Abstract
Neurodegenerative diseases (NDs) are a group of chronic, progressive disorders characterized by the gradual loss of neurons that affect specific regions of the brain, which leads to deficits in specific functions (e.g., memory, movement, cognition). The mechanism that drives chronic progression of NDs remains elusive. Among the proposed underlying pathophysiological mechanisms, aggregation and accumulation of misfolded proteins and neuroinflammation have been credited to contribute to extensive neural loss. Therapeutic agents that confer neuroprotection by downregulating these shared characteristics could therefore have beneficial effects on a wide range of NDs. In this regard, a commonly used antibiotic, doxycycline (Doxy), has been shown to reduce the progression and severity of disease in different experimental models of neurodegeneration by counteracting these common features. This review will focus on the effects reported for Doxy regarding its neuroprotective properties, the “off-target” effects, thereby supporting its evaluation as a new therapeutic approach for diseases associated with a neurodegeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurodegeneration is a pathological process leading to the loss of structure and function of neuronal tissue (Brezovakova et al. 2018). The classical examples of the neurodegenerative diseases (NDs) are Alzheimer’s disease (AD), Parkinson’s disease (PD), transmissible spongiform encephalopathies (TSEs or prion diseases), and multiple sclerosis (MS) and that occur through the progressive neuronal degeneration accumulated over a period of time. The search for treatments for NDs is a major concern in light of today’s aging population and an increasing burden on individuals, families, and society (Rotermund et al. 2018). Currently, medications for this group of disorders are limited and aim to treat the symptoms only. Although great advances have been made in the last decades to understand both environmental and genetic causes of these diseases, none of the medications available can prevent onset of the illnesses or slow progressive worsening of the diseases.
Among the proposed underlying pathophysiological mechanisms for NDs, aggregation and accumulation of misfolded proteins, neuroinflammation, oxidative stress, induction of apoptosis, and mitochondrial dysfunction have been credited to contribute to extensive neural loss (Dohm et al. 2008; Gandhi and Wood 2005; Skaper 2007; Socias et al. 2018; Soto and Estrada 2008). Therapeutic agents that confer neuroprotection by countering these shared characteristics could therefore have beneficial effects on a wide range of NDs (Noble et al. 2009).
Since pioneer studies showed that leprosy patients who received chronic treatment with antibiotics displayed significantly decreased prevalence of dementia (Chui et al. 1994), great expectations regarding the role of antibiotics as neuroprotective agents have arisen (Reglodi et al. 2017). Doxycycline (Doxy) is a broad-spectrum and second-generation semi-synthetic derivative of the bacteriostatic antibiotic tetracycline approved for use by the FDA in 1967 (Nelson and Levy 2011). In addition to their efficacy in the treatment of multidrug-resistant infections, Doxy has the advantage of a safe toxicological profile and good penetration of the blood-brain barrier (BBB) (Domercq and Matute 2004). However, their therapeutic effects are due to more than just their antimicrobial activity (Ahler et al. 2013).
Within the ancillary effects of Doxy, their neuroprotective properties against NDs are of great interest for the development of effective therapies (Forloni et al. 2001; Forloni et al. 2009; Forloni et al. 2015; Gonzalez-Lizarraga et al. 2017; Kayed et al. 2003; Lazzarini et al. 2013; Minagar et al. 2008; Santa-Cecilia et al. 2016). The aim of this paper is to overview the effects reported for Doxy regarding its neuroprotective properties in different experimental models, thereby supporting its evaluation as a new therapeutic approach for diseases associated with neurodegeneration.
Effects of Doxycycline on Alzheimer’s Disease
Alzheimer’s disease (AD) is the most common cause of acquired deterioration of cognitive functioning and emotional capacities leading to dementia (Inglese and Petracca 2013). The age-related accumulation of misfolded amyloid β (Aβ) peptides (Aβ42 and Aβ40 insoluble fibrils) and neurofibrillary tangles of hyper phosphorylated microtubule-associated protein tau (MAPT) are currently considered pathological hallmarks of AD (Socias et al. 2018). Pathological deposition of such molecules is accompanied by activation of the brain residing immune cells, mainly microglia, and release of pro-inflammatory mediators (Brezovakova et al. 2018). In this context, neuroinflammation has been widely described as a common and central pathogenic factor of the neurodegenerative process in AD (Heppner et al. 2015; McGeer and McGeer 2003).
