Abstract
We investigated redox homeostasis in cerebral and peripheral tissues of wild type (WT) and glutaryl-CoA dehydrogenase knockout mice (Gcdh−/−) submitted to inflammation induced by lipopolysaccharide (LPS) since patients with glutaric aciduria type I (GA I) manifest acute encephalopathy during catabolic events triggered by inflammation. WT and Gcdh−/− mice fed a low (0.9%) or high (4.7%) Lys chow were euthanized 4 h after LPS intraperitoneal injection. Cerebral cortex of Lys-restricted Gcdh−/− animals presented no alterations of redox homeostasis, whereas those fed a high Lys chow showed increased malondialdehyde (MDA) levels and superoxide dismutase (SOD) activity, compared to WT mice. Furthermore, Gcdh−/− mice receiving low Lys and injected with LPS presented elevated MDA levels and decreased reduced glutathione (GSH) concentrations, glutathione peroxidase (GPx), and glutathione reductase (GR) activities in cerebral cortex. LPS administration also decreased GSH values, as well as GPx and GR activities in cerebral cortex of Gcdh−/− mice receiving Lys overload. Further experiments performed in WT and Gcdh−/− mice injected with LPS and receiving either a low or high Lys chow revealed increased MDA levels and decreased GSH concentrations in cerebral cortex and striatum, but not in hippocampus, liver and heart of Gcdh−/− mice, suggesting a selective vulnerability of these cerebral structures to oxidative stress during an inflammatory process. LPS administration also increased S100B and NF-κF protein levels in brain of Gcdh−/− mice receiving high Lys. These data support the hypothesis that low Lys diet is beneficial in GA I by preventing redox imbalance, whereas a high Lys diet or systemic inflammation per se or combined induce oxidative stress in striatum and cerebral cortex that are mainly damaged in this disorder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glutaric aciduria type I (GA I, McKusick 23167; MIM #231670) is an inborn error of metabolism first described in 1975 (Goodman et al. 1975; Goodman and Kohlhoff 1975). It is caused by deficiency of the mitochondrial enzyme glutaryl-CoA dehydrogenase activity (GCDH; EC 1.3.99.7), which is involved in the catabolic pathway of lysine (Lys), hydroxylysine, and tryptophan. GCDH activity reduction leads to predominant accumulation of glutaric (GA) and 3-hydroxyglutaric (3HGA) acids in tissues, mostly brain, and biological fluids of affected patients (Goodman and Frerman 2014). The estimated worldwide frequency of GA I is 1:30,000 to 1:100,000 newborns, being one of the most prevalent organic acidurias (Wajner 2019).
Clinical symptomatology includes mild developmental delay and hypotonia in infancy and becomes much worse after encephalopathic crises that are commonly triggered by infections or immunizations (vaccine) and associated with a pro-inflammatory state (Goodman and Frerman 2014; Kölker et al. 2006). During these crises, severe symptoms such as seizures and coma that are followed by dystonia, dyskinesia, and spasticity due to bilateral striatum degeneration develop in 80–90% of untreated patients. Chronically progressive cerebral cortex and striatum abnormalities whose pathomechanisms are poorly established have been also reported in patients affected by GA I (Harting et al. 2009, 2015). Furthermore, the devastating acute basal ganglia degeneration characteristically seen in this disorder during encephalopathic crises is associated with inflammatory processes (Kölker et al. 2006; Jafari et al. 2011).
Treatment based on Lys dietary restriction and L-carnitine supplementation prevents these episodes in two-thirds or the affected patients (Kölker et al. 2006; Harting et al. 2009, 2015). Noteworthy, during these stress situations with high temperature and infections, catabolism is accelerated resulting in higher proteolysis and increased accumulation of the toxic organic acids GA and 3HGA (Kölker et al. 2006; Wajner et al. 2019). Similarly, previous studies have shown that Lys overload significantly increased GA and 3HGA concentrations in plasma and brain and simultaneously precipitate striatal degeneration in Gcdh−/− mice (Zinnanti et al. 2006, 2007; Seminotti et al. 2013).
A genetic knockout (KO) mice model of GA I (Gcdh−/−) was developed by Koeller et al. (2002) with complete loss of GCDH activity aiming to better elucidate the neuropathology of this disease. The GCDH deficient mice present a similar biochemical phenotype as that of GA I patients, with GA and 3HGA elevations, but not as high as in the human condition. They also manifest mild motor deficit and spongiform myelinopathy, but there is no striatum degeneration associated with neuronal loss and astrogliosis that are characteristically found in affected humans (Wajner et al. 2019). An improvement of this model was achieved by exposing the Gcdh−/− mice to a high lysine or protein (Lys) overload, increasing the blood and brain concentrations of the accumulating metabolites and leading to striatum degeneration (Zinnanti et al. 2006, 2007).
Inflammatory processes contribute to the appearance and progression of various neurodegenerative disorders, such as Alzheimer’s and Parkinson’s diseases (Amor et al. 2014; Guzman-Martinez et al. 2019; Hensley et al. 2006). Furthermore, mounting evidence has shown that neuroinflammation can lead to disruption of redox homeostasis and bioenergetics, as well as excitotoxicity in these diseases, therefore worsening the symptomatology and outcome (Cenini et al. 2019; Hsieh and Yang 2013; Singh et al. 2019). It was also seen that neuroinflammation and systemic inflammation can activate resident brain immune cells, such as microglial cells and astrocytes, which are involved in the perpetuation of the inflammatory state contributing to neuronal death (Palpagama et al. 2019). Noteworthy, lipopolysaccharide (LPS) has been used to mimic inflammation in experimental models, by activating immune system cells (macrophages, neutrophils, and dendritic cells) (Di Lorenzo et al. 2019) and leading to high production pro-inflammatory proteins called cytokines with potential capacity to cross the blood-brain barrier (BBB). In the brain, these cytokines act on receptors on neurons and glial cells, and more intensely in microglia, inducing neuroinflammation (Martínez Leo and Segura Campos 2019). However, although excessive release of inflammatory cytokines into the central nervous system (CNS) has been increasingly associated with neurodegeneration, the exact pathogenetic mechanisms of brain damage are not yet well determined.
Regarding GA I pathogenesis, various studies demonstrated the involvement of oxidative stress, excitotoxicity, bioenergetics disruption, vascular alterations, blood-brain barrier breakage, and altered myelination in brain of Gcdh−/− mice (Amaral et al. 2012a, b, 2015; Busanello et al. 2014; Mühlhausen et al. 2004; Olivera-Bravo et al. 2019; Sauer et al. 2005, 2006; Seminotti et al. 2012, 2013, 2014; Zinnanti et al. 2006, 2007; Wajner et al. 2019). Activation of pathways involved in neuroinflammation following intrastriatal injection of Lys and quinolinic acid to Gcdh−/− mice have been also reported (Amaral et al. 2018, 2019; Seminotti et al. 2016).
However, to the best of our knowledge, no work has evaluated the role of systemic inflammation on the brain damage in this disorder. Considering that acute encephalopathy in GA I patients is generally precipitated by systemic infections that are accompanied by an inflammatory response, it could be hypothesized that systemic inflammation may trigger still unknown deleterious mechanisms leading to striatal and cortical injury in GA I patients.
Therefore, in the present study, we evaluated important redox homeostasis parameters in striatum, cerebral cortex, hippocampus, liver, and heart of wild type (WT, Gcdh+/+) and KO (Gcdh−/−) mice fed either a restricted or a high Lys diet and submitted to LPS-induced systemic inflammation. S100B and NF-κB protein levels were also determined in cerebral cortex and striatum.
