Abstract
Although aminoglycoside antibiotics such as kanamycin are widely used clinically to treat life-threatening bacterial infections, ototoxicity remains a significant dose-limiting side effect. The prevailing view is that the hair cells are the primary ototoxic target of aminoglycosides and that spiral ganglion neurons begin to degenerate weeks or months after the hair cells have died due to lack of neurotrophic support. To test the early developmental aspects of this issue, we compared kanamycin-induced hair cell and spiral ganglion pathology in rat postnatal day 3 cochlear organotypic cultures with adult whole cochlear explants. In both adult and postnatal day 3 cultures, hair cell damage began at the base of the cochleae and progressed toward the apex in a dose-dependent manner. In postnatal day 3 cultures, spiral ganglion neurons were rapidly destroyed by kanamycin prior to hair cell loss. In contrast, adult spiral ganglion neurons were resistant to kanamycin damage even at the highest concentration, consistent with in vivo models of delayed SGN degeneration. In postnatal day 3 cultures, kanamycin preferentially damaged type I spiral ganglion neurons, whereas type II neurons were resistant. Spiral ganglion degeneration of postnatal day 3 neurons was associated with upregulation of the superoxide radical and caspase-3-mediated cell death. These results show for the first time that kanamycin is toxic to postnatal day 3 spiral ganglion neurons, but not adult neurons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aminoglycoside antibiotics continue to be used worldwide to treat gram-negative bacterial infections and tuberculosis, despite the fact that they can cause severe nephrotoxicity and ototoxicity (Van Boeckel et al. 2014). Sensitivity to aminoglycoside ototoxicity is exacerbated by certain mitochondrial mutations (Fischel-Ghodsian 1998) as well as co-administration of aminoglycosides and loop-inhibiting diuretic such as ethacrynic acid or furosemide (Ding et al. 2010a). As the dose and/or duration of aminoglycoside treatment increases, hair cell damage spreads from the high-frequency base of the cochlea toward the low-frequency apex (Sha et al. 2001a). Moreover, damage is greater and occurs earlier for outer hair cells (OHCs) than inner hair cells (IHCs) (Dallos and Harris 1978; McFadden et al. 2002). Aminoglycoside-induced hair cell degeneration is believed to be mediated by reactive oxygen species (ROS) (Hirose et al. 1997). This interpretation is supported by studies showing that aminoglycoside-induced hearing loss and hair cell loss are less severe in mice overexpressing the superoxide dismutase gene (Lautermann et al. 1997; Sha and Schacht 2000; Sha et al. 2001b).
Several weeks or months after aminoglycoside-induced hair cell loss has occurred in vivo, the spiral ganglion neurons (SGNs) begin to die off (Kopelovich et al. 2013; McFadden et al. 2004; Yu et al. 2014). SGN degeneration continues over weeks and months (Xu et al. 1993) further exacerbating the cochlear pathology. The mechanisms responsible for the initial survival of SGN as well as their delayed degeneration after hair cells are destroyed remain poorly understood. In mutant mice lacking only IHCs, SGN survival lasts for at least 6 months implying that the residual supporting cells and OHCs may provide sufficient trophic support required for SGN survival (Ding et al. 2016; Sugawara et al. 2005; Zilberstein et al. 2012). In contrast, degeneration of SGN after aminoglycoside treatment could be due to the degeneration of OHC, IHC, and support cells and loss of trophic support from a flattened sensory epithelium (Izumikawa et al. 2008). Alternatively, aminoglycoside-induced SGN degeneration could result from glutamate-induced excitotoxicity (Matsuda et al. 1999) or disruption of calcium homeostasis in the extracellular matrix (Esterberg et al. 2013; Jeong et al. 2010).
