Abstract
This study investigated the influences of lanthanum (La) exposure on learning and memory and the expression of apoptosis-related proteins in offspring rats. Wistar female rats were randomly divided into a control group (NC) and 0.25%, 0.5% and 1.0% LaCl3 treatment groups, with eight per group. La dye was transmitted to offspring rats through parental blood circulation and breast milk before delactation and through water drinking after delectation. Offspring rats were killed at 14, 28 and 42 days after birth. Hippocampal neurons were observed by microscope, and apoptosis and necrosis were tested. The expression levels of apoptosis-related proteins were detected by Western blot, and Morris water maze experiments were used to measure learning and memory abilities. LaCl3 groups showed longer escape latency periods and swimming distances than the NC group (p < 0.05). The 1.0% LaCl3 group passed across the target quadrants and platforms more times and stayed in the target quadrants for less time, than the NC group (p < 0.05). At 42 days, the apoptosis rate and necrosis in the hippocampus of the 1.0% LaCl3 group were significantly higher than those of other groups. There was a significant difference among LaCl3 groups in terms of protein expressions measured in the hippocampus. In LaCl3 groups, caspase-3 and caspase-9 were significantly higher than in the NC group (p < 0.05). Therefore, La exposure can promote neuronal apoptosis by regulating the protein expressions of Akt, Bcl-2, Bcl-xl, Bax, Bad, caspase-3 and caspase-9, thus damaging learning and memory and the hippocampal neurons of offspring rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rare earth elements comprise 15 lanthanide elements, scandium (SC) and yttrium (Y). With the extensive application of rare earth elements in agricultural, industrial, animal husbandry and medical fields and, especially, the extensive promotion of rare earth micro-fertilizers in agriculture, increasing amounts of rare earth elements are entering soil environments. Inevitably, they will enter the human body through the food chain. The short- and long-term impacts of rare earth elements on the environment, ecology and human physical health have become of wide concern (Zhuang et al. 2017). It has been reported that La is an extensively used rare earth element and can be used as a representative of their toxicity. La can accumulate in the human brain, causing nervous system impairment and dysfunction. However, the damage mechanism of La remains unknown. Apoptosis of neurons is an important potential mechanism of nervous system impairment. The apoptosis process is strictly regulated by multiple genes and involves many proteins.
As a serine/threonine protein kinase, Akt participates in the mediation of cell growth and proliferation. Akt is not only a signal medium of cell survival but is also an essential factor in cell survival (Ernens et al. 2006; Ullah et al. 2006). It can inhibit cell apoptosis through multiple pathways. In the process of cell apoptosis, proteins of the Bcl-2 family and caspase family play very important roles.
The Bcl-2 family is a key regulatory molecule in the process of cell apoptosis and forms a very complicated interaction network composed of anti- and pro-apoptotic members, which regulate cell apoptosis together. Within the Bcl-2 family, Bcl-2 and Bcl-xl can inhibit cell apoptosis, while Bad and Bax can promote it. Some studies have demonstrated that the interaction of proteins in the Bcl-2 family forms homotypic and/or heterotypic dimers. The balance between them determines the occurrence of cell apoptosis (Wang et al. 1998).
The caspase family plays a crucial role in the process of cell apoptosis. Cell apoptosis can be activated by mitochondrial stress conditions, which have an internal or mitochondrial pathway. Cytochrome c (Cyt c) is the first kind of apoptin released by mitochondria. After being released from mitochondria to the cytoplasm, Cyt c can activate caspase-9 and further activate downstream caspase-3, resulting in apoptosis (Würstle et al. 2012).
Akt can inhibit apoptosis by adjusting relevant loci of the Bcl-2 and caspase families. In this study, an offspring rat model of different concentrations of LaCl3 contamination was established to observe growth status and spatial learning and memory abilities in offspring rats and to test hippocampal neuron apoptosis and necrosis as well as changes in the expressions of p-Akt, Bcl-2, Bcl-xl, Bax, Bad, caspase-3 and caspase-9 proteins. On this basis, the role of the Akt pathway in La-induced hippocampal neuron apoptosis in offspring rats at different growth stages was elucidated.
Materials and Methods
Experimental Animals and Grouping
Wistar female rats (n = 32) were randomly divided into one control group (NC) and three LaCl3 treatment groups with eight rats per group. The three experimental groups were fed a distilled water solution containing 0.25%, 0.5% or 1.0% LaCl3. The NC group was fed pure distilled water. Male and female rats were raised in the same cage at a ratio of 1:1. Rats with sperm detected in their vagina on the next morning were recorded as being at 0 days of pregnancy. La dye was given to all experimental groups via free water drinking. La dye was transmitted to offspring rats through blood circulation from the parent’s body and through breast milk before delactation and through free water drinking after delactation. La dye induction was finished 1 month after delactation. Body weights of pregnant and offspring rats were recorded throughout the experiment. Brain tissues of offspring rats at 14, 28 and 42 days after birth were collected.