Forloni et al. (2001) found that Doxy can inhibit the aggregation pathway of Aβ42 and also disassemble the preformed fibrils. Notably, small oligomeric forms of Aβ are responsible for the toxicity and the early cognitive impairment observed in patients before the amyloid plaque deposits appear (Kayed et al. 2003). In this way, Diomede et al. (2010) examined the Doxy effects on the sequence of events leading to proteotoxicity, using transgenic Caenorhabditis elegans (C. elegans) as a simplified invertebrate model of AD. In this model, the expression of the Aβ peptide leads to the formation of β-sheet-enriched structures, the intracellular accumulation of which induces a paralysis phenotype. They found that Doxy could interact directly in vivo with different Aβ assemblies and reduce Aβ oligomer deposition, protecting transgenic C. elegans strains from the paralysis phenotype. This effect was specific, dose-related and not linked to any antibiotic activity, suggesting that Doxy might offer an effective therapeutic strategy to target soluble Aβ aggregates (Diomede et al. 2010). Furthermore, Costa et al. (2011) reinforced that Doxy can prevent Aβ toxicity both in vitro and in vivo, supporting its therapeutic potential in AD. In this work, Doxy was administered to the developing and adult Drosophilae in their culture medium. The effect of the drug on the behavior of the flies was measured in three ways, firstly by measuring the lifespan, secondly be assessing their climbing ability and at thirdly by looking for effects on a developmental retinal toxicity phenotype (the rough eye phenotype). They found that administration of Doxy to Aβ42-expressing flies did not improve their lifespan but was able to slow the progression of their locomotor deficits and partially rescued the toxicity of Aβ in the developing eye. Also, they correlated these effects with in vitro observations that showed that this drug reduced formation of amyloid aggregates, the number of fibrils and the size of Aβ species formed. Although several studies have demonstrated the role of Doxy in preventing or halting the progression of Aβ peptide, studies are required to evaluate the role of this drug in amyloid cascade of tau protein.
Balducci et al. (2018) tested for the first time the efficacy of Doxy in APP/PS1 AD mice, a transgenic mouse model that overproduces Aβ. This study showed that subchronic (20 days) and chronic (2 months) treatment with Doxy restored long-term recognition memory without Aβ plaque reduction in 15- to 16-month-old APP/PS1 mice, when they present important plaque deposition and glial activation. This was confirmed in another mouse model induced by β-amyloid oligomers (AβOs), where the AβO-mediated memory impairment was abolished by a Doxy pretreatment. Although AβOs induce memory impairment through glial activation, the memory recovery observed in both AβO-treated and APP/PS1 mice was associated with a lower neuroinflammation, promoting these positive cognitive effects of Doxy as a hopeful repositioned drug counteracting crucial neuropathological AD targets.
Recently, Lucchetti et al. (2019) carried out pharmacokinetic studies to measure plasma and brain levels of Doxy in APP23 mice, a transgenic model of AD. It was shown that the brain concentrations are lower than those required for the drug’s anti-aggregating properties as observed in cell-free studies, suggesting that other features underlie the positive cognitive effects on AD mice. Furthermore, there were no differences between Doxy concentrations in brain areas of wild-type and APP23 mice, indicating no significant changes in BBB passage of the drug in AD mice (Lucchetti et al. 2019).
