Abstract
Large amounts of waste materials are discarded in the petroleum exploration industry. This work investigates the incorporation of solid petroleum waste (SPW) as a raw material into a clay body, replacing natural clay material by up to 30 wt%. Ceramic pieces were produced at temperatures varying from 700 to 1,100°C. The technological properties of the clay ceramic pieces (e.g., linear shrinkage, apparent density, water absorption, and compressive strength) have been determined. Development of the sintered microstructure was followed by scanning electron microscopy (SEM) and X-ray diffraction analyses. The leaching toxicity of the fired pieces has also been determined. The results showed that the SPW could be used in clay-based ceramics (clay bricks and roofing tiles), in the range up to 30 wt%, as a partial replacement for natural clay material. The leaching concentrations of Ag, As, Ba, Cd, Cr (total), Hg, and Pb of the fired pieces met the Brazilian regulatory requirements. These results suggest that the SPW can be valorized for manufacturing clay-based ceramics, and at the same time, this application can help in reducing the environmental impacts of the petroleum exploration industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The petroleum exploration industry worldwide produces high amounts of waste materials named as oily sludges [1–3]. These oily sludges are considered hazardous waste materials and are composed of complex mixtures of hydrocarbons (oil), water, inorganic materials (solids), and traces of heavy metals. The final disposal of these oily sludges is a great challenge for the petroleum industry. In particular, over the years, the oily sludges produced by the petroleum exploration industry in Brazil have been mainly disposed in ponds, dykes, and biodegradation (land farming) [4]. Currently, most of the oily sludges produced in the water/oil separation process of offshore rigs and in the bottom of tanks have been treated with bentonite clay, resulting in a granular powdery material with sandy texture. This new waste material is referred hereafter as solid petroleum waste (SPW). A trial has been done to dispose the SPW in sanitary landfills. However, this solution has also displayed environmental and economic constraints such as an oil content of about 9%, while for disposal in landfills, an oil content <1% is required [3], a high cost of the SPW disposal in the landfill sites that are quickly reaching full capacity, and furthermore the landfill sites of urban solid wastes usually refuse to accept hazardous industrial wastes. Thus, efforts are needed to establish a definitive solution for the final disposal of hazardous wastes of the petroleum exploration industry.
One widely used method for the reuse of waste materials is their incorporation into clay ceramics for civil construction [5–9]. In fact, the clayey formulations used in the manufacture of clay ceramics (clay bricks and roofing tiles) are composed of natural raw materials with wide range of chemical and mineralogical compositional variation. For this reason, the ceramic industry is attractive for reuse of waste materials, including potentially hazardous and non-inert industrial wastes. In this way, several works have been published regarding the reuse of different oily sludges of the petroleum industry in the manufacture of clay bricks [4, 10–18]. However, in spite of the fact that SPW was produced in large amounts every year, insufficient attention was devoted to the reuse of SPW in clay bricks and roofing tiles.
In this work, SPW was characterized and its use in replacing non-renewable clay material to produce clay-based ceramics was evaluated.
Experimental Procedures
Raw materials and Mix Proportions
The raw materials used were common clay and SPW. The common clay used was provided by a local clay ceramic industry. The SPW sample was collected in the Brazilian oil company. Both samples in the form of granular powder were dried (110°C) and then sieved until a fraction passing in a 40 mesh (<425 μm ASTM) sieve, and coarser particles were rejected.
Chemical compositions of the raw materials were determined by X-ray fluorescence (XRF). The loss on ignition was determined by calculating the wt% difference between dry sample at 110°C and calcined sample at 1,000°C during 1 h.
Mineralogical analysis of the raw materials was done by X-ray diffraction (model URD-65 Diffractometer, Seifert), using monochromatic Cu-Kα radiation over non-oriented specimens. JCPDS-ICDD cards were used to identify the crystalline phases.
The particle size analysis of the raw materials was determined according to the NBR 7181-84 standard. The plastic properties were obtained through the Atterberg consistency limits (PI = LL−PL, in which PI is the plastic index, LL is the liquid limit, and PL is the plastic limit) according to the Brazilian standards (NBR 6459-84 and NBR 7180-84).
