Abstract
Photon buildup for some SrSO4, BaSO4, MgSO4, MnSO4, FeSO4 and ZnSO4 thermoluminescent dosimetric (TLD) compounds was investigated in the present work. Photon energy absorption buildup factors and photon exposure build factors were computed for the TLD compounds using the five-parameter geometric progression fitting method in energy range 0.015–15 MeV for penetration depths up to 40 mean free path. The buildup factors were studied as a function of photon energy, penetration depth and chemical compositions. Effective atomic numbers and air-kerma for the TLD compounds were calculated and ICRU standard tissue equivalence was discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
There are several reports on mass attenuation coefficients, effective atomic numbers and effective electron densities for thermoluminescent dosimetric materials [1–4]. Extensive studies on thermoluminescent characteristics and luminescence of MgSO4: Dy has been reported [5, 6]. A large difference in effective atomic numbers of FeSO4, thermoluminescent and phantom materials has been noted at particular energies [7]. Recently, some novel thermoluminescent radiation dosimetric materials have been discovered [8].
The intensity of X-/γ-ray beam through a medium follows Lambert’s Beer law;
where I and I 0 are transmitted and initial photon densities, µ is linear attenuation coefficient, and t is the thickness of medium. However, a buildup factor, B is required for broad beam, polychromatic and thick materials. Now the modified Eq. (1) is given as;
which include the buildup factors, B. Various researchers have used the G-P fitting method to determine the photon buildup factor for thermoluminescent dosimetric [9, 10], heavy metal oxide glasses [11], human teeth [12], polymers [13], planet samples [14], dosimetric materials [15], gel dosimeters [16], nuclear track detectors [17], and superconductors [18] etc. These investigations on buildup factors for the low- and high-Z elements containing materials show that the G-P fitting method is useful technique for low as well as high-Z element materials.
The energy absorption buildup factors (EABF), exposure buildup factors (EBF) and air-kerma for some sulfate-based thermoluminescent dosimetric(TLD) compounds such as SrSO4, BaSO4, MgSO4, MnSO4, FeSO4 and ZnSO4 are not available in the literatures. Therefore, in the present work, we have investigated the photon buildup for these compounds. The effective atomic numbers for few human organs, breast, cortical bone and soft tissue have been also calculated and compared with the selected TLD compounds. The buildup factors have been calculated using the G-P fitting method at photon energies 0.015–15 MeV for penetration depths up to 40 mean free path (mfp).
The dose rate from anisotropic point source is given by
where D is dose rate (unit of dose rate), S 0 is point source of gamma-ray (photon/s), B is buildup factor (dimensionless), µ is linear attenuation coefficient (length−1), x is thickness of shield, K is conversion factor for gamma-ray to dose rate, and r is distance from source to receptor (unit of length). The attenuation of photon is accomplished by interaction processes. The photoelectric absorption completely removes the photon, pair production remove and regenerate the photons. However, in Compton scattering process the photon interacts with the electrons and loses some part of energy. Therefore, some photons reach to the receptor by penetrating the shield which is counted as lost in the calculation of attenuation coefficient. The concept of buildup factor in dose evaluation is introduced from here. After introduction of buildup of photon in the interacting medium, the buildup factor is becoming an essential parameter for exposure and energy absorption in the field of health physics, medical physics and radiation physics for shielding and dosimetry.
2 Computational method
2.1 Effective atomic numbers
The mass attenuation coefficients for the TLD compounds were estimated using mixture rule as
where w i is the proportion by weight and (μ/ρ) i is mass attenuation coefficient of the ith element. The quantity w i is given by
with the condition ∑ n i w i = 1, where A i is the atomic weight of the ith element and n i is the number of formula units in the compounds. The µ/ρ value of individual element was taken from user-friendly Windows-based WinXcom program [19].