Effects of Doxycycline on Parkinson’s Disease
Parkinson’s disease (PD) is the second most common human neurodegenerative disorder after AD and the main clinical symptoms include bradykinesia, resting tremor, and postural instability, which result in impaired movement and other neurological dysfunctions (Kalia and Lang 2015). The neuropathological hallmarks of this disease are the progressive loss of dopaminergic neurons from the substantia nigra pars compacta and the abnormal accumulation in the surviving neurons of cytoplasmic α-synuclein (AS) protein inclusions, which are called Lewy bodies (LBs) (Forno 1996). While the pathogenic mechanism of human PD is still not fully understood, oxidative stress, neuroinflammation, protein misfolding, and mitochondrial dysfunction are thought to play an important role in the degeneration of dopaminergic neurons (Gandhi and Wood 2005). Nonetheless, AS aggregation appears to be the primary event that triggers all other injurious processes (Socias et al. 2018).
Gonzalez-Lizarraga et al. (2017) showed that Doxy interferes with this pathologic cycle at the aggregation level by binding to early multimeric AS species and inducing their reshaping into non-toxic off-pathway oligomers. Furthermore, subantibiotic doses of Doxy were also able to block the seeding capacity of AS preformed aggregates.
Accumulating evidence suggests that matrix metalloproteinases (MMPs) are associated with dopaminergic neuronal death and neuroinflammation and, as a consequence, is involved in the pathogenesis of PD (Reglodi et al. 2017). In this regard, Doxy showed neuroprotective effect on dopaminergic system and this appeared to derive from anti-inflammatory mechanisms (Bortolanza et al. 2018). Cho et al. (2009) reported that Doxy downregulated the cellular stress-induced MMP-3 expression and suppressed apoptosis and MMP-3 release in dopaminergic cells. In addition, the drug led to reduction of MMP-3 in microglial BV-2 cells exposed to lipopolysaccharide (LPS) and this was accompanied by suppression of production of nitric oxide and tumor necrosis factors alpha (TNF-α), as well as gene expression of interleukin 1 beta (IL-1β), TNF-α, inducible nitric oxide synthase (iNOS), and cyclooxygenase 2 (COX-2). In vivo, Doxy provided protection of the nigral dopaminergic neurons in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrimidine(MPTP)-induced mouse PD model and suppressed glial activation (Cho et al. 2009). In the same context, Reglodi et al. (2017) showed that Doxy has a strong inhibitory effect on LPS-induced production of MMP3 and MMP9 in primary microglial cells and, therefore, it could be useful to prevent and/or diminish neuroinflammatory-associated neurodegeneration. In addition, previous and complementary studies from our group demonstrated that Doxy could diminish the impairments produced by the intrastriatal administration of 6-hydroxydopamine (6-OHDA) by inhibiting microglial and astrocyte expression in a mouse model of PD (Lazzarini et al. 2013), which is in agreement with the neuroprotective properties observed by previously mentioned work (Cho et al. 2009).
Given that the immune response initiated by the major histocompatibility complex II (MHC II) antigen can advance chronic neurodegeneration in the process of PD, Zhang et al. (2015) demonstrated that Doxy can inhibit degeneration of LPS-induced dopaminergic neurons after injection of LPS stereotactically into the substantia nigra compacta, and this neuroprotective function is achieved by downregulating the microglia MHC II expression. Furthermore, we also demonstrated the efficiency of Doxy in the modulation of the neuroinflammatory response in LPS-activated primary microglial cells in culture. The drug showed a direct effect on microglial cell activation through the reduction of the production of pro-inflammatory cytokines (TNF-α and IL-1β), reactive nitrogen, and oxygen species and these effects were mediated by the inhibition of mitogen-activated protein kinase (MAPK) p38 and nuclear factor kappa B (NF-κB) signaling pathways (Santa-Cecilia et al. 2016).
Effects of Doxycycline on Prion Protein Diseases
Prion diseases also called transmissible spongiform encephalopathies (TSE) in human and animals are fatal neurodegenerative diseases that cause extensive loss of cerebral neurons with formation of vacuoles, giving a “sponge-like” appearance to the tissue. They include several diseases such as Creutzfeld Jakob disease (CJD), Gerstmann-Sträussler-Sheinker (GSS) diseases, Kuru, and fatal familial insomnia (FFI), with different causes (infectious/iatrogenic, sporadic, or genetic origin), recognized both in humans and animals as variations of the same disorder (Collins et al. 2004; De Luigi et al. 2008; Forloni et al. 2009; Prusiner 2001).