Test of Growth Situations of Offspring Rats
At 14, 28 and 42 days after birth, offspring rats were weighed and euthanized by anaesthesia. Next, the heads of killed offspring rats were broken to separate and weigh the brain tissues. Subsequently, the hippocampus was weighed, and brain tissue and hippocampus coefficients were calculated (brain tissue coefficient = weight of brain tissue/bodyweight × 100%; hippocampus coefficient = weight of hippocampus/bodyweight × 100%).
Nissl Stain
Dewaxing of paraffin sections: Paraffin sections were put in xylene I for 20 min, xylene II for 20 min, absolute ethyl alcohol I for 5 min, absolute ethyl alcohol II for 5 min and 75% ethyl alcohol for 5 min successively. They were washed by tap water and stained with toluidine blue for 5 min and then washed again and differentiated by 1% glacial acetic acid. After washing with water, the sections were dried in an oven. Subsequently, paraffin sections were put in clean xylene for 5 min for transparency and then sealed with neutral resin. All sections were observed under a microscope, and images were collected and analysed.
Pathologic Histology of the Hippocampus of Offspring Rats
Offspring rats were killed, and brain tissues were collected immediately after anaesthesia. The brain tissues were fixed with 2.5% glutaraldehyde for 2 h and then washed three times with PBS. Subsequently, they were fixed with 1% osmic acid for 2 h and then washed three times with normal saline. A series of ethyl alcohol dehydration and acetone dehydration was then completed. Next, brain tissues were immersed in Epon812 epoxy resin overnight and then dried in a constant temperature oven for 24 h. Then, brain tissues were cut into sections on an LKB-V ultramicrotome and dyed with uranyl acetate and lead citrate. Finally, brain tissue sections were observed under a transmission electron microscope (Hitachi H-7650).
Cell Apoptosis Assay
The rats were killed immediately after anaesthesia and decapitated for brain extraction. The cells isolated from hippocampal tissue of the brain were washed with PBS for three times, and then the concentration of the cells was adjusted to 5 × 105–1 × 106/mL. An Annexin V-FITC/PI Cell Apoptosis Detection Kit (Trans Biotech, Beijing, China) was used to determine the extent of apoptosis and necrosis. Briefly, the cells were resuspended in 100 μL 1× Annexin Binding Buffer. After the addition of 5 μL Annexin V-FITC and 5 μL PI, the cells were stained for 15 min at RT in the dark. Then, 400 μL 1× Annexin Binding Buffer was added. Flow cytometry detection was done within 1 h using CytoFLEX S flow cytometer (Beckman coulter, USA).
Neuroethology Test
Morris water maze experiments: Training and test of learning and memory ability were carried out in a round pool with a water temperature of 22 ± 2 °C. Edible melanin was added to the pool to make the water non-transparent. The round pool was divided into four quadrants, and a hidden platform with a diameter of 15 cm was placed 2.0 cm below the water surface in the centre of the fourth quadrant. Offspring rats entered the water at the centres of the four quadrants and facing the pool wall. Each offspring rat was trained four times per day for 5 days. The time taken to find the underwater platform was recorded. If the rats found the platform within 60 s, they were allowed to stay on the platform for about 15 s; otherwise, they were guided to the platform where they remained for 15 s. Offspring rats were put in a maze following the two tests 1 week after the first 5 days of training. One test was a navigation experiment, in which the hidden platform was in the centre of the fourth quadrant and offspring rats entered the water at the same position. The escape latency period and total swimming distance (length of swimming path) were evaluated. The other test was a spatial exploration experiment in which the platform was eliminated 4 h after the navigation experiment. All groups of offspring rats were placed only in the opposite quadrant to the target quadrant. Offspring rats were placed in the water in the centre of the second quadrant and swam in the pool for 60 s. The number passing across the platform, the number entering the target quadrant, the time within the target quadrant and the search paths were recorded.
Determination of p-Akt, Bcl-2, Bcl-xl, Bax, Bad, Caspase-3 and Caspase-9 Protein Expression
The protein of the seahorse organization treated was extracted with RIPA cracking solution, and the protein concentrations were measured by BCA Kit (Sigma-Aldrich, St. Louis, MO, USA). Proteins were separated by 12% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Danvers, MA, USA). Five percent of non-fat milk was used to block PVDF membranes for 1 h at 37 °C. The dilution degrees of anti primary antibody (p-Akt, Bcl-2, Bcl-xl, Bax, Bad, caspase-3, caspase-9) and β-actin were 1:2000, 1:1000, 1:1000, 1:1000, 1:1000, 1:2000, 1:1000, 1:5000, respectively. These antibodies were hybridized overnight at 4 °C and were combined with the HRP-linked secondary antibody (1:5000, BOSTER, Wuhan, China) for 2 h at RT after PVDF membranes were washed. Finally, chemiluminescence (ECL) reagent (General Electric Healthcare, Aurora, OH, USA) was used to visualize the bands of the proteins. Flour Chen 2.0 software was employed to measure integrated density value (IDV) of the protein band with GAPDH as the inside reference.
