Abstract
Changes in the concentrations of trace metals such as zinc (Zn) and selenium (Se) can pathologically lead to neurodegenerative conditions such as the Alzheimer’s disease (AD). Previous studies have shown that mitochondrial dysfunction plays an important role in the pathogenesis of AD. Several male Wistar rats were randomly divided into five groups: sham group, AD group that received 3 mg/kg of streptozotocin (STZ) intracerebroventricularly, AD + Zn group that received 10 mg/kg of Zn intraperitoneally (i.p.) for 1 week, AD + Se group that received 0.1 mg/kg of Se i.p. for 1 week, and AD + Zn + Se group that received 10 mg/kg of Zn i.p. plus 0.1 mg/kg of Se i.p. for 1 week. At end of the study, behavioral tests and mitochondrial oxidative stress and GPR39 gene expression evaluations were carried out. Co-administration of Zn and Se significantly decreased the potential collapse of mitochondrial membrane, reactive oxygen species levels, and lipid peroxidation levels while significantly increased cognitive performance, superoxide dismutase (SOD), glutathione peroxidase, and catalase activity in the brain mitochondria compared with the STZ group. In addition, no significant changes were observed in GPR39 expression in the co-treated group. Findings of the current study showed that ZnR/GPR39 receptor, mitochondrial dysfunction, and oxidative stress play important roles in the pathogenesis of AD. Co-treatment of Zn and Se improved the cognitive performance, mitochondrial dysfunction, and oxidative stress caused by STZ-induced AD. Therefore, therapeutic approaches to improve mitochondrial function could be effective in preventing the initiation and progression of AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is as a progressively chronic neurodegenerative disorder that targets elderly people through cognitive dysfunction (Bondi et al. 2017; dos Santos et al. 2018; Nobis and Husain 2018). The origin of this often sporadic disease is unclear, and several mechanisms may be involved in it, including environmental factors, mitochondrial haplotypes, free radical-mediated processes, genetic factors, gender, and age (Kandimalla et al. 2017; Tramutola et al. 2017; Wang and Brinton 2016).
Mitochondrial disorders are responsible for numerous neurodegenerative diseases, including Alzheimer’s disease, Huntington’s disease (HD), and Parkinson’s disease (PD). Recent evidence suggests that alterations in mitochondrial metabolism, especially the electron transport chain, may be involved in the pathogenesis of AD. Mitochondria are the main source of energy for cells and important regulators of cell death. Mitochondria play an important role in cellular processes including calcium homeostasis, apoptosis, reactive oxygen species (ROS) generation, signaling pathways, cell cycle regulation, and thermogenesis. Oxidative damage leads to ROS generation and mitochondrial dysfunction, which are responsible for many neurodegenerative disorders (Beal 2005; Desler et al. 2012; Fernandez-Checa and Kaplowitz 2005; Mari et al. 2009).
The “metal hypothesis” is one of the causes of the Alzheimer’s that has received much attention in the last two decades. This hypothesis focuses on diseases associated with changes in the amount of metals in the brain that eventually lead to clinical symptoms. Among the essential trace elements, zinc (Zn) plays a critical role in brain development and correct brain functions and it has many functions in hundreds of enzymes and protein domains (Yuan et al. 2014). Zn ion imbalance in the central nervous system has a prominent role in the pathogenesis and etiology of many neurological disorders such as AD, epilepsy, and depression. Zn has been identified as a ligand for activating or blocking a number of receptors in the brain. GPR39 has recently been identified as a specific zinc receptor that is expressed in various parts of the brain including the hippocampus, frontal cortex, and amygdala. Studies have shown that Zn binding to GPR39 receptor has therapeutic potentials (Khan 2016). Activation of ZnR/GPR39 stimulates the cellular pathways associated with cell growth and survival. Overall, ZnR/GPR39 is involved in regulating the activity of ion transport mechanisms that are essential for the physiological functions of neural and epithelial cells. Therefore, ZnR/GPR39 could specifically and selectively be a potential target for the therapeutic activities of Zn. A distinctive feature of Alzheimer’s disease is the accumulation of beta-amyloid (Aβ) deposits, which is associated with neurological dysfunction, spinal injury, and impaired Ca2+ hemostasis. The Aβ binds to Zn2+, a metal that is released from synaptic glutamatergic vesicles during neural activity. Zn2+ release in synapses activates mZnR/GPR39 and Ca2+ signaling in postsynaptic neurons. Studies have shown that Zn2+ signaling via ZnR/GPR39 is impaired by Aβ, which is an important pathological factor of Alzheimer’s disease (Hershfinkel 2018; Khan 2016).