Material and Methods
Chemicals
All chemicals were of analytical grade and purchased from Sigma-Aldrich (St Louis, MO, USA). Solutions were prepared on the day of the experiments and the pH was adjusted to 7.2–7.4 in the appropriate buffer for each technique.
Animals
WT (Gcdh+/+) and KO (Gcdh−/−) littermates, both of C129SvEv background, were generated from heterozygotes and maintained at Unidade Experimental Animal of the Hospital de Clínicas de Porto Alegre (Porto Alegre, Brazil). The animals were maintained on a 12:12-h light/dark cycle at a constant temperature (22 ± 1 °C) colony room, with free access to water and 20% (w/w) protein commercial chow containing 0.9% Lys. Thirty-day-old male and female Gcdh+/+ and Gcdh−/− animals were used in the experiments, at a similar age used in previous studies that allows Lys overload without significant animal death (Seminotti et al. 2013, 2016).
Ethical Statement
This study was performed in accordance with the “Guide for the Care and Use of Laboratory Animals” (National Institutes of Health, publication no. 80–23, revised 2011), the Directive 2010/63/EU and the International Guiding Principles for Bio-medical Research Involving Animals. The study was approved by the Ethical Committee for the Care and Use of Laboratory Animals of the Hospital de Clínicas de Porto Alegre (#120420) and all efforts were made to minimize suffering, stress and the number of animals necessary to produce consistent scientific data.
Dietary Lys Treatment and LPS Injection
The Gcdh+/+ (WT) and Gcdh−/− (KO) animals were submitted to a low (0.9%) or a high (4.7%) Lys chow. Dietary Lys overload was previously shown to increase plasma GA and 3HGA concentrations, similar to those of humans with GA I, as well as brain concentrations of these organic acids and leads to striatal damage in Gcdh−/− mice (Seminotti et al. 2013, 2016; Zinnanti et al. 2006).
In the first set of experiments, Gcdh+/+ and Gcdh−/− mice under a low (0.9%) or a high (4.7%) Lys chow received an intraperitoneal (i.p.) injection of 0.9% saline (Sal; vehicle) or LPS (5 mg/g) and were euthanized 4 h later (Fig. 1a). The cerebral cortex was dissected and used for the assays measuring various redox homeostasis parameters. In the second set of experiments, WT and Gcdh−/− mice fed a low or a high Lys chow received an i.p. injection of LPS (5 mg/g) (Erickson and Banks 2011; Hu et al. 2020; Zhang et al. 2020) and were euthanized 4 h later (Fig. 1b). Cerebral cortex, striatum, hippocampus, liver, and heart were isolated and used to measure the biochemical parameters.
Sample Preparation
The mice were euthanized by decapitation without anesthesia, and the various central and peripheral tissues were immediately separated onto a Petri dish placed on ice. To measure the oxidative stress parameters, the brain structures (cerebral cortex, striatum, and hippocampus), heart, and liver were homogenized in 9 volumes (1:10, w/v) of 20 mM sodium phosphate buffer, pH 7.4, containing 140 mM KCl and centrifuged at 750×g for 10 min at 4 °C to discard nuclei and cell debris (Evelson et al. 2001). The pellet was discarded and the supernatant was separated and frozen until the parameters were measured at maximal 5 days later. For western blot analysis, cerebral cortex and striatum were homogenized using RIPA (radioimmunoprecipitation assay) lysis buffer containing protease and phosphatase inhibitors (1 mM sodium orthovanadate, 1 mM aprotinin, and 1% protease inhibitor cocktail) and centrifuged at 10,000×g for 10 min at 4 °C. The supernatant was aliquoted and stored at − 80 °C until the immunoblotting was performed. The protein concentration was quantified by the method of Lowry et al. (1951).
Malondialdehyde (MDA) Levels
We evaluated lipid oxidative damage by measuring MDA levels through the thiobarbituric acid-reactive substances method (Yagi 1998) with slight modifications. Trichloroacetic acid and thiobarbituric acid were added to tissue supernatants and incubated for 2 h at 100 °C. The mixture was cooled at room temperature for 5 min. The resulting pink-stained complex was extracted with butanol. Fluorescence of the organic phase was read at 515 and 553 nm as excitation and emission wavelengths, respectively. Calibration curve was performed using 1,1,3,3-tetramethoxypropane and subjected to the same treatment as supernatants. Results were calculated as nmol MDA/mg protein.
Reduced Glutathione (GSH) Concentrations
GSH concentrations were measured according to Browne and Armstrong (1998). Tissue supernatants were deproteinized with metaphosphoric acid and centrifuged for 10 min at 7000×g. Then 30 μL of supernatants were diluted with 70 μL of 100 mM sodium phosphate buffer, pH 8.0, containing 5 mM EDTA. This preparation was incubated with o-phthaldialdehyde (1 mg/mL in methanol) at room temperature for 15 min. Fluorescence was measured using excitation (350 nm) and emission (420 nm) wavelengths. Concentrations were calculated using a calibration curve of commercial GSH and expressed as nmol GSH/mg protein.
Superoxide Dismutase (SOD) Activity
Total SOD activity was assayed according to Marklund (1985) and is based on the capacity of pyrogallol to autooxidize, a process dependent on superoxide, which is a substrate for SOD. The inhibition of autoxidation of pyrogallol occurs in the presence of SOD, whose activity can be then indirectly assayed spectrophotometrically at 420 nm. A calibration curve was performed with purified SOD as a standard to calculate the activity of SOD present in the samples. The amount of SOD that is able to inhibit pyrogallol autoxidation in 50%, is defined as the enzymatic activity unit (U). The results were reported as U/mg protein.
Glutathione Peroxidase (GPx) Activity
GPx activity was measured according to Wendel (1981) using tert-butyl hydroperoxide, GSH, and NADPH as substrates. The enzyme activity was determined in tissue supernatants by monitoring the NADPH disappearance at 340 nm. One GPx unit (U) is defined as 1 μmol of NADPH consumed per minute. The specific activity was calculated and expressed as U/mg protein.
Glutathione Reductase (GR) Activity
GR activity was measured according to Carlberg and Mannervik (1985) using oxidized glutathione (GSSG) and NADPH as substrates. The enzyme activity was determined in tissue supernatants by monitoring the NADPH disappearance at 340 nm. One GR unit (U) is defined as 1 μmol of GSSG reduced per minute. The specific activity was calculated expressed as U/mg protein.
Western Blot
Samples were denatured using 4× Laemmli buffer (250 mM Tris, 8% SDS, 40% glycerol, and 0.002% bromophenol blue, pH 6.7) and 10% 2-mercaptoethanol and then heated at 100 °C for 10 min. Equal amounts of protein (50 μg/well) were fractionated by SDS-PAGE and electroblotted onto nitrocellulose membranes. The protein loading and electroblotting efficiency were verified through Ponceau S staining, and then the membranes were blocked for 1 h with 5% albumin prepared in Tween-Tris-buffered saline (TTBS: 100 mM Tris-HCl, pH 7.5, 0.9% NaCl, 0.1% Tween-20). Membranes were incubated at 4 °C overnight with the primary antibodies anti-S100 calcium-binding protein B (S100B, 1:1000, Sigma-Aldrich, Inc., S2532), anti-nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB-p65, 1:500, Cell Signaling Technology, Inc., D14E12) and β-actin (1:20,000, Proteintech Group, Inc., HRP-conjugated 60008) according to the manufacturer’s instructions, and later washed with TTBS. Anti-rabbit or anti-mouse IgG peroxidase-linked secondary antibody (1:10,000; Santa Cruz Biotechnology, Inc, sc-2030 and sc-2031 respectively) was then incubated for 2 h with the membranes previously incubated with anti-S100B and anti-NF-κB. The membranes were washed again, and the immunoreactivity was detected by enhanced chemiluminescence using Clarity Western ECL Substrate (Bio-Rad Laboratories, Inc.). The luminescent signals were captured using a charge-coupled device (CCD) camera (ImageQuantTM LAS 4000, GE Healthcare). Densitometric analysis was performed with ImageJ software. Blots were developed to be linear in the range used for densitometry. We drew a frame around the entire row containing the bands in the gel, adjusting it to cover a minimum area that contains the whole of the largest band of the row. We next quantified the relative density of this plot. The values calculated were arbitrary numbers, represented as arbitrary units (AU). Results were expressed as ratio relative to the β-actin, as an internal control.