Reverse Transcription-Quantitative PCR (RT-qPCR)
Total RNA was isolated from the hippocampus using MiniBEST Universal RNA Extraction Kits according to the manufacturer’s protocol (TaKaRa, Tokyo, Japan). Reverse transcription was performed on 1.2 μg of total RNA using Primescript RT Reagent kits with gDNA Eraser (TaKaRa, Tokyo, Japan). A Light Cycler 480 SYBR Green I Master (Roche, Basel, Switzerland) was used to carry out SYBR Green fluorescent quantitative PCR amplification with BIO-RAD CFX Connect (Bio-Rad, Hercules, CA, USA). GAPDH acted as a control for Akt. Primers were as follows: Akt forward, 5′-GCTGGAGGACAACGACTATG-3′ and reverse, 5′-CTTCTCATGGTCCTGGTTGTAG-3′; GAPDH forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse, 5′-CGCTTCACGAATTTGCGTGTCAT-3′. All experiments were repeated three times in each group, and 2-ΔΔCt was used in the quantification of Akt mRNA.
Statistical Analysis
Data analysis of intergroup differences was carried out by SPSS17.0 software using one-way ANOVA, and the difference of the comparison group was analysed with Student-Newman-Keuls (SNK) method. All data was presented as mean ± SEM, and p < 0.05 was considered as the significance level between groups. ap < 0.05 compared with NC group. bp < 0.05 compared with the 0.25% LaCl3 group. cp < 0.05 compared with the 0.5% LaCl3 group.
Results
Effects of LaCl3 on Growth of Offspring Rats in Different Stages
It can be seen from Supplemental Table 1 that there is no statistical difference in body weight, brain weight and hippocampus of offspring rats at 14 days. Body weight of the 1.0% LaCl3 group at 28 days is lower than that of NC. At 42 days, body weight of the 1.0% LaCl3 group is lower than those of NC and the 0.25% LaCl3 group, and the brain weight is lower than NC. At 28 days, brain coefficient and hippocampus coefficient of 1.0% LaCl3 group are higher than those of other experimental groups. At 42 days, hippocampus coefficient of 1.0% LaCl3 group is higher than those of NC and 0.25% LaCl3 group.
Effects of LaCl3 on the Expression Level of Nissl Bodies in CA1, CA3 and DG Zones of Hippocampus of Offspring Rats in Different Growth Stages
It could be seen from Fig. 1 that pyramidal cells in CA1, CA3 and DG zones of hippocampus of NC were in alignment and contained a lot of Nissl bodies. The expression of Nissl bodies in CA1, CA3 and DG zones of hippocampus of offspring rats was decreased with the increase of dose of LaCl3. In Supplemental Table 2, the expression of Nissl bodies in hippocampus of LaCl3 groups in three growth stages decreased significantly (p < 0.05). In particular, the expression of Nissl bodies in CA1, CA3 and DG zones of hippocampus of 1.0% LaCl3 group dropped since the 14 days.
Effects of LaCl3 on Ultra Microstructure of CA1zone of Hippocampus of Offspring Rats in Different Growth Stages
In Fig. 2, karyotheca of neurons in CA1 zone of hippocampus of NC group in three growth stages had clear profile, uniform chromatin distribution and integral organelles (e.g. mitochondria and Golgi apparatus) in neurons. As La dye continued, the neuronal chromatin in the CA1zone of hippocampus of 0.25% LaCl3 group was increased, while the number of organelles in neuron cytoplasm decreased slightly at 42 days. At 14 days, 1.0% LaCl3 group presented uniform neuronal chromatin and integral organelles in the CA1 zone of hippocampus of 1.0% LaCl3 group, but the cell nucleus moves toward peripheral regions. Neuronal heterochromatin began to concentrate in 1.0% LaCl3 group at the 28 days. Besides, neuronal shrinkage and karyotheca sinking in the CA1 zone of hippocampus of 1.0% LaCl3 group occurred at 42 days, and most organelles in cytoplasm were missing, thus developing cavities.
Effects of LaCl3 on Hippocampal Neuron Apoptosis and Neurosis of Offspring Rats in Different Growth Stages
It could be seen from Fig. 3a and b that hippocampal neuron apoptosis of experimental groups at 14 days was higher than that of NC group and the hippocampal neuron apoptosis of 1.0% LaCl3 group was higher than those of the rest two experimental groups (p < 0.05). At 28 days and 42 days, hippocampal neuron apoptosis of 0.25% LaCl3 group was significantly higher compared with NC group, and the hippocampal neuron apoptosis of 0.5% LaCl3 group was far higher than those of 0.25% LaCl3 and NC groups. Moreover, the hippocampal neuron apoptosis of 1.0% LaCl3 group was higher than other groups (p < 0.05).
Effects of LaCl3 on Learning and Memory Ability of Offspring Rats
Navigation Experiment
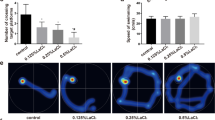
In Fig. 4a, the NC group had explicit swimming strategies starting from any quadrants and could find the hidden platform quickly. Besides, the 0.25% LaCl3 group also had a good searching route, while 0.5% and 1.0% LaCl3 groups were difficult to find the hidden platform. Some offspring rats even could only move around the wall in 60 s, but could not enter into the region of hidden platform. Based on analysis of Fig. 4b, the average escape latent period and swimming distance of offspring rats were positively related with the LaCl3 concentration (p < 0.05).