Dilatometric analysis of the raw materials were carried out on unfired test pieces with a Netzsch DIL 402C Dilatometer within the 25–1,250°C range using a heating rate of 10°C/min under air atmosphere.
Four different clay/SPW mixtures were prepared: clay without SPW (AC) and with additions of 10, 20, and 30 wt% SPW (AC10SPW, AC20SPW, and AC30SPW). The mixtures were homogenized in a cylindrical blender for 30 min. The moisture content was adjusted to 7% (moisture mass/dry mass).
Conformation and Testing of the Ceramic Pieces
Pieces in form of cylindrical disks (26.7 mm in diameter and 6 mm in height) were uniaxially pressed at 24 MPa and then dried at 110°C for 24 h. Firing was carried out at temperatures between 700 and 1,100°C, using a slow-firing cycle of total duration of 24 h (cold-to-cold). Heating and cooling rates were controlled.
The ceramic pieces were characterized in terms of linear shrinkage, water absorption, apparent density, and diametrical compressive strength. Linear shrinkage values upon drying and firing were evaluated from variation of the diameter of the specimens. Water absorption values were determined from weight differences between as-fired and water-saturated pieces (immersed in boiling water for 2 h). Apparent density was determined using the Archimedes method. The diametral compressive strength of the sintered pieces was determined by using an universal testing machine (model 5582, Instron). The crossbar speed was hold at 0.5 mm/min for all tests.
Phase and Microstructural Analysis
The crystalline phases in the fired specimens were identified by X-ray diffraction. The microstructure of the fractured surface of the specimens was observed by means of secondary electron images (SEI) using a SEM (model SSX-550, Shimadzu), at 12 kV after gold coating.
Leaching Tests
In order to assess the toxicity characteristics of the ceramic pieces fired at 700 and 1,100°C, these were subjected to leaching tests according to the Brazilian TCLP (toxicity characteristics leaching protocol) tests [19]. The fired ceramic pieces were crushed and suspended in distilled water and TCLP (acetic acid solution at pH 5.0). The content of potentially toxic metals in the filtrate of the leachate was determined by atomic emission spectrophotometry with inductively coupled plasma (ICP-AES).
Results and Discussion
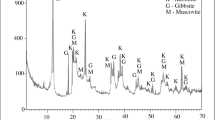
The raw materials used to prepare clay-based ceramics play an important role in the final properties and sintered microstructure. The chemical compositions of the clay, SPW, and as-mixed formulations used are summarized in Table 1. A comparison of the chemical compositions of clay and SPW shows that there are differences among them. The SPW sample has a lower alumina concentration, higher earth alkaline oxides concentration, higher barium oxide concentration, and higher loss on ignition value. In the clay sample, the dominant clay mineral, as revealed by XRD (Fig. 1), is kaolinite, with micaceous mineral as impurity. Accessory minerals are quartz, gibbsite, goethite, and microcline. The SPW sample used, as shown in Fig. 2, is a complex mixture of minerals as quartz (SiO2), barite (barium sulfate, BaSO4), calcite (calcium carbonate, CaCO3), calcium chloride (CaCl2), gypsum (calcium sulfate, CaSO4·2H2O), galena (lead sulfide, PbS), hematite (Fe2O3), halite (sodium chloride, NaCl), potassium chloride (KCl), kaolinite (2SiO2·Al2O3·2H2O), and montmorilonite [(Al1.67Na0.33Mg0.33)(Si2O5)2(OH)2]. The presence of quartz confirms that the content of SiO2 is mainly in the form of free quartz, which is probably originated from the rubble produced during the drilling step of the oil well. Barite justifies the presence of barium oxide (BaO). Although barite is a universal constituent suspended in the oceans, barite detected in XRD pattern is probably related to the drilling fluid employed in the oil extraction operation. Barite is used because its high density (4.2–4.5 g/cm3) makes it suitable for controlling hydrostatic pressure. The montmorilonite observed in SPW justifies the presences of Al2O3 and MgO. Montmorilonite is associated with bentonite clay that was used for the treatment of the crude oily sludge, which resulted in SPW. Calcite and gypsum justify the presence of CaO. Hematite found in SPW is also related to the drilling fluid and is responsible for the high content of Fe2O3. The presence of compounds such as halite, calcium chloride, and potassium chloride is also associated with drilling fluids. These compounds are added to drilling fluids as brines with the goal of carrying out diverse functions as an aqueous phase or to balance the interactions of drilling fluids with clays or soluble salts. Galena, however, is found typically in marine soils. Thus, the incorporation of SPW into clay body modifies its chemical and mineralogical compositions.