The presence of a pathological form (PrPSc) of the cellular prion protein (PrPC) is the common feature of these heterogeneous diseases (Collins et al. 2004; De Luigi et al. 2008; Forloni et al. 2013) and has been associated with either the pathogenesis or infectivity of the diseases. This causes infected brain tissue to become spongy and riddled with holes. The main target of the anti-prion strategy has been the pathological form of the cellular prion protein (PrPSc), invariably associated with TSE.
In this regard, Forloni et al. (2009) demonstrated that Doxy interacted with aggregates obtained by synthetic PrP peptides or pathological PrP (PrPSc) extracted from TSE brains, destabilized the structure of amyloid fibrils, and also inhibited the conversion of PrPC to the pathological form PrPSc. Moreover, the pretreatment of homogenates from prion-infected brains with Doxy diminished their infectivity, delayed the development of the pathology and increased the survival time (Forloni et al. 2009). This finding is in agreement with previous observations from De Luigi et al. (2008) indicating the effectiveness of Doxy as anti-prion agents in prolonging the survival of hamsters infected with the 263K scrapie strain. However, this effect was limited when Doxy was administered after animals began to show signs of disease. However, the results of randomized, double-blind, phase 2 trial of 121 CJD patients treated with Doxy versus placebo did not show significant difference in survival or disease progression between the groups. Researchers have speculated that treatment with oral Doxy after disease symptoms first appear (late stage) is relatively ineffective (Haik et al. 2014). More recently, a phase 2 study in CJD patients at an early stage demonstrated the superiority of Doxy over control (Varges et al. 2017), where slight increase in survival time in the Doxy treatment group was observed, a contrasting with the negative results of Haik et al. (2014). Another clinical trial with Doxy was reported indicating preventive treatment to persons carrying a genetic mutation associated with fatal familial insomnia (FFI) (Forloni et al. 2015).
Notably, Schmitz et al. (2016) were the first to apply real-time quaking-induced conversion (RT-QUIC), an in vitro amplification assays used to detect aggregation activity of misfolded prion protein, in different types of samples from patients with a prion disease, as a pre-screening amplification assay for anti-prion agents, and confirmed that Doxy is an efficient inhibitor of the PrP aggregation process.
Effects of Doxycycline on Multiple Sclerosis
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS) characterized, in the early phase, by infiltration of circulating mononuclear cells and extensive microglial activation that lead to axon demyelination and to neuroaxonal degeneration and brain atrophy that becomes more dominant as the disease progresses (Inglese and Petracca 2013; Lassmann and van Horssen 2011). The extent of demyelination and axonal injury are the key features of MS pathogenesis.
A clinical trial demonstrated that patients who were taking intramuscular interferon beta (IFN-β) for the treatment of MS and were submitted to a combination therapy with oral Doxy pointed effective, safe, and well-tolerated results (Minagar et al. 2008). Overexpression of MMPs in the pathogenesis of MS is associated with promotion of the inflammatory cascade in the CNS and axonal loss (Newman et al. 2001; Starckx et al. 2003). The clinical trial indicated correlations between decreased serum MMP-9 levels and enhancing lesion activity reduction. Also, Doxy decreased transendothelial migration of monocytes. Because IFN-β also decreases MMP production and activity in patients with MS, the combination of these compounds may exert a synergistic impact on MMP levels and activity (Minagar et al. 2008).
Conclusion and Further Considerations
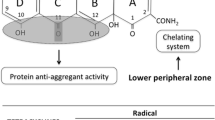
In this review, we highlighted beneficial effects of Doxy, a semi-synthetic second-generation tetracycline, together with its potential for as a typical clinical antibiotic, its ability to cross the BBB in both humans and mice, and the pleiotropic actions emerging for this class of antibiotics, mainly focused on the regulatory influence on protein aggregation and neuroinflammatory pathway (Fig. 1).
