell growth and sugar consumption were observed after 48th h (Fig. 1). Biomass concentration reached to the maximum (12.62 g/L) after 108 h, and further incubation periods did not augment biomass concentration. The initial total sugar content of LKEB medium was calculated as 53.94 g/L (60 g LKE containing 38.9 g total sugar was then supplemented with 15 g glucose). The sugar content of the medium was completely exhausted after 132 h. In contrast to cell growth, lipid accumulation in the yeast progressed more slowly and continued up to the 132th h of the cultivation with the complete depletion of sugar content in the medium. This situation might be attributed to the depletion in the medium of nitrogen source, which is essential for cell growth. That is to say the remaining sugar in the medium after the depletion of nitrogen source might be utilized for lipid synthesis. Because, investigators have reported that excessive carbon remains unutilized or is converted into storage polysaccharides in non-oleaginous species, while, it is preferentially channeled toward lipid synthesis in oleaginous species [14, 25, 32, 34]. At the end of 132-hours cultivation period, the maximum lipid concentration and lipid content were determined as 7.82 g/L and 62 %, respectively. The biomass yield and lipid yield were calculated as 0.21 (12.62 g of cell biomass/60 g of LKE) and 0.130 (7.82 g of lipid concentration/60 g of LKE).
Time profile of cell growth, lipid accumulation and sugar consumption for R. glutinis S028 in loquat kernel extract medium. Culture conditions: loquat kernel extract 60 g/L, additional carbon source (glucose) 15 g/L, additional nitrogen source (ammonium sulfate) 0.5 g/L, initial pH 6.0, temperature 30 °C and shaking speed 200 rpm
The final pH of the culture broth after 132 h was measured as 3.4. This drop in pH might be due to the production of some organic acids by the yeast. This is because some earlier studies demonstrated that R. glutinis strains could produce organic acids such as rhodotorulic acid, thereby decreasing the final pH of the culture broth [37, 38].
Determination of Fatty Acids Composition of the Yeast
The final experiments were performed to determine the fatty acids composition of lipid-rich biomass of the yeast. The major fatty acids of the yeast were palmitic acid (C16:0) (19.6 %), oleic acid (C18:1) (46.7 %) and linoleic acid (C18:2) (16.3 %). The other fatty acids were stearic acid (C18:0) (8.3 %), alpha-linolenic acid (C18:3) (5.9 %) and myristic acid (C14:0) (2.6 %). The fatty acid composition of R.glutinis SO28 was similar to those of other R.glutinis strains reported by previous studies [39–41]. The most prominent fatty acid of R. glutinis S028 was oleic acid. Total content of C16 and C18 fatty acids was determined as 96.8 °C. This is an expected result, since oleaginous yeasts contain mainly C16 and C18 fatty acids [7, 32, 42]. Considering the knowledge that C16 and C18 fatty acids are more suitable for biodiesel production [4, 15], it seems possible to use R. glutinis S028 biomass as a good feedstock for biodiesel production.
Conclusions
The cost of lipid production from the oleaginous yeast R. glutinis S028 could be reduced considerably using loquat kernel hydrolysate as substrate. Additional carbon, nitrogen and phosphorus sources were found to significantly affect the lipid accumulation potential of the yeast. The results of the present study also showed that lipids derived from the yeast R. glutinis SO28 using waste loquat kernels as substrate would be a promising alternative source for biodiesel production.