Spatial Exploration Experiment
The spatial exploration experiment of offspring rats at 42 days is shown in Fig. 5. As shown in Fig. 5a, the NC group runs through the platform region repeatedly. The 0.25% LaCl3 group also could find the hidden platform easily. However, number of entering into the platform region of the 0.5% LaCl3 group decreased significantly. The 1.0% LaCl3 group even swam bypassing the hidden platform. Figure 5b showed that the number of running through the platform, time spent in the target quadrant and the number of running through the platform of offspring rats of experimental groups were lower than that of NC. Moreover, the number of entering into the platform region of the 0.5% and 1.0% LaCl3 groups was significantly lower than that of the 0.25% LaCl3 group (F = 30.934 and p < 0.001), which proved that La exposure could deteriorate memory of offspring rats.
Effects of LaCl3 on p-Akt Protein and Akt mRNA Expression in Hippocampus of Offspring Rats in Different Stages
It could be seen from Fig. 6a and b that p-Akt protein expression level in hippocampus of the 1.0% LaCl3 group decreased at 14 days and 28 days (p < 0.05). At 42 days, p-Akt expression level in hippocampus of experimental groups was significantly lower than that of NC (p < 0.001). The p-Akt expression level in hippocampus of offspring rats decreased significantly with the increase of La dose, showing statistically significant differences (p < 0.001).
In Fig. 6c, Akt mRNA expression level in hippocampus of the 1.0% LaCl3 group cropped significantly at 14 days (p < 0.05). The Akt mRNA expression level in hippocampus of the 1.0% and 0.5% LaCl3 groups was significantly lower than that of NC at 28 days (p < 0.05). At 42 days, Akt mRNA expression level in hippocampus of three experimental groups declined observably (p < 0.05). With the increase of La dose, Akt mRNA expression level in hippocampus of offspring rats decreased gradually (p < 0.001).
Effects of LaCl3 on Bax Family in Hippocampus of Offspring Rats in Different Growth Stages
It could be seen from Fig. 7a and b that Bcl-2 expression level in hippocampus of the 1.0% LaCl3 group at 14, 28 and 42 days was significantly lower than those of rest groups. In addition, Bcl-2 expression level in hippocampus of the 0.5% LaCl3 group was remarkably lower compared with those of the 0.25% LaCl3 group and NC group (p < 0.05).
As shown in Fig. 7c and d, Bax expression level in hippocampus of the 0.25% LaCl3 group increased at 28 days and 42 days, showing statistically significant differences (p < 0.05). In these three growth stages, Bax expression levels in hippocampus of the 1.0% and 0.5% LaCl3 groups increased markedly (p < 0.05). These results suggest that Bax expression level in hippocampus of offspring rats was positively related with La dose.
Figure 7e and f showed that Bcl-xl expression level in hippocampus of the 1.0% LaCl3 group at 14 days was significantly lower than those of other experimental groups (p < 0.05). Bcl-xl expression level in hippocampus of experimental groups at 28 days and 42 days was dramatically lower than that of NC (p < 0.05). This reflects that Bcl-xl expression level in hippocampus of offspring rats was negatively related with La dose.
Bad expression level in hippocampus of experimental groups at 14, 28 and 42 days was outstanding higher than that of NC (Fig. 7g and h). At 14 days, Bad expression level in hippocampus of the 1.0% LaCl3 group was higher than those of the 0.25% and 0.5% LaCl3 groups (p < 0.05). Bad expression levels in hippocampus of the 0.5% and 1.0% LaCl3 groups at 28 days and 42 days were significantly higher compared with that of the 0.25% LaCl3 group (p < 0.05).
Effects of LaCl3 on Caspase-3 and Caspase-9 Expression in Hippocampus of Offspring Rats in Different Growth Stages
In Fig. 8a–c, the expression level of cleaved-caspase-3 protein in hippocampus of offspring rats at 14 days or 28 days increased with the increase of LaCl3 dose (p < 0.05). The variation trend of pro-caspase-3 expression level was similar with that of cleaved-caspase-3. There is no significant difference between the 0.5 and 1.0% LaCl3 groups at 14 days (p > 0.05). However, the expression level of cleaved-caspase-3 protein in hippocampus of experimental groups was significantly higher than that of NC at 42 days (p < 0.05). There is no significant difference among different experimental groups in terms of expression level of the cleaved-caspase-3 protein (p > 0.05).
It could be seen from Fig. 8d–f that expression levels of cleaved-caspase-9 and pro-caspase-9 proteins in hippocampus of experimental groups at 14, 28 and 42 days increased with the increase of LaCl3 lose and were significantly higher than that of NC (p < 0.05). At 42 days, expression levels of cleaved-caspase-9 and pro-caspase-9 in hippocampus of the 1.0% LaCl3 group were significantly higher than those of other experimental groups (p < 0.05).
