Abstract
Ferrite sample of composition Li0.45 Mn0.1Fe2.45O4 was prepared using the auto-combustion method. The sample was sintered at three different temperatures of 1080, 1032 and 984 °C. Spinel structure of the prepared ferrite sample was confirmed from the X-ray diffraction (XRD) technique. From the analysis of the XRD data, parameters such as density and crystallite size were calculated. The theoretical and the experimental densities were observed to increase with the increase in the sintering temperature. The crystallite size for the samples was in the nanometer range. Dielectric constant 10 KHz and dc resistivity at room temperature were investigated. The dielectric constant showed a decrease while the dc resistivity increased with the rise in the sintering temperature. Dispersion was observed in the variation of the dielectric constant with frequency. The Curie temperature showed a decrease. Possible reason for all the above observation was discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently there are considerable interests on the structure and various properties of ferrite and related materials [1–5]. Lithium and substituted lithium ferrite is one among them [6]. They are found useful in certain device like transformer, microwave devices, electronic and magnetic components etc. [7–9]. This is due to their interesting properties like high Curie temperature, saturation magnetization, resistivity, good hysteresis properties etc. [10, 11]. These properties can be tailored by changing the substitution, synthesis method or sintering conditions. There are various types of synthesis method like double sintering ceramic method, sol–gel, hydrothermal, chemical co-precipitation etc. [12, 13]. The auto combustion synthesis method is generally employed. This is because it is simple, economic and produces materials with enhanced properties. Moreover, it requires lower sintering temperature to obtain the required ferrite material [14, 15]. It is generally learned that the lithium volatilizes at high temperature as oxides which give rise to differing characteristic properties. It is, therefore, important to use the low temperature synthesis method. However, certain change is observed by the effect of sintering on the various materials.
In the present study the effect of sintering temperature on the structural, electrical and magnetic properties of auto combustion prepared lithium manganese ferrite has been studied.
2 Experimental details
Lithium manganese ferrites with compositional formula Li0.45Fe2.45Mn0.1O4 was prepared by the citrate precursor method, using chemicals lithium nitrate, manganese acetate, iron nitrate and citric acid. Stoichiometric amount of lithium nitrate, manganese acetate, iron nitrate and citric acid were mixed to make a solution. The ratio of metal cations to citric acid is 1:1. The synthesis method had been reported earlier [16]. The synthesized powder was mixed with polyvinyl alcohol as binder and pressed into pellets with 50 kilo Newton pressure. The samples was given sintering in air at three different temperatures of 984 °C, 1032 °C and 1080 °C for 3 h. From the X-ray diffraction (XRD) data, the theoretical density and crystallite size were calculated. Archimedes’s principle was used to measure the experimental density. Dielectric constant at room temperature was studied as a function of sintering temperature and frequency (20 Hz–1 MHz). The measurement was carried out using an Agilent HP 4284A LCR meter and the value of dielectric constant was calculated using the formula [17]
where C is the measured capacitance, d the thickness, A the cross sectional area of the sample and ε0 the permittivity of free space. The dc resistivity at room temperature was measured using four probe method. The Curie temperature was measured using the Soohoo’s method [18].
3 Results and discussion
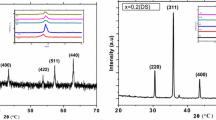
Spinel phase structure of the Li–Mn ferrite sample was confirmed from the XRD pattern as shown in (Fig. 1). All the peaks could be indexed to the standard pattern reported by the Joint Committee on Powder Diffraction Standards (JCPDS). There is no extra peak observed signifying that there is no impurity present.
The densities of the samples as measured experimentally and those calculated from the XRD data are tabulated in Table 1. An increase is observed with the increase in sintering temperatures. These results are in agreement with the observations of previous workers [19, 20]. The porosity is in the range of 24–30 % and shows a decreasing trend with increase in the sintering temperature. It has been calculated using the formula
where \( \rho_{theo} \) is theoretical or XRD density and \( \rho_{\text{expt}} \) is experimental density. The increase in densification may result in shrinking of pores and hence a decrease in the porosity is expected. The crystallite size as calculated from the XRD data using Debye–Scherrer formula is given by crystallite size = 0.9λ/β cosθ where λ is wavelength, β is full width at half maximum. It shows an increasing trend with the increase in temperature. This is because the increase in density increases slowly the crystallite size [21, 22].
The variation of the dc resistivity and dielectric constant at room temperature with sintering temperature is shown in Fig. 2. It is observed that the resistivity increases while the dielectric constant decreases with the increase in the sintering temperature. The decrease in the dielectric constant can be explained on the basis of space charge polarization and Koop’s two layer model, where the ferrite is assumed to be made up of well conducting grains separated by poor conducting layers or grain boundaries [23, 24]. The electrical conduction in ferrite is explained by the Verwey mechanism of electron hopping where conduction takes place by hopping of electrons between Fe2+ and Fe3+ ions at B sites. The electrons reach the grain boundary through hopping and due to its higher resistivity, the electrons pile up, thereby producing space charge polarization. It is learn that the increase in sintering temperature leads to the formation of Fe2+ ion due to volatilization [8]. However, in the present study there may be no loss of the lithium ions, hence the number of Fe2+ ion formation in B site does not increase. It is observed in various reported works that there is a decrease of charge carrier mobility with the increase in sintering temperature [23–25]. This is due to the fact that oxidation in air at the time of sintering increases the amount of Fe3+ ions at B site suppressing the exchange of electrons. Thus with the increase in sintering temperature a decrease in the dielectric constant is observed.