Statistical Analysis
Results are presented as mean ± standard deviation. Assays were performed in duplicate or triplicate, and the mean or median was used for statistical analysis. Data were analyzed by two-way analysis of variance (ANOVA), considering the following factors in the first set of experiments (Fig. 1a): (1) genotype: Gcdh+/+ and Gcdh−/−; (2) treatment: i.p. injection of saline or LPS; and (3) interaction between genotype x treatment. Regarding the second set of experiments (Fig. 1b), the factors were (1) genotype: Gcdh+/+ and Gcdh−/−; (2) chow: low (0.9 %) or high (4.7 %) Lys; and (3) interaction between genotype and chow. F values are presented when differences between groups were significant (P ≤ 0.05). The post-hoc Tukey’s test was performed when means from three or more groups were compared. The GraphPad Prism software version 7 (GraphPad Software, CA, USA) was used to carry out all statistical analyses, as well as for designing the graphs.
Results
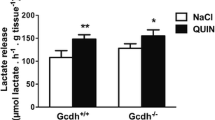
Lys Overload and LPS-Induced Systemic Inflammation Disrupt Redox Homeostasis in Cerebral Cortex of Gcdh−/− Mice
It was first observed that cerebral cortex of Gcdh−/− animals fed a restricted Lys (low, 0.9%) diet and receiving saline injection presented no alterations in redox homeostasis in cerebral cortex, as compared to WT mice (Fig. 2). However, when these animals were injected with LPS in addition to the low Lys diet, a significant increase of MDA levels (F(1,13) = 7.322, P ≤ 0.05) (Fig. 2a) and a decrease of GSH concentrations (F(1,12) = 8.306, P ≤ 0.05) (Fig. 2b) and GPx activity (F(1,11) = 6.959, P ≤ 0.05) (Fig. 2d) were observed. Furthermore, Gcdh−/− mice fed a high Lys (4.7%) chow had increased MDA levels (F(1,11) = 21.06, P ≤ 0.05) (Fig. 3a) and elevated SOD activity (F(1,10) = 36.39, P ≤ 0.01) (Fig. 3c). When these Lys overload Gcdh−/− mice received LPS injection, apart from the elevated MDA levels (F(1,11) = 4.613, P ≤ 0.05) (Fig. 3a) and increased SOD activity (F(1,10) = 18.15, P ≤ 0.01) (Fig. 3c), they also showed decreased GSH concentrations (F(1,11) = 6.1, P ≤ 0.05) (Fig. 3b) and GPx activity (F(1,11) = 6.763, P ≤ 0.05) (Fig. 3d) in the cerebral cortex, relatively to saline-injected Gcdh+/+ and Gcdh−/− mice, implying a clear influence of systemic inflammation on these parameters. In addition, GR activity was significantly decreased in cerebral cortex of Gcdh−/− mice with Lys restriction after LPS injection (F(1,17) = 13.69, P ≤ 0.05) (Fig. 2e) or overload (F(1,19) = 56.04, P ≤ 0.001) (Fig. 3e), when compared to mice that were injected with saline. These data indicate that LPS-elicited systemic inflammation is able to disturb oxidative stress parameters in cerebral cortex of Gcdh−/− mice both under a restricted or a high Lys chow, ultimately disrupting redox homeostasis.
Effect of LPS-induced inflammation on malondialdehyde (MDA) levels (a), reduced glutathione (GSH) levels (b), superoxide activity (SOD) (c), glutathione peroxidase activity (GPx) (d), and glutathione reductase activity (GR) (e) in cerebral cortex of Gcdh+/+ (WT) and Gcdh−/− (KO) mice fed a restricted Lys (0.9%) diet. Mice were fed with 0.9% Lys diet and injected with saline or LPS (5 μg/g). Values are mean ± standard deviation for 4–6 independent experiments (animals) expressed as nmol/mg protein. *P < 0.05 (two-way ANOVA as described in the text, followed by Tukey’s multiple-range test)
Effect of LPS-induced inflammation on malondialdehyde (MDA) levels (a), reduced glutathione (GSH) levels (b), superoxide activity (SOD) (c), glutathione peroxidase activity (GPx) (d), and glutathione reductase activity (GR) (e) in cerebral cortex of Gcdh+/+ (WT) and Gcdh−/− (KO) mice fed a high Lys (4.7%) diet. Mice were fed with 4.7% Lys and injected with saline or LPS (5 μg/g). Values are mean ± standard deviation for 4–6 independent experiments (animals) expressed as nmol/mg protein. *P < 0.05, **P < 0.01 (two-way ANOVA as described in the text, followed by Tukey’s multiple-range test)
High Vulnerability of Cerebral Cortex and Striatum of Gcdh−/− Mice to LPS-Induced Systemic Inflammation
In the next set of experiments, we measured MDA levels and GSH concentrations in various central (cerebral cortex, striatum, and hippocampus) and peripheral tissues (liver and heart) of Gcdh+/+ and Gcdh−/− mice fed a restricted (0.9%) or a high (4.7%) Lys chow. It was observed that systemic inflammation significantly increased MDA levels in cerebral cortex (F(1,14) = 20.38, P ≤ 0.05) (Fig. 4a) and striatum (F(1,16) = 37.35, P ≤ 0.001) (Fig. 4b), but not in hippocampus (Fig. 4c), heart (Fig. 4d), and liver (Fig. 4e) of Gcdh−/− mice both under restricted or high Lys chow. Furthermore, GSH concentrations were decreased in cerebral cortex (F(1,11) = 27.05, P ≤ 0.01) (Fig. 5a) and striatum (F(1,13) = 12.27, P ≤ 0.01) (Fig. 5b), but not in hippocampus (Fig. 5c), heart (Fig. 5d) and liver (Fig. 5e) of Gcdh−/− mice fed a high Lys chow. It should be also noted that the LPS-induced effects on the striatum were enhanced when the Gcdh−/− animals received a high Lys chow (Figs. 4b and 5b), suggesting a synergistic influence of systemic inflammatory process and Lys overload on this cerebral structure. Finally, the concentrations of GSH were only diminished in cerebral cortex of Gcdh−/− mice under a restricted Lys chow (Fig. 5a), with no changes in the other tissues. Taken together, these data strongly suggest a selective vulnerability of cerebral cortex and striatum to oxidative stress during a systemic inflammatory process in the Gcdh−/− mice.