Schematic representation of the neuroprotective effect of Doxy as a new therapeutic approach for diseases associated with a neurodegeneration. Common neurodegenerative disease-associated events known to be targeted by Doxy include protein misfolding and aggregation such as Aβ, α-synuclein, and PrPSc proteins and neuroinflammation mediated by glia cells with the release of pro-inflammatory mediators as cytokines, matrix metalloproteinases (MMP), nitric oxide (NO), and reactive oxygen species (ROS) and reactive nitrogen species (RNS). Doxy is able to act on multiple pathways counteracting the degeneration of the dopaminergic neurons in the central nervous system (CNS). Transmissible spongiform encephalopathies (TSE); pathological form of the cellular prion protein (PrPSc)
A candidate molecule for therapy in neurodegeneration must be able to freely cross the BBB, or at least at a concentration high enough to exert its action, since the pharmacologic approach may be particularly difficult and hamper the treatment (Socias et al. 2018). In this context, Doxy has good clinical safety and can easily penetrate the BBB (Domercq and Matute 2004).
Protein aggregation processes appear to involve common mechanisms during the development of the different ND pathological states. As demonstrated by the anti-aggregating activity of Doxy on Aβ, α-synuclein, and PrP disorders, there is an important opportunity for its application in multiple NDs. Furthermore, compelling evidence suggests that most, if not all, NDs involve a neuroinflammatory process, which may contribute to degeneration and neuronal death (Chen et al. 2016; Gonzalez-Lizarraga et al. 2017). Thereby, the anti-inflammatory properties of Doxy have been proposed as the mechanism involved in the neuroprotective effect (Santa-Cecilia et al. 2016). Interestingly, aggregated proteins may induce an inflammatory response mediated by glia cells as well as oxidative damage and all these processes might be interrelated in a self-sustained cycle of protein aggregation leading to neuroinflammation events that further exacerbate aggregates’ formation (Gonzalez-Lizarraga et al. 2017; Gustot et al. 2015). Overall, neuroprotective therapy with Doxy as a pleiotropic drug capable of downregulating neuroinflammation and protein aggregation at several levels constitutes a promising approach for hampering the degenerative cycle.
In fact, by adjusting the Doxy concentration, it is possible to select the antibiotic activity or its pleiotropic actions (Gonzalez-Lizarraga et al. 2017). Some clinical trials demonstrated antimicrobial effect with 200–400 mg/day of the drug, whereas subantibiotic doses (20–40 mg/day) exert anti-inflammatory activities (Di Caprio et al. 2015). Nevertheless, treatment with conventional anti-inflammatory drugs has been not proven effective in neuroprotection, suggesting that this mechanism alone would not be enough (Bortolanza et al. 2018; Gonzalez-Lizarraga et al. 2017).
Finally, long-term administration of subantimicrobial-dose Doxy of up to 2 years in clinical trials did not produce antibiotic side effects (Golub et al. 2016; Keijmel et al. 2017).
In summary, the proven feasibility and the good safety record of Doxy support its potential prospect as an effective and inexpensive protective treatment for neurodegenerative disorders without serious secondary effects. Future studies are required for a better understanding of Doxy action and unravel the molecular mechanisms involved in its effects. It seems likely that Doxy could offer novel therapeutic approaches that act on multiple pathways counteracting the degeneration of the dopaminergic neurons in the CNS.