As another trace element, selenium (Se) has received extensive consideration as an essential micronutrient. Se is present in the active site of glutathione peroxidase enzyme. Se is involved in many biochemical and physiological processes including regulation of ion flux in membranes, antibody synthesis, and coenzyme Q biosynthesis. Studies have shown that Se improved behavioral and biochemical functions in the brains of AD rats, which may be related to its antioxidant properties (Lakshmi et al. 2015; Loef et al. 2011). Pretreatment with low and high doses of Se and Zn for 6 months significantly reduced the oxidative stress and apoptosis induced by sodium fluoride in the rats’ kidneys (Deepmala et al. 2013; R.-A. Yu et al. 2006). The co-administration effects of Zn and Se that have been observed in a number of studies have shown that their combined effects had hepatoprotective, neuroprotective, and nephroprotective effects (Imed et al. 2008). This study aimed to investigate the simultaneous effect of Zn and Se administration on mitochondrial oxidative stress and the specific zinc receptor GPR39 expression in the brain tissue of AD rats.
Materials and Methods
Chemicals
4-(2-Hydroxyethyl)-1-piperazine-ethanesulfonic acid (HEPES), mannitol, ethylene glycol tetraacetic acid (EGTA), bovine serum albumin (BSA), 2,7-dichlorofluorescin diacetate (DCFH-DA), Coomassie Brilliant Blue, malondialdehyde (MDA), Tris–HCl, tetramethoxypropane (TEP), KCl, Na2HPO4, MgCl2, and ehodamine 123 (Rh123) were purchased from Sigma-Aldrich Company (St. Louis, MO). Sucrose and 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) were acquired from Merck Company (Darmstadt, Germany). All the other chemicals were of the highest analytical grade available.
Animals
Fifty male Wistar rats (6-week-old, 250 ± 20 g) were obtained from the central animal house of Ahvaz Jundishapur University of Medical Sciences (AJUMS, Ahvaz, Iran). The rats were kept under standard conditions (room temperature 20 ± 2 °C, 12-h light/dark cycle, and 50–55% humidity) with free access to water and food. Experiments were approved by the Ethics Committee of AJUMS and were in accordance with international guidelines for animal research.
Experimental Design
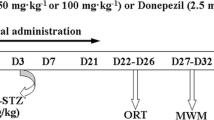
In this experimental study, 50 rats were randomly divided into five groups: The treatment schedule and the intervals for assessment of different factors are presented in Fig. 1.
Group I: Sham group which received PBS 5 ml/kg i.p. for 7 days.
Group II: AD group which received 3 mg/kg of streptozotocin (STZ) intracerebroventricularly (vehicle: artificial cerebrospinal fluid or a CSF including 147 mM NaCl, 1.6 mM MgCl2, 2.9 mM KCl, 1.7 mM CaCl2, and 2.2 mM dextrose) on the first day of the study; the recovery period of AD induction was considered to be 14 days.
Group III: Zn treatment group which received 10 mg/kg of Zn on the 14th day of the study for 7 days.
Group IV: Se treatment group which received 0.1 mg/kg of Se on the 14th day of the study for 7 days.
Group V: Zn + Se treatment group which received 10 mg/kg of Zn and 0.1 mg/kg of Se on the 14th day of the study for 7 days.
Twenty-four hours after the last injection, the rats were weighed and behavioral tests were carried out. Then, the rats were sacrificed and their brains were removed for evaluation of mitochondria isolation, mitochondrial oxidative stress tests, and GPR39 gene expression via real time PCR (Joshi et al. 2012).
Memory Assessment
Surgical Procedure
The rats were anesthetized by ketamine (60 mg/kg) and xylazine (10 mg/kg) using stainless steel 22-G gauge cannula for central drug injection and stereotaxic surgery. The needles were inserted into right and left ventricles bilaterally with the coordinates of DV 3.4 mm from the skull surface, ML ± 0.8 from the midline, and AP 1.6 mm from Bregma. Acrylic dental cement and two stainless steel screws were used to fix the needles. After the surgical procedure, the rats were placed in their own cages individually with a recovery period of 1 week prior to the drug injection (Sarkaki et al. 2019).
Morris Water Maze Test
The Morris water maze (MWM) test was performed to evaluate spatial memory in a dark circular pool with dimensions of 136 × 60 cm containing water to a depth of 25 cm at a temperature of 20 ± 1 °C. Four quadrants of north, east, south, and west were considered for the pool, and each examined animal was positioned in the water adjacent to the pool wall in one of the defined quadrants. A circular platform was placed in the middle of one of the quadrants in a way that it was 2 cm below the water’s surface. A camera was positioned above the pool in order to track the animal’s location, and then, the animal’s traveled distance and duration of movements were quantitatively measured using Ethovision video-tracking system (Noldus Information Technology, Wageningen, Netherlands). At first, each animal was placed in a random quadrant for four trial sessions (60 s) for four consecutive days in order to find the hidden platform according to a recommended program (training days). In the probe day, the platform was removed and the time percentage of swimming in the target quadrant was measured in one trial of 60 s (Sarkaki et al. 2019).
Shuttle Box Test
Briefly, the shuttle box apparatus, which was comprised of two dark and illuminated chambers (separated via a sliding door) and a floor made out of stainless steel bars (27 × 14.5 × 14 cm), was used to evaluate passive avoidance task. At first, the rats were allowed to roam freely between the chambers over a 10-min period without receiving any electric shock. After 1 day, the rats were placed in the illuminated chamber and the initial latency before moving to the dark chamber was recorded after lifting the door, upon which a single electric shock (50 Hz, 0.2 MA, 3 s) was administered to them. Twenty-four hours later, the exact same protocol was performed and the delay time before entering the dark chamber was recorded in a 300-s period (sarkaki 2019).