Effect of LPS-induced inflammation on malondialdehyde (MDA) levels in cerebral cortex (a), striatum (b), hippocampus (c), heart (d), and liver (e) of Gcdh+/+ (WT) and Gcdh−/− (KO) mice fed either a low (0.9%) or a high (4.7%) Lys diet. Values are mean ± standard deviation for 4–6 independent experiments (animals) expressed as nmol/mg protein. *P < 0.05, **P < 0.01 (Student’s t test for unpaired samples) (two-way ANOVA as described in the text, followed by Tukey’s multiple-range test)
Effect of LPS-induced inflammation on reduced glutathione (GSH) levels in cerebral cortex (a), striatum (b), hippocampus (c), heart (d), and liver (e) of Gcdh+/+ (WT) and Gcdh−/− (KO) mice fed either a low (0.9%) or a high (4.7%) Lys diet. Values are mean ± standard deviation for 4–6 independent experiments (animals) expressed as nmol/mg protein. *P < 0.05, **P < 0.01 (two-way ANOVA as described in the text, followed by Tukey’s multiple-range test)
Lys Overload and LPS-Induced Systemic Inflammation Increase S100B and NF-κB Protein Levels in Cerebral Cortex and Striatum of Gcdh−/− Mice
Figure 6 displays the protein levels of S100B in cerebral cortex (Fig. 6a) and striatum (Fig. 6b) of Gcdh+/+ and Gcdh−/− mice fed a high (4.7%) Lys chow and injected with saline or LPS. S100B levels were significantly higher in cerebral cortex (F(1,8) = 38.7, P ≤ 0.05) (Fig. 6a) and striatum (F(1,8) = 45.45, P ≤ 0.01) (Fig. 6b) of Gcdh−/− mice fed a high Lys chow after LPS injection, when compared to Gcdh−/− mice injected with saline and to the control group (saline-injected Gcdh+/+ mice) (Fig. 6a: F(1,8) = 19.77, P ≤ 0.05; Fig. 6b: F(1,8) = 3.44, P ≤ 0.01). Moreover, we observed a nonsignificant upward trend in NF-κB-p65 levels in cerebral cortex (F(1,8) = 2.773, P = 0.99) (Fig. 7a) and a significant increase of these levels in striatum of Gcdh−/− mice fed a high Lys chow combined with LPS administration (F(1,8) = 5.393, P ≤ 0.05) (Fig. 7b).
Effect of LPS-induced inflammation on S100 calcium-binding protein B (S100B) levels in cerebral cortex (a) and striatum (b) of Gcdh+/+ (WT) and Gcdh−/− (KO) mice fed a high (4.7%) Lys diet. Values are mean ± standard deviation for 3 independent experiments (animals) expressed as arbitrary units, normalized by the content of the protein β-actin. *P < 0.05, **P < 0.01 (two-way ANOVA as described in the text, followed by Tukey’s multiple-range test)
Effect of LPS-induced inflammation on nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) protein levels in cerebral cortex (a) and striatum (b) of Gcdh+/+ (WT) and Gcdh−/− (KO) mice fed a high (4.7%) Lys diet. Values are mean ± standard deviation for 3 independent experiments (animals) expressed as arbitrary units, normalized by the content of the protein β-actin. *P < 0.05 (two-way ANOVA as described in the text, followed by Tukey’s multiple-range test)
Discussion
In the present work we treated WT and Gcdh−/− mice with a restricted (0.9%) or high (4.7%) Lys chow and also injected intraperitoneally LPS in these animals to stimulate a systemic inflammatory response. Important redox homeostasis parameters, including lipid oxidative damage and non-enzymatic and enzymatic antioxidant defenses were evaluated after LPS administration in various brain structures (striatum, cerebral cortex, and hippocampus) and in peripheral tissues (liver and heart). In this context, peripheral LPS administration has been used to promote systemic inflammation and investigate brain injury (Banks and Erickson 2010; Di Lorenzo et al. 2019). Previous studies have shown that LPS induces a high production of pro-inflammatory cytokines mediated by inflammatory signaling pathways, such as NF-κB and MAPK, as well as microglia activation in many tissues, including brain (Banks and Erickson 2010; Erickson and Banks 2011; Kang et al. 2019; Noailles et al. 2018; Zhao et al. 2020).
Overall, we demonstrated that Lys overload and LPS-induced systemic inflammation per se disrupt redox homeostasis in cerebral cortex of Gcdh−/− mice and that when combined these treatments induce oxidative stress selectively in cerebral cortex and striatum, but not in hippocampus, liver and heart of these genetically modified animals. Noteworthy, a synergistic effect of high Lys chow and inflammatory response was more evident in the striatum, which is mainly damaged during encephalopathic crises associated with infection in GA I patients.
We initially observed that Gcdh−/− animals fed a restricted Lys diet presented no alterations of redox homeostasis in cerebral cortex, whereas lipid peroxidation (increased MDA levels) and decreased antioxidant defenses (reduction of GSH concentrations, as well as of GPx and GR activities) were demonstrated in the same animals under LPS-induced systemic inflammation. Of note, MDA is an end-product of the decomposition of cell membrane-containing polyunsaturated lipids resulting mainly from the attack of reactive oxygen species. Thus, MDA is considered a reliable biomarker of lipid peroxidation and oxidative stress (Ayala et al. 2014). Regarding the antioxidant defenses, the decreased levels of the major naturally occurring brain antioxidant GSH allied to alterations on important antioxidant enzymes suggest that both non-enzymatic and enzymatic antioxidant defenses were compromised in cerebral cortex of Gcdh−/− mice. The decreased activity of GPx, that detoxifies hydrogen peroxide and lipid peroxides, and that of GR, that recycles GSSG to GSH, may possibly explain the lipid oxidative damage resulting from the formation of lipid peroxides and other by-products, as well as the diminution of GSH concentrations observed (Halliwell and Gutteridge 2015; Shin et al. 2018). In case these results can be extrapolated to the human condition, it may be presumed that inflammation induces oxidative stress in the brain of GA I patients even under low Lys diet.
Furthermore, Gcdh−/− mice under Lys overload and injected with LPS presented increased SOD activity in the cerebral cortex, in addition to increased MDA levels and decreased GSH concentrations, as well as reduced GPx and GR activities that were seen in the LPS-injected Gcdh−/− mice under a restricted Lys chow. Although we did not investigate the mechanisms by which SOD activity was increased, it is conceivable that it may represent a compensatory response consisting of an upregulation of this enzyme at the transcription level in order to eliminate superoxide radicals that were probably augmented in the brain of these animals (Espinosa-Diez et al. 2015; Halliwell and Gutteridge 2015). These data indicate that Lys overload per se is able to induce oxidative stress in cerebral cortex of Gcdh−/− mice, which is further accentuated when superposed by systemic inflammation. Taken together, it was shown here that systemic inflammation per se disturb redox homeostasis in cerebral cortex of Gcdh−/− mice exposed to both a restricted or a high Lys chow, evidencing therefore a critical role of inflammation in the cortical oxidative stress induction in these animals (Fig. 8).
Our results also revealed that Lys overload associated with LPS-induced systemic inflammation increased S100B and NF-κB protein content in cerebral cortex and striatum of Gcdh−/− mice. S100B is a Ca2+−modulated protein, highly expressed and released by astrocytes, that at low levels has an important role protecting neurons against oxidative stress (Adami et al. 2004). These effects are usually mediated by S100B binding to RAGE (receptor for advanced glycation end products) in neurons and microglia, that has been shown to activate several signaling pathways, including the NF-κB pathway (Adami et al. 2004; Gonzalez-Reyes and Rubiano 2018; Reinehr et al. 2018). However, high levels of S100B are usually neurotoxic because sustained activation of the RAGE receptor leads to neuronal death associated with increased production of ROS, dysregulation of Ca2+ homeostasis, and enhanced nitric oxide production by astrocytes and microglia (Gonzalez-Reyes and Rubiano 2018; Santos et al. 2018; Steiner et al. 2011). It is important to highlight our previous reports that also showed increased content of NF-κB protein in the brain of Gcdh−/− mice under Lys overload (Seminotti et al. 2016; Amaral et al. 2019). Astrogliosis has been previously implicated in GA I pathogenesis in several studies showing increased levels of GFAP and S100B in the brain of Gcdh−/− mice, cultured cells of Gcdh−/− mice, and in chemical rat models (Amaral et al. 2018; Olivera et al. 2008; Olivera-Bravo et al. 2015; Olivera-Bravo and Barbeito 2015). Considering the role of S100B, GFAP, and NF-kB on pathological conditions linked to intracellular oxidative stress, inflammatory processes, and gliosis (Steiner et al. 2011; Tarasov et al. 2020), our present results indicate that disruption of this signaling pathway may be involved in the redox alterations observed.