References
Ghaderinezhad, F., Karimina, H.R., Yaghmaei, S.: Production of biodiesel from waste frying oil using whole cell biocatalysts: optimization of effective factors. Waste Biomass Valoriz. 5, 947–954 (2014)
Vasudevan, P.T., Fu, B.: Environmentally sustainable biofuels: advances in biodiesel research. Waste Biomass Valoriz. 1, 47–63 (2010)
Demirbas, A.: Political, economic and environmental impacts of biofuels: a review. Appl. Energy 86, S108–S117 (2009)
Katre, G., Joshi, C., Khot, M., Zinjarde, S., Ravikumar, A.: Evaluation of single cell oil (SCO) from a tropical marine yeast Yarrowia lipolytica NCIM 3589 as a potential feedstock for biodiesel. AMB Expess 2, 36 (2012)
Cheirsilp, B., Louhasakul, Y.: Industrial wastes as a promising renewable source for production of microbial lipid and direct transesterification of the lipid into biodiesel. Bioresour. Technol. 142, 329–337 (2013)
Azocar, L., Ciudad, G., Heipieper, H.J., Navia, R.: Biotechnological processes for biodiesel production using alternative oils. Appl. Microbiol. Biotechnol. 88, 621–636 (2010)
Beopoulos, A., Cescut, J., Haddouche, R., Uribelarrea, J.L., Molina-Jouve, C., Nicaud, J.M.: Yarrowia lipolytica as a model for bio-oil production. Prog. Lipid Res. 48, 375–387 (2009)
Zhang, Q., Li, Y., Xia, L.: An oleaginous endophyte Bacillus subtilis HB1310 isolated from thin-shelled walnut and its utilization of cotton stalk hydrolysate for lipid production. Biotechnol. Biofuels 7, 152 (2014)
Li, C., Ju, L.K.: Conversion of wastewater organics into biodiesel feedstock through the predator-prey interactions between phagotrophic microalgae and bacteria. RSC Adv. 4, 44026–44029 (2014)
Li, C., Xiao, S., Ju, L.K.: Cultivation of phagotrophic algae with waste activated sludge as a fast approach to reclaim waste organics. Water Res. 91, 195–202 (2016)
Meng, X., Yang, J., Xu, X., Zhang, L., Nie, Q., Xian, M.: Biodiesel production from oleaginous microorganisms. Renew. Energy 34, 1–5 (2009)
Matsakas, L., Sterioti, A.A., Rova, U., Christakopoulos, P.: Use of dried sweet sorghum for the efficient production of lipids by the yeast Lipomyces starkeyi CBS 1807. Ind. Crops Prod. 62, 332–367 (2014)
Vamvakaki, A.N., Kandarakis, I., Kaminarides, S., Komaitis, M., Papanikolaou, S.: Cheese whey as a renewable substrate for microbial lipid and biomass production by Zygomycetes. Eng. Life Sci. 10, 348–360 (2010)
Economou, C.N., Aggelis, G., Pavlou, S., Vayenas, D.V.: Single cell oil production from rice hulls hydrolysate. Bioresour. Technol. 102, 9737–9742 (2011)
Karatay, S.E., Dönmez, G.: Improving the lipid accumulation properties of the yeast cells for biodiesel production using molasses. Bioresour. Technol. 101, 7988–7990 (2011)
Mast, B., Zohrens, N., Schmidl, F., Hernandez, R., French, W.T., Merkt, N., Claupein, W., Graeff-Honninger, S.: Lipid production for microbial biodiesel by the oleagenious yeast Rhodotorula glutinis using hydrolysates of wheat straw and Miscanthus as carbon sources. Waste Biomass Valoriz. 5, 955–962 (2014)
Chang, Y.H., Chang, K.S., Lee, C.F., Hsu, C.L., Huang, C.W., Jang, H.D.: Microbial lipid production by oleaginous yeast Cryptococcus sp. in the batch cultures using corncob hydrolysate as carbon source. Biomass Bioenergy 72, 95–103 (2015)
Louhasakul, Y., Cheirsilp, B., Prasertsan, P.: Valorization ofpPalm oil mill effluent into lipid and cell-bound lipase by marine yeast Yarrowia lipolytica and their application in biodiesel production. Waste Biomass Valoriz. 7, 417–426 (2016)
Taskin, M., Erdal, S., Genisel, M.: Biomass and exopolysaccharide production by Morchella esculenta in submerged culture using the extract from waste loquat (Eriobotrya japonica L.) kernels. J. Food Process. Preserv. 35, 623–630 (2011)
Taskin, M., Ortucu, S., Unver, Y., Arslan, N.P., Algur, O.F., Saghafian, A.: l-Lactic acid production by Rhizopus oryzae MBG-10 using starch-rich waste loquat kernels as substrate. Starch/Starke 65, 322–329 (2013)
Dubois, M., Giles, U.A., Hamilton, J.K., Rebers, P.A., Smith, F.: Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356 (1956)