The particle size distribution curves of the raw materials are shown in Fig. 3. The granulometric data show that pure clay (<2, 2–20, and >20 μm fractions: 29, 23, and 48%) has a high concentration of fine particles. The SPW sample, however, presents a high concentration of coarse particles (<2, 2–20, and >20 μm fractions: 2, 17, and 81%), which is mainly due to the presence of minerals such as quartz and barite. The AC sample has high plasticity (Atterberg plastic index = 31%) and is located inside the acceptable molding region according to the extrusion prognosis [16]. The AC30SPW sample (Atterberg plastic index = 24%), however, moves the workability of the AC sample into the optimum molding region. Thus, SPW can be used as a sandy material that acts as plasticity reducer in clayey formulations, mainly due to its high content of quartz particles.
Dilatometric curves for the AC and SPW samples on heating from room temperature to 1,250°C are depicted in Fig. 4. The AC sample (pure clay) underwent expansion–shrinkage behavior very similar to kaolinitic clays [20]. It can be seen a steady and soft expansion (<1.3%) to below 500°C followed by three regions of active shrinkage at 500–650°C (predominates the dehydroxylation of kaolinite with concomitant loss of water) [20], 900–1,080°C (initial stage of sintering), and 1,080–1,260°C (predominates the viscous flow sintering with high densification). The SPW sample, however, shows a different firing behavior. Initially, a steady and small expansion up to ~800°C was observed, which is mainly due to thermal expansion of the solid particles of SPW, α-β quartz inversion, calcite decomposition to form CaO and CO2 degassing, and volatization of oil (hydrocarbons) of the structure. It can also be observed that only above 800°C, active shrinkage occurred. The shrinkage observed within the 830–900°C range may be related to the formation of liquid phase due to the melting of some components of SPW such as KCl (melting point = 776°C), CaCl2 (melting point = 782°C), and NaCl (melting point = 801°C). At 1,140°C, a sharp shrinkage occurs caused mainly by the onset of vitrification, leading to considerable shrinkage of the SPW sample. Thus, the incorporation of SPW into clay body can influence its sinterability and final properties of the clay ceramic pieces.
Figure 5a, b shows the XRD patterns of the AC and AC30SPW samples fired between 700 and 1,100°C. It can be seen in Fig. 5a that at 700°C, kaolinite peaks disappeared due to the transformation of kaolinite to amorphous metakaolinite. Gibbsite has likely been transformed into a transition alumina phase. It has been observed that the peaks of micaceous mineral and microcline remain up to 900°C, while the peaks of quartz are present during all firing temperatures. At 1,100°C, however, cristobalite begins to develop. It can also be observed that the quartz peaks decreases slightly at high temperature due to its partial dissolution. Primary mullite (Al2O3:SiO2 = 2:1) and hematite also were detected at 1,100°C.
Specimens containing SPW (AC30SPW sample) showed a quite different phase evolution as revealed by the XRD patterns (Fig. 5b). Quartz, gypsum (anhydrite calcium sulfate), and barite peaks can be observed during the whole firing cycle. This is important because the barite particles can act as stress concentrators for crack nucleation, one of those responsible for the decrease in mechanical strength of clay ceramics. At 700°C, quartz, barite, micaceous mineral, calcite, and gypsum peaks appear. There are also traces of halite and calcium chloride. Some of the quartz present in the specimen, however, was transformed into cristobalite at 1,100°C. Primary mullite peaks also were detected in specimens fired at 1,100°C. The incorporation of SPW into clay body, however, resulted in the decrease in the peak intensities of mullite. According to Table 1, the incorporation of SPW decreased the alumina content in the system, which in part explains the decreased primary mullite crystallization in AC30SPW sample on firing.