Mitochondrial Complex Estimation
Isolation of Mitochondria
The brains of all the rats were removed to prepare samples for isolation of mitochondria according to a previously described procedure (Brown et al. 2004). Homogenization of the brains was performed in an isolation buffer containing mannitol (215 mM), sucrose (75 mM), BSA (0.1%), HEPES (20 mM), and EGTA (1.0 μM) with a pH value of 7.4. The differential centrifugation method was used for isolation of mitochondria. Thus, the centrifugation of homogenates was performed at 4 °C at 1000×g for 8 min followed by collection of supernatants and re-centrifugation at 4 °C at 10,000×g for 10 min. In the next step, obtained pellets were suspended in an isolation buffer and re-centrifuged at 4 °C at 12,300×g for 10 min followed by re-collection of supernatants, re-suspension in the isolation buffer, and re-centrifugation at 4 °C at 12,300×g for 10 min. The final precipitate was resuspended in the isolation buffer to determine the amount of suspended protein.
Protein Content
The protein content in the mitochondria was measured using Coomassie Brilliant Blue protein assay (Bradford 1976) by the BSA as standard. The mitochondria’s protein levels (0.5 mg/ml) were used to normalize all mitochondrial assays (Bradford 1976).
Lipid Peroxidation Measurement
The protocol proposed by Zhang et al. was followed to measure the malondialdehyde (MDA) content. Thus, 0.5 mg of mitochondrial fractions per ml was incubated in the presence of different uranyl acetate levels at 37 °C for 1 h. Then, 0.25 ml of sulfuric acid, 0.05 M, and subsequently 0.3 ml of thiobarbituric acid (TBA) 0.2% were appended to 0.2 ml of mitochondrial fractions. The microtubes were first boiled in a water bath for 30 min and subsequently in an ice bath, which were followed by adding n-butanol (0.4 ml) to each tube and centrifugation for 10 min at 3500×g. The optical density (OD) of the supernatant was read by an ELISA reader (Tecan, Rainbow Thermo, Austria) at a wavelength of 532 nm to measure the formed MDA content in each sample, and the results were reported as nmol/mg protein in comparison with tetramethoxypropane (TEP) as the standard (Zhang et al. 2008).
GSH Measurement
The GSH content of the isolated mitochondria was measured by Ellman’s reagent (5,5′-dithiobis-(2-nitrobenzoic acid)) or DTNB. In the next step, 0.5 mg of mitochondrial fractions per ml was incubated in the presence of different uranyl acetate levels at 37 °C for 1 h. Then, the mitochondrial fraction (0.1 ml) was appended into phosphate buffer (0.1 M) and DTNB (0.04%) in the final volume of 3.0 ml with a pH value of 7.4. The OD of appeared yellow color was read at a wavelength of 412 nm using a spectrophotometer (UV-1601 PC, Shimadzu Corporation, Japan). The GSH concentration was reported in μmol/mg of protein (Sadegh and Schreck 2003).
Mitochondrial ROS Level Measurement
The mitochondrial ROS level was measured by fluorescent probe (DCFH). Afterward, the obtained brain mitochondria were poured into the respiration buffer, and then, the DCFH (10 μM) was appended to mitochondria, and the mixture was left for 10 min. Next, a fluorescence spectrophotometer (Shimadzu RF-5000U, λEm = 527 nm and λEx = 488 nm) was used to measure the fluorescence intensity of DCF (2,7-dichlorofluorescein) (Talari et al. 2014).
Mitochondrial Membrane Potential Measurement
The mitochondrial membrane potential (MMP, ΔΨm) was measured by mitochondrial uptake of the cationic fluorescent dye, rhodamine 123 (Rh123) (Pourahmad and O’Brien 2000). Therefore, 0.5 mg of mitochondrial protein fractions per ml was incubated in the presence of Rh123 (10 μM) in MMP assay buffer. Then, a fluorescence spectrophotometer (Shimadzu RF-5000U, λEm = 535 nm and λEx = 490 nm) was used to monitor the fluorescence (Baracca et al. 2003).
CAT Measurement
The proposed method for measuring catalase activity (CAT) was using a reaction mixture consisting of mitochondrial suspension, Tris-HCl (0.05 mM), and H2O2 (0.01 M), which were incubated for 10 min. After adding ammonium molybdate (4%) and measuring the absorbance rate at the wavelength of 410 nm, the obtained results were reported as U/mg protein.
SOD and GPX Measurements
Mitochondrial SOD and GPX activities were evaluated using ZellBio Company’s standard diagnostic kits while following the manufacturer’s instructions.