References
Ahler E, Sullivan WJ, Cass A, Braas D, York AG, Bensinger SJ, Graeber TG, Christofk HR (2013) Doxycycline alters metabolism and proliferation of human cell lines. PLoS One 8:e64561. https://doi.org/10.1371/journal.pone.0064561
Balducci C, Santamaria G, la Vitola P, Brandi E, Grandi F, Viscomi AR, Beeg M, Gobbi M, Salmona M, Ottonello S, Forloni G (2018) Doxycycline counteracts neuroinflammation restoring memory in Alzheimer’s disease mouse models. Neurobiol Aging 70:128–139. https://doi.org/10.1016/j.neurobiolaging.2018.06.002
Bortolanza M, Nascimento GC, Socias SB, Ploper D, Chehin RN, Raisman-Vozari R, Del-Bel E (2018) Tetracycline repurposing in neurodegeneration: focus on Parkinson’s disease. J Neural Transm 125:1403–1415. https://doi.org/10.1007/s00702-018-1913-1
Brezovakova V, Valachova B, Hanes J, Novak M, Jadhav S (2018) Dendritic cells as an alternate approach for treatment of neurodegenerative disorders. Cell Mol Neurobiol 38:1207–1214. https://doi.org/10.1007/s10571-018-0598-1
Chen WW, Zhang X, Huang WJ (2016) Role of neuroinflammation in neurodegenerative diseases (review). Mol Med Rep 13:3391–3396. https://doi.org/10.3892/mmr.2016.4948
Cho Y, Son HJ, Kim EM, Choi JH, Kim ST, Ji IJ, Choi DH, Joh TH, Kim YS, Hwang O (2009) Doxycycline is neuroprotective against nigral dopaminergic degeneration by a dual mechanism involving MMP-3. Neurotox Res 16:361–371. https://doi.org/10.1007/s12640-009-9078-1
Chui DH, Tabira T, Izumi S, Koya G, Ogata J (1994) Decreased beta-amyloid and increased abnormal Tau deposition in the brain of aged patients with leprosy. Am J Pathol 145:771–775
Collins SJ, Lawson VA, Masters CL (2004) Transmissible spongiform encephalopathies. Lancet 363:51–61. https://doi.org/10.1016/S0140-6736(03)15171-9
Costa R, Speretta E, Crowther DC, Cardoso I (2011) Testing the therapeutic potential of doxycycline in a Drosophila melanogaster model of Alzheimer disease. J Biol Chem 286:41647–41655. https://doi.org/10.1074/jbc.M111.274548
De Luigi A et al (2008) The efficacy of tetracyclines in peripheral and intracerebral prion infection. PLoS One 3:e1888. https://doi.org/10.1371/journal.pone.0001888
Di Caprio R, Lembo S, Di Costanzo L, Balato A, Monfrecola G (2015) Anti-inflammatory properties of low and high doxycycline doses: an in vitro study Mediators of inflammation. Mediat Inflamm 2015:329418. https://doi.org/10.1155/2015/329418
Diomede L, Cassata G, Fiordaliso F, Salio M, Ami D, Natalello A, Doglia SM, de Luigi A, Salmona M (2010) Tetracycline and its analogues protect Caenorhabditis elegans from beta amyloid-induced toxicity by targeting oligomers. Neurobiol Dis 40:424–431. https://doi.org/10.1016/j.nbd.2010.07.002
Dohm CP, Kermer P, Bahr M (2008) Aggregopathy in neurodegenerative diseases: mechanisms and therapeutic implication. Neurodegener Dis 5:321–338. https://doi.org/10.1159/000119459
Domercq M, Matute C (2004) Neuroprotection by tetracyclines. Trends Pharmacol Sci 25:609–612. https://doi.org/10.1016/j.tips.2004.10.001
Forloni G, Colombo L, Girola L, Tagliavini F, Salmona M (2001) Anti-amyloidogenic activity of tetracyclines: studies in vitro. FEBS Lett 487:404–407
Forloni G, Salmona M, Marcon G, Tagliavini F (2009) Tetracyclines and prion infectivity. Infect Disord Drug Targets 9:23–30
Forloni G, Artuso V, Roiter I, Morbin M, Tagliavini F (2013) Therapy in prion diseases. Curr Top Med Chem 13:2465–2476