Figure 6a–d shows the microstructure of the fracture surfaces of pure clay specimens (AC sample) fired at different temperatures. SEM micrographs show the evolution of the sintering process with the rise of the firing temperature. At 700°C (Fig. 6a), there is only poor sintering due to solid-state reactions among adjacent particles of metakaolinite and accessory minerals, which result in slight porosity reduction. Interconnectivity between particles is limited. The presence of microscopic pores and voids is clearly visible. At 900°C (Fig. 6b), the structure is less porous, but kaolinite platelets are still observed. After firing at 1,100°C (Fig. 6c), the vitrification of the specimens can be clearly detected due to the partial fusion of the clay particles in the matrix. In this temperature range, a glassy phase starts to emerge, enables the joining of particles, and reduces the porosity of the ceramic ware. It can be observed in the AC30SPW sample fired at 1,100°C (Fig. 6d), however, that the presence of quartz and barite particles produces microcracking of the interface with the matrix during cooling. On cooling the quartz, inversion from the β-polymorph to the low-temperature α-polymorph occurs. This inversion is accompanied by residual stress formation and microcracking in the clay ceramic structure as α-quartz has a higher coefficient of thermal expansion than the surrounding glassy phase [21]. In addition to the barite and quartz particles, the combustion of the organic fraction of SPW also contributes to generate pores in the fired structure.
The quality of the ceramic pieces was evaluated by measuring four physical–mechanical properties: linear shrinkage, apparent density, water absorption, and diametral compressive strength (Figs. 7, 8, 9, 10).
The level of shrinkage is an important factor influencing the quality of clay-based ceramics. The linear shrinkage of the pieces, as shown in Fig. 7, is influenced by both firing temperature and SPW addition. The results revealed that with increase in firing temperature, the shrinkage increased. Linear shrinkage of the fired pieces, however, behaves differently below and above 900°C. This behavior is due to the predomination of distinct sintering mechanisms. Between 700 and 900°C, there is a low linear shrinkage (1.05–3.64%), in which predominates solid-state sintering mechanisms. Necks between contacting particles grow, and smoothening of the particle surface occurs. Above 900°C, the linear shrinkage accelerates, reaching a maximum (10.48%) after firing at 1,100°C. A glassy phase was formed due to the sources of flux materials. The glassy phase formation acts to densify the structure by liquid-phase sintering. The driving force for densification is the lowering of surface free energy by the elimination of interfaces. Viscous flow is the dominant sintering mechanism [22]. It can also be seen in Fig. 7 that mainly above 1,000°C, the addition of the SPW decreased the linear shrinkage of the pieces. A similar result was observed during the dilatometry assay of SPW (Fig. 4), and it could be explained by the high concentration of quartz and barite particles, which lowers the clay plasticity. Thus, the incorporation of SPW tends to improve the dimensional stability of the clay ceramic pieces.
Apparent density of the fired pieces (Fig. 8) appears to follow the same behavior as the linear shrinkage. Between 700 and 900°C, the pieces presented low density (1.62–1.78 g/cm3), mainly due to the combined inverse effects of sintering and weight loss. The green pieces, on heating up to 900°C, underwent an intense process of mass transfer, in which hydrocarbon volatization of SPW and complex physical–chemical reactions such as dehydration of hydroxides, dehydroxylation of clay minerals, and decomposition of calcite occurred. At temperatures above 900°C, however, a substantial increase in density is observed, reaching a maximum (2.21 g/cm3) after firing at 1,100°C. This behavior is related to vitrification, which results in denser ceramic pieces. For temperatures above 1,000°C, pieces containing SPW underwent a lower densification rate, which can be interpreted in terms of the high concentration of non-plastic mineral particles in the SPW sample.
Figure 9 shows the results of water absorption of the fired pieces. This physical property is related to the microstructure of the sintered ceramic matrix and determines the level of open porosity of the fired pieces. As can be observed in Fig. 9, the role of the firing temperature was to decrease the water absorption of the pieces, independently of the added SPW amount. This is caused by the open-pore partial closure. The results also showed that the addition of SPW reduced the water absorption, except on temperatures above 1,000°C. This observation is in good agreement with the sintered microstructure of pieces containing SPW (Fig. 6d). In fact, at a higher temperature (1,100°C), cooling microcracks were formed and resulted in higher value of open porosity of the ceramic pieces.