Gene Expression
Real-Time Quantitative PCR
Purification and concentration of total RNA extracted from the brains were measured with a spectrophotometer at 260 and 280 nm. The cDNA was constructed by total RNA (5 μg) of target gene GPR39, and the housekeeping reference gene HPRT was based on the guidelines of RNeasy plus universal mini kit and Quantinova reverse transcription kit. Duplicate final volumes (10 μl) of amplicons (5 μl SYBR green master mix, 1 μl ROX dye, 1.4 μl specific primers, 1.6 RNase free water, and 1 μl template cDNA) were placed in one-step real-time and cycled according to the instructions of Quantinova SYBER green PCR kit including PCR initial heat activation (2 min, 95 °C), denaturation (5 s, 95 °C), and combined annealing/extension (10 s, 60 °C) for 30–40 cycle. Specific primers HPRT (forward primer: GCTTCCTCCTCAGACCGCTT and reverse primer: CATCATCACTAATCACGACGCTG) as well as GPR39 (forward primer: CTCCACACGTTCCTCTTTGAG and reverse primer: ACCCATACAAAGCCGATCAG) were used in this protocol. At the end of the process, the specificity of PCR products was examined via the melting curve and the relative quantification of gene expression, and it was determined via the comparative threshold cycle (Ct) method using an arithmetic formula (2−∆∆Ct) (Egerod et al. 2007).
Statistical Analysis
Data was evaluated using one-way ANOVA followed by Turkey’s post hoc test; it was analyzed using GraphPad Prism software program (Inc., La Jolla, CA; Version 5), and expressed as mean ± SEM (n = 10). A value of P < 0.05 was considered statistically significant. Statistical differences in behavioral data were analyzed by two-way ANOVA.
Result
Memory Assessment
Spatial memory test: over training days of MWM, bilateral i.c.v. infusion of STZ significantly increased the latency time of AD rats to find the hidden platform versus the sham-operated group (P < 0.01 and P < 0.001). Rats treated with Zn + Se showed a significant decrease in days 3 and 4 compared with the AD rats (P < 0. 05). However, the same results were not observed in the groups that were injected with only one of the compounds. The time spent in the target quadrant (in percent) in the probe trials displayed a significant difference between the AD group and the sham group (P < 0.001), while the co-treatment group only showed a significant increase versus the AD group (P < 0. 05). Similarly, step-through latency (STL) had a significant decrease in the AD rats versus the sham group, while a significant increase was observed in the co-treatment group versus the AD group (Fig. 2).
Effects of Zn (10 mg/kg, i.p., vehicle: PBS 1 week) or/and Se (0.1 mg/kg, i.p., vehicle: PBS, 1 week) treatment on the spatial memory test (MWM) via measuring of latency time in the training days (a) and the time spent in the target quadrant in the probe trial test (b), as well as passive avoidance memory via evaluating step-through latency (c) after 2 weeks of recovery period from microinjection of STZ (3 mg/kg/10 μl/rat, i.c.v., vehicle: aCSF, once) for induction of the AD model in the rats. Data is presented as mean ± SEM (n = 10); ***P < 0.001 vs. sham group and #P < 0.05 vs. AD group
Mitochondrial Complex Estimation
Mitochondrial Membrane Damage and ROS Level Measurement
The data obtained from assessment of mitochondrial membrane damage and ROS levels demonstrated a significant increase in AD rats versus the sham group (P < 0.01). Meanwhile, the co-treatment group showed a significant decrease versus the AD group (P < 0.05) (Fig. 3).
Effects of Zn (10 mg/kg, i.p., vehicle: PBS, 1 week) or/and Se (0.1 mg/kg, i.p., vehicle: PBS, 1 week) treatment on the damage level of mitochondrial membrane (a) and ROS level (b) after 2 weeks of recovery period from microinjection of STZ (3 mg/kg/10 μl/rat, i.c.v., vehicle: aCSF, once) that induced the AD model. Data is presented as mean ± SEM (n = 10); **P < 0.01 vs. sham group and #P < 0.05 vs. AD group
Mitochondrial GSH and MDA Measurement
The mitochondrial GSH level showed a significant decrease in the AD rats versus the sham group (P < 0.01). While co-treatment with Zn + Se led to an increase in mitochondrial GSH level compared with the AD group. However, the increase was not statistically significant. The mitochondrial MDA level showed a significant increase in the AD rats versus the sham group (P < 0.01). While co-treatment with Zn + Se demonstrated a significant decrease in mitochondrial MDA level compared with the AD group (P < 0.05) (Fig. 4).
Effects of Zn (10 mg/kg, i.p., vehicle: PBS, 1 week) or/and Se (0.1 mg/kg, i.p., vehicle: PBS, 1 week) treatment on mitochondrial GSH level (a) and mitochondrial MDA level (b) after 2 weeks of recovery period from microinjection of STZ (3 mg/kg/10 μl/rat, i.c.v., vehicle: aCSF, once) that induced the AD model. Data is presented as mean ± SEM (n = 10); **P < 0.01 vs. sham group and #P < 0.05 vs. AD group
Mitochondrial SOD, GPX, and CAT Activities
Mitochondrial SOD, GPX, and CAT activities were significantly decreased in the AD rats versus the sham group (P < 0.001 and P < 0.05). Meanwhile, co-treatment with Zn + Se led to a significant increase in the activity of these enzymes compared with the AD group (P < 0.01 and P < 0.05). Zn treatment group showed a significant increase in mitochondrial SOD activity compared with the AD group (P < 0.05) (Fig. 5).