Noteworthy, Lys is able to easily cross the blood-brain barrier and enter the neural cells (Sauer et al. 2006) leading to increased brain production of GA and 3HGA in Gcdh−/− mice (Seminotti et al. 2012; Zinnanti et al. 2006, 2007). Therefore, it may be presumed that the effects observed in redox homeostasis due to Lys overload could be attributed to increased GA and 3HGA concentrations in the brain of the Gcdh−/− mice. In accordance with this, there is robust in vitro and in vivo evidence showing that GA and 3HGA provoke lipid and protein oxidation and reduce the antioxidant defenses in the brain of young WT rats (de Oliveira et al. 2003; Latini et al. 2002, 2005, 2007; Olivera et al. 2008), as well as extensive cerebral damage in Gcdh−/− mice treated with Lys overload (Amaral et al. 2019; Olivera-Bravo et al. 2019; Rodrigues et al. 2015; Seminotti et al. 2012, 2013, 2014; Wajner et al. 2019). Altogether, these findings reinforce the assumption that the metabolites accumulating in GA I, especially GA, mediate the brain damage in this disorder.
Mounting evidence showed that efficient management of episodes of metabolic decompensation triggered by infectious/inflammatory processes in patients with inherited metabolic diseases is critical for their survival and to prevent brain damage and long-term cerebral complications (Guerrero et al. 2018; Saudubray and Garcia-Cazorla 2018; Scriver et al. 2001; Tarasenko and McGuire 2017). In this particular, an inflammatory response usually leads to cytokines release and immune cells mobilization, such as microglial cells, consisting of the primary inflammatory cell type in the central nervous system (Miller et al. 2013). The secretion of cytokines may potentially provoke serious consequences to the normal functioning of the brain since they disturb neurotransmitter systems, impacting neurocircuits and altering BBB permeability (Galic et al. 2012; Yarlagadda et al. 2009). Of note, a recent study with GA I patients showed an increase of the cytokines IL-6, IL-8, GM-CSF, and TNF-α in plasma, indicating a pro-inflammatory state in these patients (Guerreiro et al. 2018). Other studies in Gcdh−/− mice also demonstrated gliosis, neuronal loss, white mater injury, and BBB breakdown (Amaral et al. 2019; Olivera-Bravo et al. 2019; Zinnanti et al. 2014). These data allied to our present findings showing that LPS induces oxidative stress support the hypothesis that inflammation plays a major role in the pathogenesis of the neurological alterations found in GA I patients.
Other interesting findings of the present study were the observations that systemic inflammation significantly increased lipid oxidative damage (elevated MDA levels) and decreased the antioxidant defenses (reduction of GSH concentrations) in cerebral cortex and striatum but not in hippocampus, liver and heart of Gcdh−/− mice under restricted or high Lys chow, implying a selective vulnerability of cerebral cortex and striatum to oxidative stress during a systemic inflammatory process. Interestingly, we observed a synergic effect of LPS injection and high Lys dietary intake disrupting redox status in the striatum, since the alterations caused by LPS administration were worsened in the Gcdh−/− mice under a high Lys chow. These findings lead us to hypothesize that brain damage in GA I patients during encephalopathic crises precipitated by inflammatory processes associated with infections may be in part due to oxidative stress.
Although to the best of our mind, this is the first study using LPS to induce inflammation in Gcdh−/− mice, a previous report investigated the effects of intrastriatal administration of quinolinic acid, an intermediate of kynurenine pathway that is also activated during infections by inflammatory cytokines, to Gcdh−/− mice (Amaral et al. 2018). Quinolinic acid was shown to cause profound histopathological changes, including marked vacuolation, lymphocyte infiltration, nitrosative stress, and reactive astrogliosis (increase of GFAP and S100B staining) in the striatum of Gcdh−/− mice. The same treatment was demonstrated to disturb redox status and bioenergetics in the striatum of Gcdh−/− mice fed a high Lys chow, by decreasing the activities of complex IV and creatine kinase, as well as inducing generation of reactive oxygen species (ROS) and reducing GSH concentrations (Seminotti et al. 2016).
The observations that cerebral cortex and striatum of Gcdh−/− animals were particularly sensitive to oxidative stress, when compared to the liver and heart, may be due to the fact that the brain is especially susceptible to free radical attack due to its high rate of oxidative metabolism coupled to ROS production, lower antioxidant defenses, and higher peroxidation potential due to its high polyunsaturated fatty acids content (Halliwell and Gutteridge 2015) (Fig. 6). It should be also emphasized that the liver has a much higher regenerative capacity and adaptability to metabolic alterations than the brain (Hallbergson et al. 2003; Tarlá et al. 2006). However, this does not explain why hippocampus was not affected in our animal model and further investigation is necessary to elucidate this point.
In conclusion and to the best of our knowledge, we showed for the first time that LPS-induced systemic inflammation disrupts cellular redox status particularly in striatum and cerebral cortex of Gcdh−/− mice. Based on the present data and on previous studies, it is presumed that disruption of redox homeostasis is involved in the neuropathological alterations observed in GA I patients mainly during encephalopathic crises triggered by systemic infections.