The effective atomic numbers for the TLD compounds were calculated using effective atomic cross section and electronic cross section. The details of the calculation procedures for σ a and σ e are given below. The total atomic cross sections (σ t ) for the compounds are obtained from the total mass attenuation coefficients, μ m values using the following relation;
where M = ∑ n i n i A i is the molecular weight of the compound and N A is the Avogadro’s number. The effective atomic cross section (σ a ) is calculated using the following equation:
The effective electronic cross section (σ e ) is calculated using the following equation;
where \( f_{i} = \frac{{n_{i} }}{{\sum\nolimits_{i} {n_{i} } }} \) denotes the mole fraction or fractional abundance of element i with respect to the number of atoms such that ∑ n i f i = 1, Z i is the atomic number of ith element. The effective atomic number Z eff of a compound is given as by the following relation;
2.2 Buildup factors
The ANSI/ANS-6.4.3, 1991 report [20] is published by the American Nuclear Society; a compilation of buildup factors at energies 0.015–15 MeV, and for penetration depths up to 40 mean free paths (mfp) for the elements Z = 4–92.Footnote 1 Harima et al. [24] have developed a five-parameter fitting formula, called geometric progression (G-P), which gives the buildup factors for compounds and mixtures, can be referred also the historical review by Harima [25]. The buildup factors B (E, x) are calculated from the following equations [25]:
where E is the source energy, x is the penetration depth in units of mfp, and a, b, c, d and X K are the G-P fitting parameters.
The buildup factor of a given compound can be calculated by using the detailed procedure found else elsewhere [12–18]. The equivalent atomic numbers and G-P fitting parameters for the TLD compounds are given in Tables 1, 2, 3, 4, 5, 6 and 7, respectively.
The mfp parameter, or the relaxation length is reciprocal of linear attenuation coefficients (x = 1/µ), where μ is linear attenuation coefficient (cm−1). The mfp is the average distance of a single particle travels through a given material or compound before interacting with another material and mfp is the depth at which a fraction, 1/e (~37 %), of a large homogeneous population of particles in a beam can penetrate.
2.3 Air-kerma
Air-kinetic energy released per unit mass (kerma) is defined as the initial kinetic energy of all secondary charged particles liberated per unit mass at a point of interest by uncharged radiation [26]. The computation of air-kerma is calculation of mass energy absorption coefficient, µ en/ρ of a compound or mixture to the air. The values of μ en/ρ of the elements are given in the compilation of Hubbell and Seltzer report [27].
3 Standardization of method
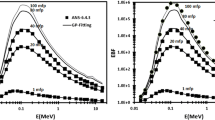
The present G-P fitting method has been compared by calculating the buildup factors for water (low-Z) and lead (high-Z) in the energy range of 0.015–15 MeV for selected penetration depth up to 40 mfp. The results obtained are compared with the ANSI/ANS-6.4.3, 1991 [20] standard the energy absorption buildup factors and the exposure buildup factors for randomly selected penetration depths up to energies 15 MeV. As shown in Figs. 1, 2 and 3, the energy absorption buildup factors and the exposure buildup factors computed using the present G-P fitting method are in good agreement with the ANSI/ANS-6.4.3, 1991 standard data except at K-edge for lead. The differences in buildup factors for lead are slightly higher than water but insignificant. This provides confidence in our results obtained for TLD compounds.
4 Results and discussion
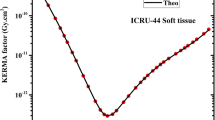
The effective atomic numbers for the selected TLD compounds along with ICRU tissues is shown in Fig. 4. The energy absorption buildup factors (EABF) and the exposure buildup factors (EBF) for the TLD compounds are shown in Figs. 5(a)–5(f) and 6(a)–6(f), respectively. The EABF and EBF for the TLD compounds are plotted graphically for photon energy 0.015–15 MeV up to penetration depth 40 mfp. The air-kerma for the TLD compounds is shown in Fig. 7 for photon energies 1 keV–15 MeV. The EABF and EBF for the TLD compounds are discussed for photon energy, penetration depth and chemical composition dependency.
4.1 Effective atomic numbers of TLD compounds
The variation of effective atomic number (Z eff) for the TLD compounds in energy range 1 keV–15 MeV is shown in Fig. 4. In Fig. 4, Z eff for some tissues like breast, cortical bone and soft tissue [28] are also shown. The Z eff for MgSO4 is found to be the lowest among the selected TLD compounds, which is comparable with the values of Z eff for the cortical bone. It is observed that the Z eff values for BaSO4 are the highest in the selected region of interested energy. The Z eff values for all the TLD compounds are <20 in the Compton scattering region. The Z eff values for the TLD compounds in Compton scattering region at photon energy 1 MeV are found to be 14.47, 18.07, 10, 12.19, 12.36 and 13.05 for SrSO4, BaSO4, MgSO4, MnSO4, FeSO4 and ZnSO4, respectively. A very high peak of 53.29 is observed for BaSO4 at near K-edge of Ba. Since the Z eff values for MgSO4 are nearer to cortical bone which resembles that the photon interaction characteristics of each other; therefore, it can be considered suitable for dose monitoring.