Discussion
La is a representative of the light rare earth elements and can react with almost all elements and tissue components. Due to its high chemical activity and low atomic weight, La has a certain toxicity to the immune, reproductive and nervous systems. Therefore, La is often used as a representative substance to study the neurotoxicity of rare earth elements. It has been reported that LaCl3 exposure from drinking water can decrease body weight and influence the growth of offspring rats (Zheng et al. 2013). According to the experimental results of the present study, the body weights of the 1.0% LaCl3 group at 28 days and 42 days were significantly lower than that of NC rats, indicating that LaCl3 can influence the growth of offspring rats.
In the nervous system, La can enter into and accumulate in brain tissues and has strong bioactivity, thus influencing the development of the nervous system and causing neurodevelopmental disorders. According to the experiments, La exposure between pregnancy and 30 days after birth changed some neurobehaviours of offspring rats, such as the opening of ears and eyes, sense of touch, visual positional response, swimming and walking (Briner et al. 2000). The 0.25% La exposure between pregnancy and 50 days after birth damaged the learning and memory abilities of rats, indicating that La is a potential neurobehavioural teratogen (Feng et al. 2006). Moreover, La can enter offspring rats through the placenta and lactation, which might be caused by excessive La absorption due to imperfect development of the intestinal wall in young animals.
Nissl bodies, known as tiger spots, are a special type of nerve cell structure. A kind of alkaline matter, Nissl bodies have an extensive distribution in various neurons and mainly synthesize proteins. They are essential in neural activities as they update certain cell components and produce proteins and enzymes related to neurotransmitters. Most proteins at the axon terminals come from the Nissl bodies of soma. During conduction of excitation, neurons may consume some protein substances, and Nissl bodies can synthesize new proteins to supplement the consumed protein substances. Under physiological conditions, Nissl bodies are large in size and quantity, which reflects the strong protein synthesis of nerve cells. The quantity of Nissl bodies decreases and even disappears in impaired neurons. In this study, the numbers of cells in the CA1, CA3 and DG zones of the hippocampus of offspring rats varied. According to Nissl dye testing, nerve cells in the CA1, CA3 and DG zones of the hippocampus of NC rats had normal structures and were in alignment. There were abundant Nissl bodies in cytoplasm. With increases in La dose, the number of hippocampal neurons decreased gradually, and hippocampal neurons were arranged sparsely and accompanied by increasing intercellular spaces, decreasing Nissl bodies in cytoplasm and ambiguous boundaries. The expression levels of Nissl bodies in the CA1, CA3 and DG zones of the hippocampus in the 1.0% LaCl3 group at 14, 28 and 42 days were significantly lower than those in the NC group, indicating that La exposure can cause damage to neurons and toxicity to cells. According to the morphologies of cells in different growth stages, there was a great deal of cell alignment in the three hippocampal zones of offspring rats at 14 days. With growth of the offspring rats, the number of neurons at 42 days decreased gradually and the distribution density of neurons declined. This demonstrates that LaCl3 can accumulate to some extent and stay in the body for a long time, thus causing great toxicity.
After LaCl3 dye application, rats may have high La accumulations in the cerebral cortex, opisthencephalon and cortex. Meanwhile, the learning and memory abilities of rats are damaged (Zheng et al. 2013). Recently, Morris water maze tests (divided into navigation and spatial exploration experiments) have been widely applied to test learning and memory in rats and mice (Barnhart et al. 2015; Higaki et al. 2018; Ning et al. 2017). Some studies have proven that the integrity of hippocampal structures plays an important role in learning and memory. When the hippocampus is damaged, these abilities are damaged accordingly (Bruno et al. 2014; Sakamoto et al. 2014). The escape latency period and swimming distance of offspring rats were analysed in the navigation experiment. The numbers of offspring rats passing across the platforms and target quadrants, and the staying times in the target quadrants, were investigated in the spatial exploration experiment. Both experiments reflect the spatial learning and memory abilities of offspring rats in an experimental environment. This study found that in the navigation experiment, the 0.5% and 1.0% LaCl3 groups showed longer escape latency periods and took longer swimming distances to search the target quadrants than the NC group. In the spatial exploration experiment, the treatment groups took fewer runs across the target quadrants and platforms than the NC group and stayed in the target quadrant for shorter periods. This demonstrates that La exposure damaged the spatial learning and memory abilities of the offspring rats. Although LaCl3 can influence animal learning and memory and damage cells, the mechanism remains unknown and requires further exploration.
Cell apoptosis is an automatically ordered process of cell death in living organisms that is under genetic control. It is not only a special type of cell death; it also has important biological significance and complicated molecular biological mechanisms. In this study, the apoptosis rate and necrosis in the hippocampus of the 1.0% LaCl3 group at 42 days were significantly higher than those of the other groups, indicating that La exposure can cause hippocampal neuron apoptosis.