Forloni G, Tettamanti M, Lucca U, Albanese Y, Quaglio E, Chiesa R, Erbetta A, Villani F, Redaelli V, Tagliavini F, Artuso V, Roiter I (2015) Preventive study in subjects at risk of fatal familial insomnia: innovative approach to rare diseases. Prion 9:75–79. https://doi.org/10.1080/19336896.2015.1027857
Forno LS (1996) Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol 55:259–272
Gandhi S, Wood NW (2005) Molecular pathogenesis of Parkinson’s disease. Hum Mol Genet 14:2749–2755. https://doi.org/10.1093/hmg/ddi308
Golub LM, Elburki MS, Walker C, Ryan M, Sorsa T, Tenenbaum H, Goldberg M, Wolff M, Gu Y (2016) Non-antibacterial tetracycline formulations: host-modulators in the treatment of periodontitis and relevant systemic diseases. Int Dent J 66:127–135. https://doi.org/10.1111/idj.12221
Gonzalez-Lizarraga F et al (2017) Repurposing doxycycline for synucleinopathies: remodelling of alpha-synuclein oligomers towards non-toxic parallel beta-sheet structured species. Sci Rep 7:41755. https://doi.org/10.1038/srep41755
Gustot A, Gallea JI, Sarroukh R, Celej MS, Ruysschaert JM, Raussens V (2015) Amyloid fibrils are the molecular trigger of inflammation in Parkinson’s disease. Biochem J 471:323–333. https://doi.org/10.1042/BJ20150617
Haik S et al (2014) Doxycycline in Creutzfeldt-Jakob disease: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Neurol 13:150–158. https://doi.org/10.1016/S1474-4422(13)70307-7
Heppner FL, Ransohoff RM, Becher B (2015) Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci 16:358–372. https://doi.org/10.1038/nrn3880
Inglese M, Petracca M (2013) Imaging multiple sclerosis and other neurodegenerative diseases. Prion 7:47–54. https://doi.org/10.4161/pri.22650
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386:896–912. https://doi.org/10.1016/S0140-6736(14)61393-3
Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG (2003) Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300:486–489. https://doi.org/10.1126/science.1079469
Keijmel SP, Delsing CE, Bleijenberg G, van der Meer JWM, Donders RT, Leclercq M, Kampschreur LM, van den Berg M, Sprong T, Nabuurs-Franssen MH, Knoop H, Bleeker-Rovers CP (2017) Effectiveness of long-term doxycycline treatment and cognitive-behavioral therapy on fatigue severity in patients with Q fever fatigue syndrome (Qure study): a randomized controlled trial. Clin Infect Dis 64:998–1005. https://doi.org/10.1093/cid/cix013
Lassmann H, van Horssen J (2011) The molecular basis of neurodegeneration in multiple sclerosis. FEBS Lett 585:3715–3723. https://doi.org/10.1016/j.febslet.2011.08.004
Lazzarini M, Martin S, Mitkovski M, Vozari RR, Stuhmer W, Bel ED (2013) Doxycycline restrains glia and confers neuroprotection in a 6-OHDA Parkinson model. Glia 61:1084–1100. https://doi.org/10.1002/glia.22496
Lucchetti J, Fracasso C, Balducci C, Passoni A, Forloni G, Salmona M, Gobbi M (2019) Plasma and brain concentrations of doxycycline after single and repeated doses in wild-type and APP23 mice. J Pharmacol Exp Ther 368:32–40. https://doi.org/10.1124/jpet.118.252064
McGeer EG, McGeer PL (2003) Inflammatory processes in Alzheimer’s disease. Prog Neuro-Psychopharmacol Biol Psychiatry 27:741–749. https://doi.org/10.1016/S0278-5846(03)00124-6
Minagar A, Alexander JS, Schwendimann RN, Kelley RE, Gonzalez-Toledo E, Jimenez JJ, Mauro L, Jy W, Smith SJ (2008) Combination therapy with interferon beta-1a and doxycycline in multiple sclerosis: an open-label trial. Arch Neurol 65:199–204. https://doi.org/10.1001/archneurol.2007.41