Effects of Zn (10 mg/kg, i.p., vehicle: PBS, 1 week) or/and Se (0.1 mg/kg, i.p., vehicle: PBS, 1 week) treatment on SOD (a), GPX (b), and catalase activities (c) in mitochondria after 2 weeks of recovery period from microinjection of STZ (3 mg/kg/10 μl/rat, i.c.v., vehicle: aCSF, once) that induced the AD model. Data is presented as mean ± SEM (n = 10); ***P < 0.001 and *P < 0.05 vs. sham group and ##P < 0.01 #P < 0.05 vs. AD group
Real-Time PCR of GPR39
Statistical analyses presented a significant decrease in GPR39 expression in the brains of AD rats in comparison with the sham group (P < 0. 01). Meanwhile, other treatment groups did not have statistically significant differences compared with the AD group (Fig. 6).
Effects of Zn (10 mg/kg, i.p., vehicle: PBS, 1 week) or/and Se (0.1 mg/kg, i.p., vehicle: PBS, 1 week) pretreatment on GPR39 expression after 2 weeks of recovery period from microinjection of STZ (3 mg/kg/10 μl/rat, i.c.v., vehicle: aCSF, once) that induced the AD model. Data is presented as mean ± SEM (n = 6); **P < 0.01 vs. sham analyzed via one-way ANOVA followed by the Tukey’s post hoc test for multiple comparisons
Discussion
A considerable number of studies have suggested that mitochondrial dysfunction and oxidative stress are involved in the Alzheimer’s disease progression. Mitochondrial pathology could be the main cause in the clinical manifestations of certain neurological disorders such as the Alzheimer’s disease. For this very reason, treatment modalities based on enhancing mitochondrial functions can be useful in treatment or prevention of these diseases (Hroudová et al. 2014). Studies have shown that mitochondria-targeting compounds and antioxidants including manganese, selenium, zinc, omega-3 polyunsaturated fatty acids, carnitine, vitamin C, vitamin E, coenzyme Q10, simvastatin, methylene blue, curcumin, and piracetam have potential effects on Alzheimer’s disease (Eckert et al. 2012; Paglia et al. 2016). Findings of the present work showed that co-treatment of rats with Zn and Se improved their performance in behavioral tests, decreased the mitochondrial membrane damage as an important factor in mitochondrial function, mitochondrial ROS levels as an central indicator of oxidative stress, and mitochondrial MDA levels as the final product of lipid peroxidation as well as increased GSH levels as an significant antioxidant in the mitochondrial and augmented the activity of SOD, GPX, and CAT enzymes as mitochondrial antioxidants against reactive free radicals. However, co-treatment with Zn + Se did not have a statistically significant effect on GPR39 expression in the brains of AD rats.
Oxidative stress and ROS generation are potential suppliers of AD’s progress. Oxidative stress is a state of imbalance between ROS generation and the antioxidant defense level, which causes oxidative damage to the brain. Brain cells are more susceptible to oxidative damage than other tissues because of their high amounts of unsaturated fatty acids, a weak antioxidant defense, and high oxygen content. Free radical overgeneration can change membrane lipid and lead to lipid peroxidation and eventually cell death (Xu et al. 2014). In the present study, ROS and MDA amounts were significantly increased in AD rats, while co-treatment with Zn and Se significantly decreased lipid peroxidation and ROS levels. These results indicated that the simultaneous use of Zn and Se increased their antioxidant capacity for preventing the occurrence and progression of AD, which was not observed in either treatment alone.
The mitochondrial membrane potential (ΔΨm) affects not only ROS production, but also other important mitochondrial functions such as ATP synthesis and calcium sequestration, thus being an important indicator of overall health or mitochondrial injury in neurons (Perry et al. 2011). The ability of Zn/Se co-treatment in preserving ΔΨm demonstrated that these metals could preserve the functional integrity of neuronal mitochondria in the brains of AD rats. Consistent with the current study, it has been reported that the mitochondria-targeted antioxidant MitoQ decreased ROS level, MDA amount, and mitochondrial membrane’s potential collapse, and inhibited the injury of spatial memory and oxidative stress in the brains of AD mice (McManus et al. 2011).