Akt is a member of the AGC serine/threonine protein kinase family. Its molecular structure is composed of a PH structural domain at the amino terminal, a middle catalysis domain and a regulatory domain at the carboxyl terminal. Due to the high homology of the catalysis region with protein kinase and protein kinase C, Akt is also called protein kinase B (Pearce et al. 2010). Akt is an important protein in the cascading reaction downstream pathway, which is mediated by growth factor receptor, tyrosine kinase and G protein-coupled receptors. Similar to many other protein kinases, Akt is at the intersection of cell death and survival. Therefore, Akt is crucial to cell growth and transfer, glucose and lipid metabolism, skeletal muscle and myocardial contraction, formation of new vessels and self-updating of stem cells, among others (Nitulescu et al. 2018). Akt regulates cell proliferation and growth. In this experiment, expression levels of p-Akt protein in the hippocampus of the 1.0% LaCl3 group at 14, 28 and 42 days were lower than those of NC rats. Moreover, p-Akt protein expression in hippocampus of offspring rats decreased gradually with increases in La dose, indicating that LaCl3 can inhibit p-Akt protein expression.
Akt can regulate vital movement through phosphorylation of downstream target proteins and thereby inhibit cell apoptosis. For instance, Akt can regulate the Bcl-2 and caspase families.
The Bcl-2 relevant protein was one of the first-discovered members of the BcL-2 family that can regulate cell apoptosis. It mainly exists in the mitochondrial nuclear membrane and adventitia. Bcl-2 relevant protein can maintain Ca2+ stability in cells by inhibiting the release of pro-apoptotic molecules (e.g. cytochrome C), inhibiting the concentration and pyrolysis of chromatin and weakening the accumulation of free radicals, thus inhibiting apoptosis (Hata et al. 2015). Bcl-2 can not only inhibit cell apoptosis but also prolong the life of cells (De Siqueira et al. 2015). The Bcl-2, Bcl-xl and Bax members of the Bcl-2 family can all form membrane channels on intracellular membranes. The formation of membrane channels also lays a foundation by which Bcl-2 proteins can develop their important physiological functions. Bcl-2 protein inhibits apoptosis by adjusting mitochondrial functions. Therefore, changes in mitochondrial functions are an important link in the cell apoptosis process.
Bax promotes apoptosis as an apoptosis-upregulating gene and is one of the primary pro-apoptosis genes in the human body. Bax can form a homodimer (Bax-Bax) in this coding region, which is the main structural basis for Bax to participate in cell apoptosis, thus promoting it directly (Liu et al. 2017). Bax has an extensive distribution in nerve nuclei, cytoplasm and cell membranes. Therefore, Bas is important to the development and dynamic balance of the central nervous system (Khazaei et al. 2017). Bax is a humongous protein of Bcl-2 and can inhibit cell apoptosis by forming a heterodimer (Bcl-2-Bax).
Bcl-xl is the first member of the Bcl-2 family to have its spatial structure interpreted (Kvansakul and Hinds 2013). Moreover, Bcl-xl is an important anti-apoptosis protein in the human body and mainly participates in cell apoptosis that regulates the mitochondrial pathway. Bcl-xl can protect the integrity of mitochondrial outer membranes (Llambi and Green 2011). In the endogenous apoptosis pathway, Bcl-xl can bind with pro-apoptosis protein Bax into a heterodimer, thus realizing the goal of anti-apoptosis. The relevant mechanism can be explained from two aspects. On the one hand, Bcl-xl restricts the permeabilization of Bax protein to mitochondria to improve the integrity of mitochondrial membranes and inhibit the release of Cyt C (Bleicken et al. 2016). On the other hand, Bcl-xl promotes Bax transfer from the mitochondria to cytoplasm (Renault et al. 2015) or makes Bax that is transferred from cytoplasm to mitochondria and back to cytoplasm (Edlich et al. 2011), thus developing the anti-apoptosis function. Bcl-xl can also develop an anti-apoptosis function that is independent of cytoplasm C.
The Bad protein is a member of the Bcl-2 family which participates in cell apoptosis. It is not only one of the earliest AKT target proteins but is also a member of the BH3 structural domain subfamily of the Bcl-2 family (Berg and Soreide 2012). Unlike most members of the Bcl-2 family, Bad contains no targeted C-terminal transmembrane domain of external mitochondrial membranes and karyotheca (Radisavljevic 2013). At phosphorylation by Akt, Bad forms the Bad-(14-3-3) protein heterodimer. The pro-apoptosis effect of Bad relies on the combination of Bcl-2 and Bcl-xl to regulate cell apoptosis. Bad can combine with Bcl-2 or Bcl-xl, thus hindering anti-apoptosis activity. Akt can make the Serl36 loci of Bad phosphorylated, thus restricting its interaction with Bcl-2 and Bcl-xl on the mitochondrial outer membranes of anti-apoptosis proteins. As a result, Bad combines with protein 14-3-3, and then it is kept out of the cytoplasm, thus inhibiting apoptosis. After induction of apoptosis via a similar way, Ser 184 loci on Bax is phosphorylated by Akt, which hinders Bax transfer onto mitochondria and prevents conformation changes to Bax (Karunagaran et al. 2005). According to the experiment, the expression levels of Bad in the hippocampus of the 1.0% LaCl3 group at 14, 28 and 42days were higher than those of the NC and 0.25% LaCl3 groups, while the expression levels of Bcl-2 were lower.