4.2 Energy absorption buildup factor and exposure buildup factor of TLD compounds
The variation trend of the buildup factors is observed similar up to photon energy of 3 MeV. In low energy, the buildup factors are small because the photons are completely removed through absorption by photoelectric absorption, gradually increase with the energy due to multiple scattering as dominance of Compton scattering in the intermediate-energy and finally again reduces in high-energy region as dominance of pair production as shown in Fig. 5(a)–5(f). In high-energy photon region (>3 MeV) another absorption process, pair/triplet production dominates in bringing down EABF and EBF. The EABF and EBF for BaSO4 (having highest Z eff) are found to be the lowest among the selected the TLD compounds. The EABF for the TLD compounds are found to be 31.44, 587.34, 9.96, 14.13, 14.72 and 18.11 for SrSO4, BaSO4, MgSO4, MnSO4, FeSO4 and ZnSO4, respectively, for penetration depth 40 mfp at energy 15 MeV. The EBF for the TLD compounds are found to be 37.18, 1094.10, 10.64, 17.33, 22.80 and 26.68 for SrSO4, BaSO4, MgSO4, MnSO4, FeSO4 and ZnSO4, respectively, for penetration depth 40 mfp at energy 15 MeV.
The variation of EABF and EBF for the TLD compounds with penetration depths are of very importance in the field of dosimetry. It is found that the EABF and EBF for the TLD compounds increase with the penetration depths. It is to be noted that difference in buildup factors of TLD compounds increases with decrease in energy. The buildup factors for BaSO4 are observed to be the lowest in low and intermediate energies among all the selected TLD compounds, whereas it shows increasing trend in high energies different from the other compounds.
In general the EABF and EBF for all the TLD compounds decrease with increase in penetration depth and photon energy, whereas BaSO4 shows reverse behavior. This can be explained using the partial photon interaction processes namely the photoelectric effect, the Compton scattering and the pair production. In low energy, the small values of buildup factors are due to dominance of photoelectric effect which results in the fast removal photons thereby not allowing these photons to buildup in the medium. The EABF and EBF at 0.015 MeV energy are roughly constant (unity) and for all the penetration depths.
The Z eq for TLD compounds plays the similar roll of a composite material as Z of an element. Therefore, it can be explained that the EABF and EBF of the TLD compounds are minimum in low energy due to dominance of photoelectric effect as the interaction cross section is directly proportional to Z 4−5eq . With increase in photon energy, buildup factors increase due to multiple scattering as Compton scattering dominates with Z eq. In high-energy region (>3 MeV), pair production takes over the Compton scattering process as dependent upon Z 2eq . The reason for large EABF and EBF for BaSO4 above 3 MeV is the dominance of pair production due to the highest Z eq. In the pair production, the electron–positron pairs generate and buildup in large penetration as well as originate the secondary photons (0.511 MeV) by annihilation of positron with electron at rest. These secondary photons may escape from the TLD compounds of low penetration depth, whereas buildup for large penetration depths.
4.3 Air-kerma of TLD compounds
The variation of air-kerma of the TLD compounds for photon energy 1 keV to 15 MeV is shown in Fig. 7. It is to be noted that the air-kerma values for MgSO4 are the lowest among all the TLD compounds. We have found that the air-kerma values for all the TLD compounds reach to unity for photon energy region 1–3 MeV. The air-kerma values for MgSO4 are in range of 0.91–4.62 in the photon energy range from 1 to 150 keV and above 150 keV, it is in range of unity. The air-kerma values for all the TLD compounds vary due to dominance of photoelectric effect in photon energy region 1–150 keV. It is observed that the air-kerma values for TLD compounds are independent upon chemical compositions in the photon energy range of 800 keV to 4 MeV.
5 Conclusions
The photon interaction for some thermoluminescent dosimetric compounds has been studied for effective atomic numbers, energy absorption buildup factors, exposure buildup factors and air-kerma. The G-P fitting method has been used for calculation of the buildup factors in the energy range 0.015–15 MeV, penetration depth up to 40 mfp. The effective atomic numbers for the TLD compounds are found to be higher than the tissue substitutes.