The values of water absorption are used for specification of clay-based ceramic products for civil construction. In correlation with the Brazilian specifications for water absorption (wa) of clay-based products for bricks and roofing tiles are used pieces with wa of 8–25% and <18%, respectively. According to observed results (Fig. 9) and firing temperature applied for the production of the pieces containing SPW, the ceramic products that can be obtained are dense and hollow bricks (700–1,100°C) and roofing tiles (1,000–1,100°C).
The diametral compressive strength of the fired pieces is shown in Fig. 10. The mechanical behavior is quite correlated with all the other studied properties. The effect of the firing temperature was to increase the diametral compression strength (5.76–23.16 MPa). This behavior is related to the denser structure of the clay ceramic pieces. The diametral compressive strength of the SPW-containing pieces, as shown in Fig. 10, was lower than that of the AC sample (pure clay). The data indicate a tendency toward worse mechanical strength with a higher SPW concentration as a consequence of hydrocarbons vaporization of SPW that resulted in pore generation in the fired structure and induction of flaws in the sintered ceramic matrix due to the presence of high concentrations of quartz and barite particles in SPW (Fig. 6d). Furthermore, at higher temperatures, the deleterious effect of the SPW additions on mechanical strength is more pronounced. In this case, the quartz and barite particles act as filler material that can initiate catastrophic failure reducing the mechanical strength of the fired pieces. It implies, therefore, that additions of very high SPW concentrations in clay-based ceramics should be avoided, because it impairs the mechanical strength of the pieces.
The results of the leaching tests on the ceramic pieces fired at 700 and 1,100°C used in this study are shown in Table 2. The values of the maximum concentrations accepted by the Brazilian standard [23] for the leaching extract are also shown. It was found that the leachate concentrations of Ag, As, Ba, Cd, Cr (total), Hg, and Pb are lower than the limits required by the Brazilian standard, independently of the firing temperature and added SPW amount. At higher temperature (1,100°C), however, the fired pieces had better chemical resistance against leaching, most probably due to the bounding of heavy metals ions in the glassy matrix formed at high temperature. These results indicate that the produced pieces could be taken as non-hazardous materials. This is considered to be a major advantage for employing SPW as an alternative raw material in the manufacture of clay-based ceramic products.
Conclusions
The following conclusions may be drawn form the experimental results and their discussion.
This study has demonstrated that solid petroleum waste (SPW) could be used as an alternative raw material for partial replacement to natural clay in the production of clay-based ceramics. The obtained results indicated that the composition of used SPW sample is chemically rich in SiO2, Al2O3, Fe2O3, MgO, CaO, and BaO, which are usually present in the raw materials used in the manufacture of clay-based ceramics. The SPW powder behaved as a non-plastic material.
Up to 30 wt% of SPW can be incorporated into clay ceramic formulations to result in good final properties. Results showed that specifications for dense and hollow bricks have been achieved for sintering between 700 and 1,100°C, whereas roofing tiles between 1,000 and 1,100°C.
The leaching tests of the clay ceramic pieces incorporated with SPW showed that they do not cause any serious environmental impact.
It is feasible to valorize SPW for the production of clay-based ceramics. The replacement of natural clay with SPW, in the range up to 30 wt%, allows the production of clay-based ceramics (hollow bricks and roofing tiles) with properties compatible with those specified in the Brazilian standardization. This is important because the valorization of the referred waste in ceramic bodies can be a technological solution to the negative environmental impact caused by the petroleum industry.