The caspase family is a group of proteolytic enzymes that cause cell apoptosis through the specific cutting of peptide bonds on aspartic acid residues. The caspase family can be divided into two types. One is the initial caspase, which acts in the early stage of cell apoptosis. The other one is executive caspase, which acts in the late stage of cell apoptosis. The precursor of initial caspase is cut and activated under the influence of external signals. The activated caspase can cut the precursor of executive caspase to activate it. The activated executive caspase triggers irreversible apoptosis through hydrolysis of target proteins (Flusberg and Sorger 2015; Green and Llambi 2015). In the caspase family, caspase-3 is essential in most apoptosis signal transduction pathways and assumes the core control effect. Moreover, caspase-3 plays the key activation role in the specific morphological and physiological changes involved in apoptosis. Caspase-3 is the most important member of the caspase family and is the key effector molecule of the final execution of apoptosis. Most factors that trigger apoptosis act via the caspase-3-mediated signal conduction pathway (Zhang et al. 2015). Nevertheless, caspase-3, an executor of apoptosis, is activated through activation of the apoptosis promoter of caspase-9. Caspase-9, which is one of the matrixes of Akt, is the promoter of the mitochondrial apoptosis pathway and plays an important role in apoptosis. In the mitochondrial pathway, apoptotic stimulator and apoptotic protease-activating factor (Apaf-1) form poly-complexes and then combine with the precursor of caspase-9 in the cytoplasm to form a great complex. Due to this great complex, the proenzyme of caspase-9 is decomposed into active forms, and caspase-9 activates downstream caspase-3 to trigger cascading reactions, executing the apoptosis program and finally inducing apoptosis (Means et al. 2015). On the contrary, the activity of caspase-9 can be weakened through phosphorylation by pro-apoptosis protein kinases including Akt. Phosphorylation of the Ser196 loci of caspase-3 and caspase-9 can be realized via Akt, which inactivates caspase-3 and caspase-9 and thereby inhibits the pro-apoptosis of caspase (Salvesen and Riedl 2008). According to the experimental results, caspase-3 contents in the hippocampal neurons of the three experimental groups were significantly higher than that in NC rats, indicating that LaCl3 can increase caspase-3 contents. Moreover, the expression level of caspase-3 increased with LaCl3 concentration.
In summary, La exposure can damage spatial learning and memory abilities, as well as the hippocampal neurons of offspring rats. It can inhibit the expression level of p-Akt, downregulate expressions of Bcl-2 and Bcl-xl and upregulate expressions of Bad, Bax, caspase-3 and caspase-9, finally promoting apoptosis of neurons.
Availability of Data and Material
All data generated or analysed during this study are included in this article.
References
Barnhart CD, Yang D, Lein PJ (2015) Using the Morris water maze to assess spatial learning and memory in weanling mice. PLoS One 10:e0124521
Berg M, Soreide K (2012) EGFR and downstream genetic alterations in KRAS/BRAF and PI3K/AKT pathways in colorectal cancer: implications for targeted therapy. Discov Med 14:207–214
Bleicken S, Hofhaus G, Ugarteuribe B, Schröder R, GarcíaSáez AJ (2016) Cbid, Bax and Bcl-xL exhibit opposite membrane remodeling activities. Cell Death Dis 7:e2121
Briner W, Rycek RF, Moellenberndt A, Dannull K (2000) Neurodevelopmental effects of lanthanum in mice. Neurotoxicol Teratol 22:573–581
Bruno TF, Luanna DMPF, Tavares NPA, Raiol DSMA, Walace GL et al (2014) Masticatory deficiency as a risk factor for cognitive dysfunction. Int J Med Sci 11:209–214
Edlich F, Banerjee S, Suzuki M, Cleland M, Arnoult D et al (2011) Bcl-xL retrotranslocates Bax from the mitochondria into the cytosol. Cell 145:104–116
Ernens I, Goodfellow SJ, Innes F, Kenneth NS, Derblay LE, White RJ, Scott PH (2006) Hypoxic stress suppresses RNA polymerase III recruitment and tRNA gene transcription in cardiomyocytes. Nucleic Acids Res 34:286–294
Feng L, Xiao H, He X, Li Z, Li F et al (2006) Long-term effects of lanthanum intake on the neurobehavioral development of the rat. Neurotoxicol Teratol 28:119–124
Flusberg DA, Sorger PK (2015) Surviving apoptosis: life–death signaling in single cells. Trends Cell Biol 25:446–458
Green DR, Llambi F (2015) Cell death signaling. Cold Spring Harb Perspect Biol 7:a006080
Hata AN, Engelman JA, Faber AC (2015) The BCL2 family: key mediators of the apoptotic response to targeted anticancer therapeutics. Cancer Discov 5:475–487
Higaki A, Mogi M, Iwanami J, Min LJ, Bai HY et al (2018) Predicting outcome of Morris water maze test in vascular dementia mouse model with deep learning. PLoS One 13:1–12