White, T.J., Bruns, T., Lee, S., Taylor, J.: Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J. (eds.) PCR Protocols: a guide to methods and applications, pp. 315–322. Academic Press, New York (1990)
Hall, T.A.: BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98 (1999)
Zhang, Z., Schwartz, S., Wagner, L., Miller, W.: A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 7, 203–214 (2000)
Taskin, M., Saghafian, A., Aydogan, M.N., Arslan, N.P.: Microbial lipid production by cold-adapted oleaginous yeast Yarrowia lipolytica B9 in non-sterile whey medium. Biofuels Bioprod Biorefin. 9, 595–605 (2015)
Taskin, M.: A new strategy for improved glutathione production from Saccharomyces cerevisiae: use of cysteine- and glycine-rich chicken feather protein hydrolysate as a new cheap substrate. J. Sci. Food Agric. 93, 535–541 (2013)
Taskin, M., Unver, Y., Firat, A., Ortucu, S., Yildiz, M.: Sheep wool protein hydrolysate: a new peptone source for microorganisms. J. Chem. Technol. Biotechnol. 91, 1675–1680 (2016)
Yang, B.Y., Montgomery, R.: Alkaline degradation of glucose: effect of initial concentration of reactants. Carbohydr. Res. 280, 27–45 (1996)
Woo, K.S., Kim, H.Y., Hwang, I.G., Lee, S.H., Jeong, H.S.: Characteristics of the thermal degradation of glucose and maltose solutions. Prev. Nutr. Food Sci 20, 102–109 (2015)
Palmqvist, E., Hahn-Hägerdal, B.: Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresour. Technol. 74, 25–33 (2000)
Batt, C.A., Tortorello, M.Y.: Encyclopedia of Food Microbiology, 2nd edn, pp. 133–136. Academic press, Cambridge (2014)
Amaretti, A., Raimondi, S., Sala, M., Roncaglia, L., Lucia, M.D., Leonardi, A., Rossi, M.: Single cell oils of the cold-adapted oleaginous yeast Rhodotorula glacialis DBVPG 4785. Microb. Cell Fact. 9, 73 (2010)
Granger, L.M., Perlot, P., Goma, G., Pareilleux, A.: Efficiency of fatty acid synthesis by oleaginous yeasts: prediction of yield and fatty acid cell content from consumed C/N ratio by a simple method. Biotechnol. Bioeng. 42, 1151–1156 (1993)
Wu, S., Hu, C., Jin, G., Zhao, X., Zhao, Z.K.: Phosphate-limitation mediated lipid production by Rhodosporidium toruloides. Bioresour. Technol. 101, 6124–6129 (2010)
Gill, C.O., Hall, M.J., Ratledge, C.: Lipid accumulation in an oleaginous yeast (Candida 107) growing on glucose in single-stage continuous culture. Appl. Environ. Microbiol. 33, 231–239 (1977)
Granger, L.M., Perlot, P., Goma, G., Pareilleux, A.: Effect of various nutrient limitations on fatty acid production by Rhodotorula glutinis. Appl. Microbiol. Biotechnol. 38, 784–789 (1993)
Calvente, V., de Orellano, M.E., Sansone, G., Benuzzi, D.: Sanz de Tosetti, M.I.: effect of nitrogen source and pH on siderophore production by Rhodotorula strains and their application to biocontrol of phytopathogenic moulds. J. Ind. Microbiol. Biotechnol. 26, 226–229 (2001)
Cho, D.H., Chae, H.J., Kim, E.Y.: Synthesis and characterization of a novel extracellular polysaccharide by Rhodotorula glutinis. Appl. Biochem. Biotechnol. 95, 183–193 (2001)
Misra, S., Ghosh, A., Dutta, J.: Production and composition of microbial fat from Rhodotorula glutinis. J. Sci. Food Agric. 35, 59–65 (1984)
Zhang, G., French, W.T., Hernandez, R., Alley, E., Paraschivescu, M.: Effects of furfural and acetic acid on growth and lipid production from glucose and xylose by Rhodotorula glutinis. Biomass Bioenergy 35, 734–740 (2010)
Sargeant, L.A., Chuck, C.J., Donnelly, J., Bannister, C.D., Scott, R.J.: Optimizing the lipid profile, to produce either a palm oil or biodiesel substitute, by manipulation of the culture conditions for Rhodotorula glutinis. Biofuels 5, 33–43 (2014)
Gohel, H.R., Ghosh, S.K., Braganza, V.J.: Yeast as a viable and prolonged feedstock for biodiesel production. IJRER 3, 126–131 (2013)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ortucu, S., Yazici, A., Taskin, M. et al. Evaluation of Waste Loquat Kernels as Substrate for Lipid Production by Rhodotorula glutinis SO28. Waste Biomass Valor 8, 803–810 (2017). https://doi.org/10.1007/s12649-016-9615-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-016-9615-0