GSH level is extremely important in cellular free radical detoxification in the brain tissue. Mitochondrial SOD (MnSOD), as the first antioxidant enzyme in response to free radicals in mitochondria, converts superoxide anion radicals to hydrogen peroxide and oxygen. As two key enzymes in mitochondria, mitochondrial CAT and GPX eliminate the excess H2O2 (Khodayar et al. 2018). In the present study, the activities of mitochondrial GPX, CAT, and MnSOD enzymes as well as mitochondrial GSH amounts were significantly decreased in the brains of AD rats compared with the sham group. Meanwhile, co-treatment with Zn and Se significantly increased the activities of mitochondrial GPX, CAT, and MnSOD enzymes in comparison with the AD rats. However, co-treatment with Zn and Se did not result in a statistically significant change in mitochondrial GSH amounts compared with the AD rats. In agreement with the current study, it has been reported that zinc chloride (5 mg/kg for 3 days, i.p.) completely reversed the effects of depression and oxidative stress induced by malathion (a toxic organophosphate) including increased lipid peroxidation and decreased GPX or glutathione reductase activity in the cerebral cortex or hippocampus, respectively (Brocardo et al. 2007). Consistent with the present study, previous studies have shown that Se, as a key trace element, is involved in different metabolic and reduction/oxidation processes that could reportedly prevent the AD initiation and progression. However, no evidence supported the role of Se in AD treatment (Hatfield et al. 2014; Loef et al. 2011). The separate roles of Se and Zn in protecting brain mitochondrial antioxidants and electron transport chain enzymes following postnatal protein malnutrition have been demonstrated in another study. The consequences of protein malnutrition were a significant increase in lipid peroxidation level, ROS production, glutathione oxide level, mitochondrial swelling, and CAT activity in both the cortex and cerebellum of rats. Consumption of Zn and Se restored all the changes mentioned above (Adebayo et al. 2016).
Various studies have reported a significant reduction in Se levels in the patients suffering from the Alzheimer’s disease. Oxidative stress has been recognized as a consequence of the AD progress. Several antioxidants provide protection against the oxidative damage to brain tissue that is caused by stress. Se plays an essential role in the brain function. As an antioxidant, it is inserted in brain tissue selenoproteins such as selenoprotein P, glutathione peroxidase, and thioredoxin reductases, and other selenoprotein oxidoreductases in the antioxidant defense. In the pathology of AD, selenoprotein P is known as a donor of Se in the synthesis of other selenoproteins due to its direct and indirect antioxidant roles. Studies on selenoprotein P and other selenoproteins in AD have prominently indicated that these status may be related to activities of selenoprotein antioxidants in the brain tissue. Therefore, it can be concluded that the antioxidant defense is weakened by a reduction in the amount of SE and its availability, which results in cognitive disturbances in neurological disorders such as AD (Tamtaji et al. 2019; Varikasuvu et al. 2019).
The pathogenesis of AD is closely associated with synaptic Zn content and Zn homeostasis (Lee et al. 2002; W. H. Yu et al. 2001). GPR39 is known as a Zn-sensing receptor that recognizes extracellular Zn content and regulates the cell growth processes and survival signaling pathways (Hershfinkel 2018). Thus, the ZnR/GPR39 as a pivotal parameter for regulating cell growth can trigger downstream pathway of ion transport, absorption, and ion gradients (Azriel-Tamir et al. 2004; Ranasinghe et al. 2015; Saadi et al. 2012; Sharir et al. 2010; Sunuwar et al. 2017). Therefore, ZnR/GPR39 is an important target for the therapeutic purpose of Zn (Dittmer et al. 2008). In this study, GPR39 expression was significantly decreased in the brains of AD rats compared with the sham group. Meanwhile, co-treatment with Zn and Se and treatment with Zn and Se alone did not significantly change the GPR39 expression. Due to the lack of increased expression of GPR39 receptor that was observed in the treatment and co-treatment groups of Zn, it is possible that Zn is associated with a large number of proteins in the cells or extracellular domain and it modulates their activity, and as a result, changes in Zn concentration may affect cellular function and activity of many proteins and not just ZnR/GPR39 activity (Hershfinkel 2018).
Conclusion
The findings of the current study showed that ZnR/GPR39 receptor, mitochondrial dysfunction, and oxidative stress have critical roles in the pathogenesis of AD. The data suggested that co-treatment of Zn and Se improved cognitive performance in MWM and shuttle box. In addition, co-treatment could improve the oxidative stress in the mitochondria extracted from the brains of STZ-induced AD rats. Therefore, therapeutic approaches to enhance and improve mitochondrial function could be effective in preventing the initiation and progression of AD.