Karunagaran D, Rashmi R, Kumar TJ (2005) Induction of apoptosis by curcumin and its implications for cancer therapy. Curr Cancer Drug Targets 5:117–129
Khazaei S, Ramachandran V, Abdul Hamid R, Mohd Esa N, Etemad A et al (2017) Flower extract of Allium atroviolaceum triggered apoptosis, activated caspase-3 and down-regulated antiapoptotic Bcl-2 gene in HeLa cancer cell line. Biomed Pharmacother 89:1216–1226
Kvansakul M, Hinds MG (2013) Disease structural biology of the Bcl-2 family and its mimicry by viral proteins. Cell Death Dis 4:e909
Llambi F, Green DR (2011) Apoptosis and oncogenesis: give and take in the BCL-2 family. Curr Opin Genet Dev 21:12–20
Liu LS, Bai XQ, Gao Y, Wu Q, Ren Z, Li Q, Pan LH, He NY, Peng J, Tang ZH (2017) PCSK9 promotes oxldl-induced pc12 cell apoptosis through the bcl-2/Bax-caspase 9/3 signaling pathway. J Alzheimers Dis 57:723–734
Means JC, Venkatesan A, Gerdes B, Fan JY, Bjes ES, Price JL (2015) Drosophila spaghetti and doubletime link the circadian clock and light to caspases, apoptosis and tauopathy. PLoS Genet 11(5):e1005171
Ning H, Cao D, Wang H, Kang B, Xie S et al (2017) Effects of haloperidol, olanzapine, ziprasidone, and PHA-543613 on spatial learning and memory in the Morris water maze test in naïve and MK-801-treated mice. Brain Behav 7:1–6
Nitulescu GM, Van De Venter M, Nitulescu G, Ungurianu A, Juzenas P et al (2018) The Akt pathway in oncology therapy and beyond (review). Int J Oncol 53:2319–2331
Pearce LR, Komander D, Alessi DR (2010) The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol 11:9–22
Radisavljevic Z (2013) AKT as locus of cancer angiogenic robustness and fragility. J Cell Physiol 228:21–24
Renault TT, Teijido O, Missire F, Ganesan YT, Velours G, Arokium H, Beaumatin F, Llanos R, Athané A, Camougrand N, Priault M, Antonsson B, Dejean LM, Manon S (2015) Bcl-xL stimulates Bax relocation to mitochondria and primes cells to ABT-737. Int J Biochem Cell Biol 64:136–146
Sakamoto S, Hara T, Kurozumi A, Oka M, Kuroda-Ishimine C, Araki D, Iida S, Minagi S (2014) Effect of occlusal rehabilitation on spatial memory and hippocampal neurons after long-term loss of molars in rats. J Oral Rehabil 41:715–722
Salvesen GS, Riedl SJ (2008) Caspase mechanisms. Adv Exp Med Biol 615:13–23
Siqueira EC, Souza FT, Diniz MG, Gomez RS, Gomes CC (2015) Hsp27 (HSPB1) differential expression in normal salivary glands and pleomorphic adenomas and association with an increased Bcl2/Bax ratio. Tumour Biol 36:213–217
Ullah MS, Davies AJ, Halestrap AP (2006) The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1α-dependent mechanism. J Biol Chem 281:9030–9037
Wang L, Ma W, Markovich R, Chen JW, Wang PH (1998) Regulation of cardiomyocyte apoptotic signaling by insulin-like growth factor I. Circ Res 83:516–522
Würstle ML, Laussmann MA, Rehm M (2012) The central role of initiator caspase-9 in apoptosis signal transduction and the regulation of its activation and activity on the apoptosome. Exp Cell Res 318:1213–1220
Zhang XJ, Mei WL, Tan GH, Wang CC, Zhou SL, Huang FR, Chen B, Dai HF, Huang FY (2015) Strophalloside induces apoptosis of SGC-7901 cells through the mitochondrion-dependent caspase-3 pathway. Molecules 20:5714–5728
Zheng L, Yang J, Liu Q, Yu F, Wu S, Jin C, Lu X, Zhang L, du Y, Xi Q, Cai Y (2013) Lanthanum chloride impairs spatial learning and memory, downregulates NF-κB Signalling pathway in rats. Arch Toxicol 87:2105–2117
Zhuang M, Zhao J, Li S, Liu D, Wang K et al (2017) Concentrations and health risk assessment of rare earth elements in vegetables from mining area in Shandong, China. Chemosphere 2017(168):578–582
Funding
The study was supported by the National Natural Science Foundation of China (81602891), Science Project of Education Department in Province Liaoning (ldxy2017017), Program for Liaoning Innovation Talents in University (LR2016053), LiaoNing Revitalization Talents Program (XLYC1807194) and College Student Innovation and Entrepreneurship Training Program of Liaoning Province (2018117790053).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
The animal study has been approved by the Local Ethical Committee on Animal Testing at the Eastern Liaoning University in China. All studies involving animals are reported according to the ARRIVE (Animal Research: Reporting of In vivo Experiments) guidelines.
Consent to Participate
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
Consent for Publication
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing, we confirm that we have followed the regulations of our institutions concerning intellectual property.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOC 54.5 kb).
Rights and permissions
About this article
Cite this article
Wang, J., Wu, T., Ma, L. et al. Action of Akt Pathway on La-Induced Hippocampal Neuron Apoptosis of Rats in the Growth Stage. Neurotox Res 38, 434–446 (2020). https://doi.org/10.1007/s12640-020-00206-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-020-00206-z