Nelson ML, Levy SB (2011) The history of the tetracyclines. Ann N Y Acad Sci 1241:17–32. https://doi.org/10.1111/j.1749-6632.2011.06354.x
Newman TA, Woolley ST, Hughes PM, Sibson NR, Anthony DC, Perry VH (2001) T-cell- and macrophage-mediated axon damage in the absence of a CNS-specific immune response: involvement of metalloproteinases. Brain 124:2203–2214
Noble W, Garwood CJ, Hanger DP (2009) Minocycline as a potential therapeutic agent in neurodegenerative disorders characterised by protein misfolding. Prion 3:78–83
Prusiner SB (2001) Shattuck lecture--neurodegenerative diseases and prions. N Engl J Med 344:1516–1526. https://doi.org/10.1056/NEJM200105173442006
Reglodi D, Renaud J, Tamas A, Tizabi Y, Socias SB, Del-Bel E, Raisman-Vozari R (2017) Novel tactics for neuroprotection in Parkinson’s disease: role of antibiotics, polyphenols and neuropeptides. Prog Neurobiol 155:120–148. https://doi.org/10.1016/j.pneurobio.2015.10.004
Rotermund C, Machetanz G, Fitzgerald JC (2018) The therapeutic potential of metformin in neurodegenerative diseases. Front Endocrinol 9:400. https://doi.org/10.3389/fendo.2018.00400
Santa-Cecilia FV et al (2016) Doxycycline suppresses microglial activation by inhibiting the p38 MAPK and NF-kB signaling pathways. Neurotox Res 29:447–459. https://doi.org/10.1007/s12640-015-9592-2
Schmitz M, Cramm M, Llorens F, Candelise N, Müller-Cramm D, Varges D, Schulz-Schaeffer WJ, Zafar S, Zerr I (2016) Application of an in vitro-amplification assay as a novel pre-screening test for compounds inhibiting the aggregation of prion protein scrapie. Sci Rep 6:28711. https://doi.org/10.1038/srep28711
Skaper SD (2007) The brain as a target for inflammatory processes and neuroprotective strategies. Ann N Y Acad Sci 1122:23–34. https://doi.org/10.1196/annals.1403.002
Socias SB, González-Lizárraga F, Avila CL, Vera C, Acuña L, Sepulveda-Diaz JE, del-Bel E, Raisman-Vozari R, Chehin RN (2018) Exploiting the therapeutic potential of ready-to-use drugs: repurposing antibiotics against amyloid aggregation in neurodegenerative diseases. Prog Neurobiol 162:17–36. https://doi.org/10.1016/j.pneurobio.2017.12.002
Soto C, Estrada LD (2008) Protein misfolding and neurodegeneration. Arch Neurol 65:184–189. https://doi.org/10.1001/archneurol.2007.56
Starckx S, Van den Steen PE, Verbeek R, van Noort JM, Opdenakker G (2003) A novel rationale for inhibition of gelatinase B in multiple sclerosis: MMP-9 destroys alpha B-crystallin and generates a promiscuous T cell epitope. J Neuroimmunol 141:47–57
Varges D, Manthey H, Heinemann U, Ponto C, Schmitz M, Schulz-Schaeffer WJ, Krasnianski A, Breithaupt M, Fincke F, Kramer K, Friede T, Zerr I (2017) Doxycycline in early CJD: a double-blinded randomised phase II and observational study. J Neurol Neurosurg Psychiatry 88:119–125. https://doi.org/10.1136/jnnp-2016-313541
Zhang GB, Feng YH, Wang PQ, Song JH, Wang P, Wang SA (2015) A study on the protective role of doxycycline upon dopaminergic neuron of LPS-PD rat model rat. Eur Rev Med Pharmacol Sci 19:3468–3474
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Santa-Cecília, F.V., Leite, C.A., Del-Bel, E. et al. The Neuroprotective Effect of Doxycycline on Neurodegenerative Diseases. Neurotox Res 35, 981–986 (2019). https://doi.org/10.1007/s12640-019-00015-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-019-00015-z