References
Adebayo OL, Adenuga GA, Sandhir R (2016) Selenium and zinc protect brain mitochondrial antioxidants and electron transport chain enzymes following postnatal protein malnutrition. Life Sci 152:145–155
Azriel-Tamir H, Sharir H, Schwartz B, Hershfinkel M (2004) Extracellular zinc triggers ERK-dependent activation of Na+/H+ exchange in colonocytes mediated by the zinc-sensing receptor. J Biol Chem 279(50):51804–51816
Baracca A, Sgarbi G, Solaini G, Lenaz G (2003) Rhodamine 123 as a probe of mitochondrial membrane potential: evaluation of proton flux through F0 during ATP synthesis. Biochimica et Biophysica Acta (BBA)-Bioenergetics 1606(1–3):137–146
Beal MF (2005) Mitochondria take center stage in aging and neurodegeneration. Ann Neurol 58(4):495–505
Bondi MW, Edmonds EC, Salmon DP (2017) Alzheimer’s disease: past, present, and future. J Int Neuropsychol Soc 23(9–10):818–831
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Brocardo PS, Assini F, Franco JL, Pandolfo P, Müller YM, Takahashi RN et al (2007) Zinc attenuates malathion-induced depressant-like behavior and confers neuroprotection in the rat brain. Toxicol Sci 97(1):140–148
Brown MR, Sullivan PG, Dorenbos KA, Modafferi EA, Geddes JW, Steward O (2004) Nitrogen disruption of synaptoneurosomes: an alternative method to isolate brain mitochondria. J Neurosci Methods 137(2):299–303
Deepmala J, Deepak M, Srivastav S, Sangeeta S, Kumar SA, Kumar SS (2013) Protective effect of combined therapy with dithiothreitol, zinc and selenium protects acute mercury induced oxidative injury in rats. J Trace Elem Med Biol 27(3):249–256
Desler C, Hansen TL, Frederiksen JB, Marcker ML, Singh KK, Juel Rasmussen L (2012) Is there a link between mitochondrial reserve respiratory capacity and aging? J Aging Res 2012:1–9
Dittmer S, Sahin M, Pantlen A, Saxena A, Toutzaris D, Pina A-L, Geerts A, Golz S, Methner A (2008) The constitutively active orphan G-protein-coupled receptor GPR39 protects from cell death by increasing secretion of pigment epithelium-derived growth factor. J Biol Chem 283(11):7074–7081
dos Santos P, Leide C, Ozela PF, de Fatima de Brito Brito M, Pinheiro AA, Padilha EC et al (2018) Alzheimer’s disease: a review from the pathophysiology to diagnosis, new perspectives for pharmacological treatment. Curr Med Chem 25(26):3141–3159
Eckert GP, Renner K, Eckert SH, Eckmann J, Hagl S, Abdel-Kader RM, Kurz C, Leuner K, Muller WE (2012) Mitochondrial dysfunction—a pharmacological target in Alzheimer’s disease. Mol Neurobiol 46(1):136–150
Egerod KL, Holst B, Petersen PS, Hansen JB, Mulder J, Hökfelt T, Schwartz TW (2007) GPR39 splice variants versus antisense gene LYPD1: expression and regulation in gastrointestinal tract, endocrine pancreas, liver, and white adipose tissue. Mol Endocrinol 21(7):1685–1698
Fernandez-Checa JC, Kaplowitz N (2005) Hepatic mitochondrial glutathione: transport and role in disease and toxicity. Toxicol Appl Pharmacol 204(3):263–273
Hatfield DL, Tsuji PA, Carlson BA, Gladyshev VN (2014) Selenium and selenocysteine: roles in cancer, health, and development. Trends Biochem Sci 39(3):112–120
Hershfinkel M (2018) The zinc sensing receptor, ZnR/GPR39, in health and disease. Int J Mol Sci 19(2):439
Hroudová J, Singh N, & Fišar Z (2014) Mitochondrial dysfunctions in neurodegenerative diseases: relevance to Alzheimer’s disease. BioMed Res Int, 2014
Imed M, Fatima H, Abdelhamid K (2008) Protective effects of selenium (Se) and zinc (Zn) on cadmium (Cd) toxicity in the liver and kidney of the rat: histology and Cd accumulation. Food Chem Toxicol 46(11):3522–3527
Joshi, M., Akhtar, M., Najmi, A., Khuroo, A., & Goswami, D. (2012). Effect of zinc in animal models of anxiety, depression and psychosis. Human & experimental toxicology, 31(12), 1237–1243
Kandimalla R, Thirumala V, Reddy PH (2017) Is Alzheimer’s disease a type 3 diabetes? A critical appraisal. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 1863(5):1078–1089
Khan MZ (2016) A possible significant role of zinc and GPR39 zinc sensing receptor in Alzheimer disease and epilepsy. Biomed Pharmacother 79:263–272
Khodayar MJ, Kalantari H, Khorsandi L, Rashno M, Zeidooni L (2018) Betaine protects mice against acetaminophen hepatotoxicity possibly via mitochondrial complex II and glutathione availability. Biomed Pharmacother 103:1436–1445
Lakshmi B, Sudhakar M, Prakash KS (2015) Protective effect of selenium against aluminum chloride-induced Alzheimer’s disease: behavioral and biochemical alterations in rats. Biol Trace Elem Res 165(1):67–74
Lee J-Y, Cole TB, Palmiter RD, Suh SW, Koh J-Y (2002) Contribution by synaptic zinc to the gender-disparate plaque formation in human Swedish mutant APP transgenic mice. Proc Natl Acad Sci 99(11):7705–7710