References
P Önder, A Tursucu, D Demir and A Gürol Nucl. Inst. Methods Phys. Res. B 292 1 (2012)
S Gowda, S Krishnaveni, T Yashoda, T K Umesh and G Ramakrishna Pramana 63 529 (2004)
S B Kaginelli, T Rajeshwari, Sharanabasappa, B R Kerur and A S Kumar Med. Phys. 34 176 (2009)
R Singh, M K Dhasmana and R B S Rawat Int. J. Eng. Sci. 2 16 (2013)
D Luo, C Zhang, Z Deng, L Guozhen Radiat. Meas. 30 59 (1999)
R S Kher, A K Upadhyay, S J Dhoble and M S K Khokhar Indian J. Pure Appl. Phys. 46 607 (2008)
I O Olarinoye Res. J. Chem. Sci. 1(2) 64 (2011)
A H Khalid, B M Nadjima, R Z Nurul, M N Roslan, M A Yusoff and D A Bradley Appl. Radiat. Isot. 82126 (2013).
S R Manohara, S M Hanagodimath and L Gerward Radiat. Phys. Chem. 79 575 (2010)
N Kucuk, S R Manohara, S M Hanagodimath and L Gerward Radiat. Phys. Chem. 86 10–22 (2013)
S R Manohara, S M Hanagodimath, L Gerward and K C Mittal J. Korean Phys. Soc. 59 2039 (2011)
M Kurudirek and S Topcuoglu Nucl. Inst. Methods Phys. Res. Sect. B: Beam Interact. Mater. Atoms 269 1071 (2011)
M Kurudirek and Y Özdemir J. Radiol. Prot. 31 117 (2011)
M Kurudirek, B Dogan, Y Özdemir, A C Moreira and C R Appoloni Radiat. Phys. Chem. 80 235 (2011)
K S Mann, M Kurudirek and G S Sidhu Appl. Radiat. Isot. 70 681 (2012)
V P Singh and N M Badiger Radiat. Phys. Chem. 103 234 (2014)
V P Singh, M E Medhat and N M Badiger Radiat. Phys. Chem. 106 83 (2015)
V P Singh, M E Medhat, N M Badiger and A Z M S Rahman Radiat. Phys. Chem. 106 175 (2015)
L Gerward, N Guilbert, K B Jensen and H Levring Radiat. Phys. Chem. 71 653 (2004)
ANS Gamma ray attenuation coefficient and buildup factors for engineering materials. ANSI/ANS-6.4.3 (American Nuclear Society. La Grange Park, Illinois) (1991)
J C Ryman et al. Trans. Am. Nucl. Soc. 99 613 (2008)
L P Ruggieri and C E Sanders Trans. Am. Nucl. Soc. 99 618 (2008)
D Luis Update to ANSI/ANS-6.4.3-1991 for low-Z materials and compound materials and review of particle transport theory’ (UNLV, Las Vegas) NV 89154 (2009)
Y Harima, Y Sakamoto, S Tanka and M Kawai. Nucl. Sci. Eng. 94 24 (1986)
Y Harima Radiat. Phys. Chem. 41 631 (1993)
ICRU Radiation Quantities and Units Report 33 of the International Commission on Radiation Units and Measurements (Bethesda, MD) (1980)
J H Hubbell and S M Seltzer. Tables of X-ray mass attenuation coefficients and mass energy absorption coefficients from 1 keV to 20 MeV for elements Z = 1 to 92 and 48 additional substances of dosimetric interest, Available at http://www.nist.gov/pml/data/xraycoef. Accessed on 22 Aug 2014 (1995)
ICRU Tissue substitutes in radiation dosimetry and measurement Report 44 of the International Commission on Radiation Units and Measurements, (Bethesda, MD) (1989)
Acknowledgments
Authors would like to thank Prof. L. Gerward, Department of Physics, Technical University of Denmark for providing WinXcom program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, V.P., Badiger, N.M. Studies on photon buildup for some thermoluminescent dosimetric compounds. Indian J Phys 90, 259–269 (2016). https://doi.org/10.1007/s12648-015-0756-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12648-015-0756-y