Availability of Data and Material
Not applicable
References
Adami C, Bianchi R, Pula G, Donato R (2004) S100B-stimulated NO production by BV-2 microglia is independent of RAGE transducing activity but dependent on RAGE extracellular domain. Biochim Biophys Acta 1742:169–177. https://doi.org/10.1016/j.bbamcr.2004.09.008
Amaral AU, Seminotti B, Cecatto C, Fernandes CG, Busanello EN, Zanatta A, Kist LW, Bogo MR, de Souza DO, Woontner M, Goodman S, Koeller DM, Wajner M (2012a) Reduction of Na+, K+-ATPase activity and expression in cere- bral cortex of glutaryl-CoA dehydrogenase deficient mice: a possible mechanism for brain injury in glutaric aciduria type I. Mol Genet Metab 107:375–382. https://doi.org/10.1016/j.ymgme.2012.08.016
Amaral AU, Cecatto C, Seminotti B, Zanatta A, Fernandes CG, Busanello EN, Braga LM, Ribeiro CA, de Souza DO, Woontner M, Koeller DM, Goodman S, Wajner M (2012b) Marked reduction of Na(+), K(+)-ATPase and creatine kinase activities induced by acute lysine administration in glutaryl-CoA dehydrogenase deficient mice. Mol Genet Metab 107:81–86. https://doi.org/10.1016/j.ymgme.2012.04.015
Amaral AU, Cecatto C, Seminotti B, Ribeiro CA, Lagranha VL, Pereira CC, de Oliveira FH, de Souza DG, Goodman S, Woontner M, Wajner M (2015) Experimental evidence that bioenergetics disruption is not mainly involved in the brain injury of glutaryl-CoA dehydrogenase deficient mice submitted to lysine overload. Brain Res 1620:116–129. https://doi.org/10.1016/j.brainres.2015.05.013
Amaral AU, Seminotti B, da Silva JC, de Oliveira FH, Ribeiro RT, Vargas CR, Leipnitz G, Santamaría A, Souza DO, Wajner M (2018) Induction of neuroinflammatory response and histopathological alterations caused by quinolinic acid administration in the striatum of glutaryl-CoA dehydrogenase deficient mice. Neurotox Res 33:593–606. https://doi.org/10.1007/s12640-017-9848-0
Amaral AU, Seminotti B, da Silva JC, de Oliveira FH, Ribeiro RT, Leipnitz G, Souza DO, Wajner M (2019) Acute lysine overload provokes marked striatum injury involving oxidative stress signaling pathways in glutaryl-CoA dehydrogenase deficient mice. Neurochem Int 129:104467. https://doi.org/10.1016/j.neuint.2019.104467
Amor S, Peferoen LA, Vogel DY, Breur M, Valk P, Baker D, Noort JM (2014) Inflammation in neurodegenerative diseases - an update. Immunology 142:151–166. https://doi.org/10.1111/imm.12233
Ayala A, Muñoz MF, Argüelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med Cell Longev 2014:360438. https://doi.org/10.1155/2014/360438
Banks WA, Erickson MA (2010) The blood-brain barrier and immune function and dysfunction. Neurobiol Dis 37:26–32. https://doi.org/10.1016/j.nbd.2009.07.031
Browne RW, Armstrong D (1998) Reduced glutathione and glutathione disulfide. Methods Mol Biol 108:347–352. https://doi.org/10.1385/0-89603-472-0:347
Busanello EN, Fernandes CG, Martell RV, Lobato VG, Goodman S, Woontner M, de Souza DO, Wajner M (2014) Disturbance of the glutamatergic system by glu- taric acid in striatum and cerebral cortex of glutaryl-CoA dehydrogenase-deficient knockout mice: possible implications for the neuropathology of glutaric acidemia type I. J Neurol Sci 346:260–267. https://doi.org/10.1016/j.jns.2014.09.003
Carlberg I, Mannervik B (1985) Glutathione reductase. Methods Enzymol 113:484–490
Cenini G, Lloret A, Cascella R (2019) Oxidative stress in neurodegenerative diseases: from a mitochondrial point of view. Oxidative Med Cell Longev 9:2105607. https://doi.org/10.1155/2019/2105607
de Oliveira MF, Hagen ME, Pederzolli CD, Sgaravatti AM, Durigon K, Testa CG, Wannmacher CM, de Souza Wyse AT, Wajner M, Dutra-Filho CS (2003) Glutaric acid induces oxidative stress in brain of young rats. Brain Res 964:153–158. https://doi.org/10.1016/S0006-8993(02)04118-5
Di Lorenzo F, De Castro C, Silipo A, Molinaro A (2019) Lipopolysaccharide structures of gram-negative populations in the gut microbiota and effects on host interactions. FEMS Microbiol Rev 43:257–272. https://doi.org/10.1093/femsre/fuz002
Erickson MA, Banks WA (2011) Cytokine and chemokine responses in serum and brain after single and repeated injections of lipopolysaccharide: multiplex quantification with path analysis. Brain Behav Immun 25:1637–1648. https://doi.org/10.1016/j.bbi.2011.06.006
Espinosa-Diez C, Miguel V, Mennerich D, Kietzmann T, Sánchez-Pérez P, Cadenas S, Lamas S (2015) Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol 6:183–197. https://doi.org/10.1016/j.redox.2015.07.008
Evelson P, Travacio M, Repetto M, Escobar J, Llesuy S, Lissi EA (2001) Evaluation of total reactive antioxidant potential (TRAP) of tissue homogenates and their cytosols. Arch Biochem Biophys 388:261–266. https://doi.org/10.1006/abbi.2001.2292
Galic MA, Riazi K, Pittman QJ (2012) Cytokines and brain excitability. Front Neuroendocrinol 33:116–125. https://doi.org/10.1016/j.yfrne.2011.12.002
Gonzalez-Reyes RE, Rubiano MG (2018) Astrocyte´s RAGE: more than just a question of mood. Cent Nerv Syst Agents Med Chem 18:39–48. https://doi.org/10.2174/1871524916999160505105121
Goodman SI, Markey SP, Moe PG, Miles BS, Teng CC (1975) Glutaric aciduria; a “new” disorder of amino acid metabolism. Biochem Med 12:12–21. https://doi.org/10.1016/0006-2944(75)90091-5
Goodman SI, Kohlhoff JG (1975) Glutaric aciduria: inherited deficiency of glutaryl-CoA dehydrogenase activity. Biochem Med 13:138–140. https://doi.org/10.1016/0006-2944(75)90149-0
Goodman SI, Frerman FE (2014) Organic acidemias due to defects in lysine oxidation: 2-ketoadipic acidemia and glutaric acidemia. In: Valle D, Beaudet AL, Vogelstein B, Kinzler KW, Antonarakis SE, Ballabio A, Gibson K, Mitchell G (eds) The Online Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill, New York http://ommbid.mhmedical.com/content.aspx?bookid = 971§ionid = 62677296. Accessed 3 June 2019
Guerreiro G, Faverzani J, Jacques CED, Marchetti DP, Sitta A, de Moura CD, Kayser A, Kok F, Athayde L, Manfredini V, Wajner M, Vargas CR (2018) Oxidative damage in glutaric aciduria type I patients and the protective effects of L-carnitine treatment. J Cell Biochem 119:10021–10032. https://doi.org/10.1002/jcb.27332
Guerrero RB, Salazar D, Tanpaiboon P (2018) Laboratory diagnostic approaches in metabolic disorders. Ann Transl Med 6:470. https://doi.org/10.21037/atm.2018.11.05
Guzman-Martinez L, Maccioni RB, Andrade V, Navarrete LP, Pastor MG, Ramos-Escobar N (2019) Neuroinflammation as a common feature of neurodegenerative disorders. Front Pharmacol 10:1008. https://doi.org/10.3389/fphar.2019.01008
Hallbergson AF, Gnatenco C, Peterson DA (2003) Neurogenesis and brain injury: managing a renewable resource for repair. J Clin Invest 112:1128–1133. https://doi.org/10.1172/JCI200320098
Halliwell B, Gutteridge JMC (2015) Cellular responses to oxidative stress: adaptation, damage, repair, senescence and death. In: Halliwell B, Gutteridge JMC (eds) Free radicals in biology and medicine, 5th edn. Oxford University Press Inc, Oxford, pp 199–283