Loef M, Schrauzer GN, Walach H (2011) Selenium and Alzheimer’s disease: a systematic review. J Alzheimers Dis 26(1):81–104
Mari M, Morales A, Colell A, García-Ruiz C, Fernández-Checa JC (2009) Mitochondrial glutathione, a key survival antioxidant. Antioxid Redox Signal 11(11):2685–2700
McManus MJ, Murphy MP, Franklin JL (2011) The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer’s disease. J Neurosci 31(44):15703–15715
Nobis L, Husain M (2018) Apathy in Alzheimer’s disease. Curr Opin Behav Sci 22:7–13
Paglia G, Miedico O, Cristofano A, Vitale M, Angiolillo A, Chiaravalle AE et al (2016) Distinctive pattern of serum elements during the progression of Alzheimer’s disease. Sci Rep 6(1):1–12
Perry SW, Norman JP, Barbieri J, Brown EB, Gelbard HA (2011) Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniques 50(2):98–115
Pourahmad J, O’Brien PJ (2000) Contrasting role of Na+ ions in modulating Cu+ 2 or Cd+ 2 induced hepatocyte toxicity. Chem Biol Interact 126(2):159–169
Ranasinghe P, Wathurapatha W, Ishara M, Jayawardana R, Galappatthy P, Katulanda P, Constantine G (2015) Effects of zinc supplementation on serum lipids: a systematic review and meta-analysis. Nutr Metab 12(1):26
Saadi R, He K, Hartnett K, Kandler K, Hershfinkel M, Aizenman E (2012) SNARE-dependent upregulation of potassium chloride co-transporter 2 activity after metabotropic zinc receptor activation in rat cortical neurons in vitro. Neuroscience 210:38–46
Sadegh C, Schreck RP (2003) The spectroscopic determination of aqueous sulfite using Ellman’s reagent. MURJ 8:39–43
Sarkaki A, Farbood Y, Mansouri SMT, Badavi M, Khorsandi L, Dehcheshmeh MG, Shooshtari MK (2019) Chrysin prevents cognitive and hippocampal long-term potentiation deficits and inflammation in rat with cerebral hypoperfusion and reperfusion injury. Life Sci 226:202–209
Sharir H, Zinger A, Nevo A, Sekler I, Hershfinkel M (2010) Zinc released from injured cells is acting via the Zn2+−sensing receptor, ZnR, to trigger signaling leading to epithelial repair. J Biol Chem 285(34):26097–26106
Sunuwar L, Asraf H, Donowitz M, Sekler I, Hershfinkel M (2017) The Zn2+−sensing receptor, ZnR/GPR39, upregulates colonocytic Cl− absorption, via basolateral KCC1, and reduces fluid loss. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 1863(4):947–960
Talari M, Seydi E, Salimi A, Mohsenifar Z, Kamalinejad M, & Pourahmad J (2014) Dracocephalum: novel anticancer plant acting on liver cancer cell mitochondria. BioMed Res Int, 2014
Tamtaji OR, Heidari-Soureshjani R, Mirhosseini N, Kouchaki E, Bahmani F, Aghadavod E et al (2019) Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: a randomized, double-blind, controlled trial. Clin Nutr 38(6):2569–2575
Tramutola A, Lanzillotta C, Perluigi M, Butterfield DA (2017) Oxidative stress, protein modification and Alzheimer disease. Brain Res Bull 133:88–96
Varikasuvu SR, Prasad S, Kothapalli J, Manne M (2019) Brain selenium in Alzheimer’s disease (BRAIN SEAD study): a systematic review and meta-analysis. Biol Trace Elem Res 189(2):361–369
Wang Y, Brinton RD (2016) Triad of risk for late onset Alzheimer’s: mitochondrial haplotype, APOE genotype and chromosomal sex. Front Aging Neurosci 8:232
Xu P-x, Wang S-w, Yu X-l, Su Y-j, Wang T, Zhou W-w et al (2014) Rutin improves spatial memory in Alzheimer’s disease transgenic mice by reducing Aβ oligomer level and attenuating oxidative stress and neuroinflammation. Behav Brain Res 264:173–180
Yu R-A, Xia T, Wang A-G, Chen X-M (2006) Effects of selenium and zinc on renal oxidative stress and apoptosis induced by fluoride in rats. Biomed Environ Sci 19(6):439–444
Yu WH, Lukiw WJ, Bergeron C, Niznik HB, Fraser PE (2001) Metallothionein III is reduced in Alzheimer’s disease. Brain Res 894(1):37–45
Yuan Y, Niu F, Liu Y, Lu N (2014) Zinc and its effects on oxidative stress in Alzheimer’s disease. Neurol Sci 35(6):923–928
Zhang F, Xu Z, Gao J, Xu B, Deng Y (2008) In vitro effect of manganese chloride exposure on energy metabolism and oxidative damage of mitochondria isolated from rat brain. Environ Toxicol Pharmacol 26(2):232–236
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
This study was supported by a grant (APRC-9603) from the Physiology Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Farbood, Y., Sarkaki, A., Mahdavinia, M. et al. Protective Effects of Co-administration of Zinc and Selenium Against Streptozotocin-Induced Alzheimer’s Disease: Behavioral, Mitochondrial Oxidative Stress, and GPR39 Expression Alterations in Rats. Neurotox Res 38, 398–407 (2020). https://doi.org/10.1007/s12640-020-00226-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-020-00226-9