The frequency dependence of dielectric constant is shown in Fig. 3. It is observed that the dielectric constant shows dispersion with increasing frequency. This is a normal behavior of ferrites. The value of dielectric constant is high at low frequencies, and then rapidly decreases with increase in the frequency. Similar observation has been reported by several workers [26–28]. The observed decrease in dielectric constant with frequency can be explained in terms of the space charge polarization and Koop’s two layer model as has been mentioned. At low frequency of applied field the high resistivity grain boundary hinders the hopping motion of electrons creating space charge polarization, leading to a high dielectric constant. As the frequency of the applied field is increased the electronic exchange is not able to follow the alternating field and the electrons reverse the direction of motion thus, decreasing the probability of electrons reaching the grain boundary. This leads to a decrease in the value of dielectric constant. At still higher frequency, the polarisability is very small and becomes independent of frequency [23].
The dc resistivity at room temperature is observed to increase with the increase in the sintering temperature which conforms to that of dielectric constant measurement.
The measured Curie temperature obtained for different sintering temperature are shown in Table 1. It is observed in the table that the Curie temperature decreases with increase in sintering temperature. This may be due to weakening of the magnetic exchange interaction with the increase in the sintering temperature.
4 Conclusions
Lithium manganese ferrite has been successfully prepared by the auto-combustion method. The increase in sintering temperature gives more densification while the porosity decreases. The dielectric constant at room temperature decreases with increase in sintering temperature. Dispersion behaviour is observed for the variation of dielectric constant with frequency. The dielectric constant is high at low frequency and decreases with the increase in the frequency. The dc resistivity at room temperature shows an increase with the increase in sintering temperature. The Curie temperature is observed to decrease with the increase in the sintering temperature.
References
K B Modi, T K Pathak, N H Vasoya, V K Lakhani, G J Baldha and P K Jha Indian J. Phys. 85 411 (2011)
L N Borah, Sanjay and A Pandey Indian J. Phys. 84 699 (2010)
B N Dash, P Dash, H Rath, P Mallick, R Biswal, P K Kulriya and N C Mishra Indian J. Phys. 84 1315 (2010)
L A Najam, N Y Jamil and R M Yousif Indian J. Phys. 86 267 (2012)
N C Pop and O F Căltun Indian J. Phys. 86 283 (2012)
N K Saxena, N Kumar and P K S Pourush Indian J. Phys. 84 1193 (2010)
A G Fox, S E Miller and M T Weiss Bell Syst. Tech. J. 34 5 (1955)
J Smit and H P J Wijn Ferrites (Eindhoven: Phillips Technical Library) (1959)
P D Baba, G M Argentina, W E Courtney, G F Dionne and D H Temme IEEE Trans. Magn. 8 83 (1972)
C Kuroda and T Kawashima IEEE Trans. Magn. 3 192 (1969)
C E Patton, C A Edmondson and Y H Liu J. Appl. Phys. 53 2431 (1982)
G S Gopalakrishna, M J Mahesh, K G Ashamanjari, M S Bhargava Ramu and K Milina Indian J. Phys. 84 143 (2010)
J Manam, S Das and A Isaac Indian J. Phys. 83 1407 (2009)
M Pechini US Patent 3909327 (1975)
M Galceran, M C Pujol, M Aguilo and F Diaz J. Sol-Gel Sci. Technol. 42 79 (2007)
I Soibam, S Phanjoubam, H B Sharma and H N K Sarma Indian J. Phys. 82 611 (2008)
L L Hench and J K West Principles of Electronics Ceramics (New York: Wiley) (1990)
R F Soohoo Theory and Application of Ferrites (Englewood Cliffs: Prentice Hall) (1960)
A Dias, N D S Mohallen and R L Moreira Mater. Res. Bull. 33 475 (1998)
S S Jadhav, S E Shirsath, B G Toksha, D R Shengule and K M Jadhav J. Optoelectron. Adv. Mater. 10 2644 (2008)
M N Rahman Ceramic Processing and Sintering (New York: Marcel Dekkar) (1995)
T K Gupta J. Am. Ceram. Soc. 55 276 (1972)
C G Koops Phys. Rev. 83 121 (1951)
I Soibam, S Phanjoubam, H B Sharma, H N K Sarma and C Prakash Indian J. Phys. 83 285 (2009)
A Tawfik and M M Barakat J. Mater. Sci. Lett. 7 1098 (1988)
C Shivaji, S Phanjoubam, H N K Sarma, R Laishram and C Prakash Mod. Phys. Lett. B 19 899 (2005)
R K Kotnala, V Verma, V Pandey, V P S Awana, R P Aloysius and P C Kothari Solid State Commun. 143 527 (2007)
I Soibam, S Phanjoubam, H B Sharma, H N K Sarma, R Laishram and C Prakash Solid State Commun. 148 399 (2008)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soibam, I., Phanjoubam, S. Effect of sintering temperature on Li0.45Fe2.45Mn0.1O4 ferrite. Indian J Phys 87, 121–124 (2013). https://doi.org/10.1007/s12648-012-0194-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12648-012-0194-z