Harting I, Neumaier-Probst E, Seitz A, Maier EM, Assmann B, Baric I, Troncoso M, Mühlhausen C, Zschocke J, Boy NP, Hoffmann GF, Garbade SF, Kolker S (2009) Dynamic changes of striatal and extrastriatal abnormalities in glutaric aciduria type I. Brain 132:1764–1782. https://doi.org/10.1093/brain/awp112
Harting I, Boy N, Heringer J, Seitz A, Bendszus M, Pouwels PJ, Kölker S (2015) 1H-MRS in glutaric aciduria type 1: impact of biochemical phenotype and age on the cerebral accumulation of neurotoxic metabolites. J Inherit Metab Dis 38:829–838. https://doi.org/10.1007/s10545-015-9826-8
Hensley K, Mhatre M, Mou S, Pye QN, Stewart C, West M, Williamson KS (2006) On the relation of oxidative stress to neuroinflammation: lessons learned from the G93A-SOD1 mouse model of amyotrophic lateral sclerosis. Antioxid Redox Signal 8:2075–2087. https://doi.org/10.1089/ars.2006.8.2075
Hsieh HL, Yang CM (2013) Role of redox signaling in neuroinflammation and neurodegenerative diseases. Biomed Res Int 2013:484613. https://doi.org/10.1155/2013/484613
Hu AL, Song S, Li Y, Xu SF, Zhang F, Li C, Liu J (2020) Mercury sulfide-containing Hua-Feng-Dan and 70 W (Rannasangpei) protect against LPS plus MPTP-induced neurotoxicity and disturbance of gut microbiota in mice. J Ethnopharmacol 254:112674. https://doi.org/10.1016/j.jep.2020.112674
Jafari P, Braissant O, Bonafé L, Ballhausen D (2011) The unsolved puzzle of neuro- pathogenesis in glutaric aciduria type I. Mol Genet Metab 104:425–437. https://doi.org/10.1016/j.ymgme.2011.08.027
Kang JB, Park DJ, Shah MA, Kim MO, Koh PO (2019) Lipopolysaccharide induces neuroglia activation and NF-κB activation in cerebral cortex of adult mice. Lab Anim Res 35:19. https://doi.org/10.1186/s42826-019-0018-9
Koeller DM, Woontner M, Crnic LS, Kleinschmidt-DeMasters B, Stephens J, Hunt EL, Goodman SI (2002) Biochemical, pathologic and behavioral analysis of a mouse model of glutaric acidemia type I. Hum Mol Genet 11:347–357. https://doi.org/10.1093/hmg/11.4.347
Kölker S, Sauer SW, Okun JG, Hoffmann GF, Koeller DM (2006) Lysine intake and neurotoxicity in glutaric aciduria type I: towards a rationale for therapy? Brain 129:e54. https://doi.org/10.1093/brain/awl137
Latini A, Borba Rosa R, Scussiato K, Llesuy S, Belló-Klein A, Wajner M (2002) 3-Hydroxyglutaric acid induces oxidative stress and decreases the antioxidant defenses in cerebral cortex of young rats. Brain Res 956:367–373. https://doi.org/10.1016/S0006-8993(02)03573-4
Latini A, Scussiato K, Leipnitz G, Dutra-Filho CS, Wajner M (2005) Promotion of oxidative stress by 3-hydroxyglutaric acid in rat striatum. J Inherit Metab Dis 28:57–67. https://doi.org/10.1007/s10545-005-3677-7
Latini A, Ferreira GC, Scussiato K, Schuck PF, Solano AF, Dutra-Filho CS, Vargas CR, Wajner M (2007) Induction of oxidative stress by chronic and acute glutaric acid administration to rats. Cell Mol Neurobiol 27:423–438. https://doi.org/10.1007/s10571-006-9134-9
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Marklund SL (1985) Pyrogallol autoxidation. Handbook for Oxygen Radical Research. CRC Press, Boca Raton, pp 243–247
Martínez Leo EE, Segura Campos MR (2019) Effect of ultra-processed diet on gut microbiota and thus its role in neurodegenerative diseases. Nutrition 71:110609. https://doi.org/10.1016/j.nut.2019.110609
Miller AH, Haroon E, Raison CL, Felger JC (2013) Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety 30:297–306. https://doi.org/10.1002/da.22084
Mühlhausen C, Ergün S, Strauss KA, Koeller DM, Crnic L, Woontner M, Goodman SI, Ullrich K, Braulke T (2004) Vascular dysfunction as an additional pathomechanism in glutaric aciduria type I. J Inherit Metab Dis 27:829–834. https://doi.org/10.1023/B:BOLI.0000045766.98718.d6
Noailles A, Maneu V, Campello L, Lax P, Cuenca N (2018) Systemic inflammation induced by lipopolysaccharide aggravates inherited retinal dystrophy. Cell Death Dis 9:350. https://doi.org/10.1038/s41419-018-0355-x
Olivera S, Fernandez A, Latini A, Rosillo JC, Casanova G, Wajner M, Cassina P, Barbeito L (2008) Astrocytic proliferation and mitochondrial dysfunction induced by accumulated glutaric acidemia I (GAI) metabolites: possible implications for GAI pathogenesis. Neurobiol Dis 32:528–534. https://doi.org/10.1016/j.nbd.2008.09.011
Olivera-Bravo S, Barbeito L (2015) A role of astrocytes in mediating postnatal neurodegeneration in Glutaric acidemia-type 1. FEBS Lett 589:3492–3497. https://doi.org/10.1016/j.febslet.2015.09.010
Olivera-Bravo S, Ribeiro CA, Isasi E, Trías E, Leipnitz G, Díaz-Amarilla P, Woontner M, Beck C, Goodman SI, Souza D, Wajner M, Barbeito L (2015) Striatal neuronal death mediated by astrocytes from the Gcdh-/- mouse model of glutaric acidemia type I. Hum Mol Genet 24:4504–4515. https://doi.org/10.1093/hmg/ddv175
Olivera-Bravo S, Seminotti B, Isasi E, Ribeiro CA, Leipnitz G, Woontner M, Goodman SI, Souza D, Barbeito L, Wajner M (2019) Long lasting high lysine diet aggravates white matter injury in glutaryl-CoA dehydrogenase deficient (Gcdh-/-) mice. Mol Neurobiol 56:648–657. https://doi.org/10.1007/s12035-018-1077-x
Palpagama TH, Waldvogel HJ, Faull RLM, Kwakowsky A (2019) The role of microglia and astrocytes in Huntington’s Disease. Front Mol Neurosci 12:258. https://doi.org/10.3389/fnmol.2019.00258
Reinehr S, Reinhard J, Gandej M, Gottschalk I, Stute G, Faissner A, Dick HB, Joachim SC (2018) S100B immunization triggers NFκB and complement activation in an autoimmune glaucoma model. Sci Rep 8:9821. https://doi.org/10.1038/s41598-018-28183-6
Rodrigues MD, Seminotti B, Amaral AU, Leipnitz G, Goodman SI, Woontner M, de Souza DO, Wajner M (2015) Experimental evidence that overexpression of NR2B glutamate receptor subunit is associated with brain vacuolation in adult glutaryl-CoA dehydrogenase deficient mice: a potential role for glutamatergic-induced excitotoxicity in GA I neuropathology. J Neurol Sci 359:133–140. https://doi.org/10.1016/j.jns.2015.10.043
Santos G, Barateiro A, Gomes CM, Brites D, Fernandes A (2018) Impaired oligodendrogenesis and myelination by elevated S100B levels during neurodevelopment. Neuropharmacology 129:69–83. https://doi.org/10.1016/j.neuropharm.2017.11.002
Saudubray JM, Garcia-Cazorla À (2018) Inborn errors of metabolism overview: Pathophysiology, manifestations, evaluation, and management. Pediatr Clin N Am 65:179–208. https://doi.org/10.1016/j.pcl.2017.11.002
Sauer SW, Okun JG, Schwab MA, Crnic LR, Hoffmann GF, Goodman SI, Koeller DM, Kölker S (2005) Bioenergetics in glutaryl-coenzyme A dehydrogenase deficiency: a role for glutaryl-coenzyme A. J Biol Chem 280:21830–21836. https://doi.org/10.1074/jbc.M502845200
Sauer SW, Okun JG, Fricker G, Mahringer A, Müller I, Crnic LR, Mühlhausen C, Hoffmann GF, Hörster F, Goodman SI, Harding CO, Koeller DM, Kölker S (2006) Intracerebral accumulation of glutaric and 3-hydroxyglutaric acids secondary to limited flux across the blood-brain barrier constitute a biochemical risk factor for neurodegeneration in glutaryl-CoA dehydrogenase deficiency. J Neurochem 97:899–910. https://doi.org/10.1111/j.1471-4159.2006.03813.x