References
Curran, L.M.: Waste minimization practices in the petroleum refining industry. J. Hazard. Mater. 29, 189–197 (1992)
Elektorowicz, M., Habibi, S.: Sustainable waste management: recovery of fuels from petroleum sludge. Can. J. Civ. Eng. 32, 164–169 (2005)
Andrade, P.F., Azevedo, T.F., Gimenez, I.F., Filho, A.G.S., Barreto, L.S.: Conductive carbon-clay nanocomposites from petroleum oily sludge. J. Hazard. Mater. 167, 879–884 (2009)
Amaral, S.P., Domingues, G.H.: Application of oily sludge for manufacturing of ceramic materials. In: Proceedings of 4th Brazilian Congress on Petroleum, Rio de Janeiro, Brazil (1990)
Dondi, M., Marsigli, M., Fabbri, B.: Recycling of industrial and urban wastes in brick production: a review. Tile Brick Int 13, 218–225 (1997)
Segadães, A.M.: Use of phase diagrams to guide ceramic production from wastes. Adv. Appl. Ceram. 105, 46–54 (2006)
Lin, K.: Use of thin film transistor liquid crystal display (TFT-LCD) waste glass in the production of ceramic tiles. J. Hazard. Mater. 148, 91–97 (2007)
Chiang, K., Chien, K., Hwang, S.: Study on the characteristics of building bricks produced from reservoir sediment. J. Hazard. Mater. 159, 499–504 (2008)
Souza, A.J., Pinheiro, B.C.A., Holanda, J.N.F.: Recycling of gneiss rock waste in the manufacture of vitrified floor tiles. J. Environ. Manag. 91, 685–689 (2010)
Meejoda, N.J., Huang, D.R., Dubose, B.H., Chen, Y., Chuang, K.Y.: Use of petroleum-contaminated soils in construction material production. New Jersey Department of Environmental protection and Energy Technical, Report No. 2-1993 (1993)
Saikia, N.J., Sengupta, P., Dutta, D.K., Saikia, P.C., Borthakur, P.: Oil field sludge used to make brick. Am. Ceram. Soc. Bull. 79, 71–74 (2000)
Taha, R., Ba-Omar, M., Pillay, A.E., Roos, G., Al-Hamdi, A.: Recycling of petroleum-contaminated sand. J. Environ. Monit. 3, 417–420 (2001)
Sengupta, P., Saikia, N., Borthakur, P.C.: Bricks from petroleum effluent treatment plant sludge: properties and environmental characteristics. J. Environ. Eng. 128, 1090–1094 (2002)
Souza, G.P., Holanda, J.N.F.: Densification behaviour of petroleum waste bearing clay-based ceramic bodies. Ceram. Int. 30, 99–104 (2004)
Alves, M.R.F.V., Holanda, F.S.R.: Reciclagem de borra oleosa através de incorporação em blocos cerâmicos de vedação. Cerâm. Ind. 10, 41–46 (2005)
Monteiro, S.N., Vieira, C.M.F., Ribeiro, M.M., Silva, F.A.N.: Red ceramic industrial products incorporated with oily wastes. Constr. Build. Mater. 21, 2007–2011 (2007)
Acchar, W., Rulf, B.M., Segadães, A.M.: Effect of the incorporation of a spent catalyst reject from the petroleum industry. Appl. Clay Sci. 42, 657–660 (2009)
Pinheiro, B.C.A., Holanda, J.N.F.: Processing of red ceramic incorporated with encapsulated petroleum waste. J. Mater. Process. Technol. 209, 5606–5610 (2009)
Brazilian Association of Technical Standards: Solid Wastes—Leaching Test. NBR 10005/04, Rio de Janeiro (2004)
Souza, G.P., Sanchez, R., Holanda, J.N.F.: Thermal and structural characterization of Brazilian south-eastern kaolinitic clays. J. Therm. Anal. Calorim. 73, 293–305 (2003)
Souza, G.P., Messer, P.F., Lee, W.E.: Effect of varying quartz size and firing atmosphere on densification of Brazilian clay-based stoneware. J. Am. Ceram. Soc. 89, 1993–2002 (2006)
Reed, J.S.: Principles of ceramic processing, 2nd edn. Wiley Interscience, New York (1995)
Brazilian Association of Technical Standards: Solid Wastes—Classification. NBR 10004/04, Rio de Janeiro (2004)
Acknowledgments
The authors would like to thank National Council for Scientific and Technological Development (CNPq) and Foundation for Research of the State of Rio de Janeiro (FAPERJ) for the financial support. Authors also would like to thank Brazilian Oil Company (PETROBRAS) for the supply of SPW.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Souza, A.J., Pinheiro, B.C.A. & Holanda, J.N.F. Valorization of Solid Petroleum Waste as a Potential Raw Material for Clay-Based Ceramics. Waste Biomass Valor 2, 381–388 (2011). https://doi.org/10.1007/s12649-011-9090-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-011-9090-6