Seminotti B, da Rosa MS, Fernandes CG, Amaral AU, Braga LM, Leipnitz G, de Souza DO, Woontner M, Koeller DM, Goodman S, Wajner M (2012) Induction of oxidative stress in brain of glutaryl-CoA dehydrogenase deficient mice by acute lysine administration. Mol Genet Metab 106:31–38. https://doi.org/10.1016/j.ymgme.2012.03.002
Seminotti B, da Rosa MS, Fernandes CG, Leipnitz G, Olivera-Bravo S, Barbeito L, Ribeiro CA, de Souza DO, Woontner M, Goodman SI, Koeller DM, Wajner M (2013) Disruption of brain redox momeostasis in glutaryl-CoA dehydrogenase deficient mice treated with high dietary lysine supplementation. Mol Genet Metab 108:30–39. https://doi.org/10.1016/j.ymgme.2012.11.001
Seminotti B, Ribeiro RT, Amaral AU, da Rosa MS, Pereira CC, Leipnitz G, Koeller DM, Goodman S, Woontner M, Wajner M (2014) Acute lysine overload provokes protein oxidative damage and reduction of antioxidant defenses in the brain of infant glutaryl-CoA dehydrogenase deficient mice: a role for oxidative stress in GA I neuropathology. J Neurol Sci 344:105–113. https://doi.org/10.1016/j.jns.2014.06.034
Seminotti B, Amaral AU, Ribeiro RT, Rodrigues MD, Colin-Gonzalez AL, Leipnitz G, Santamaria A, Wajner M (2016) Oxidative stress, disrupted energy metabolism, and altered signaling pathways in glutaryl-CoA dehydrogenase knockout mice: potential implications of quinolinic acid toxicity in the neuropathology of glutaric acidemia type I. Mol Neurobiol 53:6459–6475. https://doi.org/10.1007/s12035-015-9548-9
Scriver C, Beaudt A, Sly W, Valle D (2001) The metabolic and molecular bases of inherited disease, 8th edn. McGraw- Hill, NY
Shin EJ, Hwang YG, Pham DT, Lee JW, Lee YJ, Pyo D, Jeong JH, Lei XG, Kim HC (2018) Glutathione peroxidase-1 overexpressing transgenic mice are protected from neurotoxicity induced by microcystin-leucine-arginine. Environ Toxicol 33:1019–1028. https://doi.org/10.1002/tox.22580
Singh A, Kukreti R, Saso L, Kukreti S (2019) Oxidative stress: a key modulator in neurodegenerative diseases. Molecules 24:1583. https://doi.org/10.3390/molecules24081583
Steiner J, Bogerts B, Schroeter ML, Bernstein HG (2011) S100B protein in neurodegenerative disorders. Clin Chem Lab Med 49:409–424. https://doi.org/10.1515/CCLM.2011.083
Tarasenko TN, McGuire PJ (2017) The liver is a metabolic and immunologic organ: a reconsideration of metabolic decompensation due to infection in inborn errors of metabolism (IEM). Mol Genet Metab 121:283–288. https://doi.org/10.1016/j.ymgme.2017.06.010
Tarasov VV, Svistunov AA, Chubarev VN, Sologova SS, Mukhortova P, Levushkin D, Somasundaram SG, Kirkland CE, Bachurin SO, Aliev G (2020) Alterations of astrocytes in the context of schizophrenic dementia. Front Pharmacol 10:1612. https://doi.org/10.3389/fphar.2019.01612
Tarlá MR, Ramalho F, Ramalho LN, Silva T d C, Brandão DF, Ferreira J, Silva O d C, Zucoloto S (2006) Cellular aspects of liver regeneration. Acta Cir Bras 21:63–66. https://doi.org/10.1590/S0102-86502006000700015
Wajner M (2019) Neurological manifestations of organic acidurias. Nat Rev Neurol 15:253–271. https://doi.org/10.1038/s41582-019-0161-9
Wajner M, Amaral AU, Leipnitz G, Seminotti B (2019) Pathogenesis of brain damage in glutaric acidemia type I: Lessons from the genetic mice model. Int J Dev Neurosci 78:215–221. https://doi.org/10.1016/j.ijdevneu.2019.05.005
Wendel A (1981) Glutathione peroxidase. Methods Enzymol 77:325–332. https://doi.org/10.1016/s0076-6879(81)77046-0
Yagi K (1998) Simple procedure for specific assay of lipid hydroperoxides in serum or plasma. Methods Mol Biol 108:107–110. https://doi.org/10.1385/0-89603-472-0:107
Yarlagadda A, Alfson E, Clayton AH (2009) The blood brain barrier and the role of cytokines in neuropsychiatry. Psychiatry (Edgmont) 6:18–22
Zhang Y, Jia H, Jin Y, Liu N, Chen J, Yang Y, Dai Z, Wang C, Wu G, Wu Z (2020) Glycine attenuates LPS-induced apoptosis and inflammatory cell infiltration in mouse liver. J Nutr. https://doi.org/10.1093/jn/nxaa036
Zhao Z, Wang Y, Zhou R, Li Y, Gao Y, Tu D, Wilson B, Song S, Feng J, Hong JS, Yakel JL (2020) A novel role of NLRP3-generated IL-1β in the acute-chronic transition of peripheral lipopolysaccharide-elicited neuroinflammation: implications for sepsis-associated neurodegeneration. J Neuroinflammation 17:64. https://doi.org/10.1186/s12974-020-1728-5
Zinnanti WJ, Lazovic J, Wolpert E, Antonetti DA, Smith MB, Connor JR, Woontner M, Goodman SI, Cheng KC (2006) A diet-induced mouse model for glutaric aciduria type I. Brain 129:899–910. https://doi.org/10.1093/brain/awl009
Zinnanti WJ, Lazovic J, Housman C, LaNoue K, O’Callaghan JP, Simpson I, Woontner M, Goodman SI, Connor JR, Jacobs RE, Cheng KC (2007) Mechanism of age-dependent susceptibility and novel treatment strategy in glutaric acidemia type I. J Clin Invest 117:3258–3270. https://doi.org/10.1172/JCI31617
Zinnanti WJ, Lazovic J, Housman C, Antonetti DA, Koeller DM, Connor JR, Steinman L (2014) Mechanism of metabolic stroke and spontaneous cerebral hemorrhage in glutaric aciduria type I. Acta Neuropathol Commun 2:13. https://doi.org/10.1186/2051-5960-2-13
Funding
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, #425914/2016-0), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS, #2266-2551/14-2), Fundo de Incentivo à Pesquisa e Eventos/Hospital de Clínicas de Porto Alegre (FIPE/HCPA, #120420), Financiadora de Estudos e Projetos/Rede Instituto Brasileiro de Neurociência (FINEP, #01.06.0842-00), Instituto Nacional de Ciência e Tecnologia em Excitotoxicidade e Neuroproteção (INCT-EN, #573677/2008-5), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Methodology performed by Bianca Seminotti and Alexandre U. Amaral. Experiments were performed by Bianca Seminotti, Alexandre U. Amaral, Mateus Grings, and César Augusto J. Ribeiro. Data analysis was carried out by Bianca Seminotti, Guilhian Leipnitz, and Moacir Wajner. The first draft of the manuscript was written by Bianca Seminotti and all authors revised previous versions of the manuscript. Funding acquisition and resources were provided by Moacir Wajner. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
International, national, and institutional guidelines for the care and use of animals were followed. The experimental protocol was approved by the Ethical Committee for the Care and Use of Laboratory Animals of the Hospital de Clínicas de Porto Alegre (RS, Brazil), number 2012-0420. This article does not contain studies with human participants.
Consent to Participate
Not applicable
Consent for Publication
Not applicable
Code Availability
Not applicable
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Seminotti, B., Amaral, A.U., Grings, M. et al. Lipopolysaccharide-Elicited Systemic Inflammation Induces Selective Vulnerability of Cerebral Cortex and Striatum of Developing Glutaryl-CoA Dehydrogenase Deficient (Gcdh−/−) Mice to Oxidative Stress. Neurotox Res 38, 1024–1036 (2020). https://doi.org/10.1007/s12640-020-00291-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-020-00291-0