Streptomycin, an aminoglycoside antibiotic that can rapidly cross the blood-labyrinth barrier, impairs synaptic transmission at neuromuscular junction and afferent synapse beneath vestibular hair cells (Pittinger and Adamson 1972; Zucca et al. 1992). After systemic streptomycin injection, an acute neurotoxic effect rapidly occurs to the cochlear afferent and efferent terminals prior to the hair cell degeneration; this significantly increased the latency of the compound action potential and wave I and the interwave intervals of the auditory brain stem response 20 min after streptomycin treatment (Ding and Salvi 2005; Ding et al. 2010b). Some studies also suggest that kanamycin (KM) exerts neurotoxic effects on neurons in the dorsal cochlear nucleus (Fan et al. 2013). In cases where gentamicin was infused into the cochlea, a noticeable loss of SGN occurred in the first week post-treatment; this was followed by an even greater loss of SGN over the next 30 weeks (Dodson and Mohuiddin 2000). Other in vivo studies report that KM causes a retraction of auditory nerve terminals from hair cells prior to IHC loss (Kong et al. 2010) suggesting that aminoglycosides may have a direct neurotoxic effect on SGN. In contrast, others report that most postnatal SGN survive in aminoglycoside-treated cochlear cultures for 5–6 days, but survival declines to about 50% after 10 days unless the cultures are treated with neurotrophic factors (Lalwani et al. 2002; Staecker et al. 1996). Thus, there is some ambiguity in the literature regarding the time course of SGN degeneration.
Because of the growing concern that SGN and their peripheral afferents are particularly vulnerable to a variety of ototraumatic agents (Kujawa and Liberman 2006, 2015; Sergeyenko et al. 2013), KM was applied to cochlear organotypic cultures from postnatal day 3 (P3) and adult rats to investigate the mechanisms of cell death and time course of hair cell and neural degeneration.
Methods
Subjects
Adult Sprague-Dawley rats (~4 months old) and their P3 rat pups were obtained from Charles River Laboratories (Wilmington, MA, USA).
Postnatal Organotypic Cultures
Our procedures for preparing organotypic cultures from postnatal and adult animals have been described in prior publications (Ding et al. 2012, 2013b; Wei et al. 2010). In brief, animals were euthanized with an overdose of CO2 and decapitated and the cochleae quickly removed. The organ of Corti from P3 rat pups, which contained the hair cells and SGN, was placed on a droplet (15 μl) of rat tail collagen gel in a tissue culture dish. The cultures were subsequently incubated overnight in serum-free medium (0.01 mg/ml bovine serum albumin [Sigma A-4919], 1% serum-free supplement [Sigma I-1884], 2.4 of 20% glucose [Sigma G-2020], 0.2% Penicillin G [Sigma P-3414], 1% 200 mM glutamine [Sigma G-6392], and 95.4% 1× Basal Medium Eagle [Sigma B-1522]) in a CO2 incubator (37 °C, 5% CO2, 95% humidity). On the second day, the cultures were treated for 24 h with 2 ml of fresh culture medium containing various concentrations of KM (Sigma-Aldrich, K4000). Some P3 cultures were treated with 1 mM KM for 3, 6, 12, or 24 h and used to detect the expression of the superoxide radical or caspase-3, an executioner caspase, whereas other P3 cultures were treated for 24 or 48 h with 1 mM KM and subsequently labeled with an antibody against peripherin which only labels type II neurons or a neurofilament 200 kDa antibody that labels both type I and type II neurons.
Adult Cochlear Explants
Our procedures for preparing and culturing adult whole cochlear explants have been described previously (Ding et al. 2012). In adult rats, the bony cochlea was removed from the temporal bone after decapitation, and perforations were carefully made along the bony surface of cochlear spiral shell in order to permit the flow of culture medium into the tissue. The whole cochlear explant was immersed into serum-free culture medium which was maintained in an incubator at 37 °C and 5% CO2 for subsequent treatment with various concentrations of KA. For observations using adult SGN, the SGN was dissected out from Rosenthal’s canal in the modiolus, placed on the collagen gel in a culture dish, and cultured using methods similar to those for P3 rat organ cultures as previously described (Ding et al. 2013a; Fu et al. 2013).
Histology
Tissues were fixed with 10% formalin for 2 h and rinsed three times in 0.01 M phosphate-buffered saline (PBS, Sigma P-3813). To label neurofilaments in SGN and nerve fibers, tissues were initially incubated for 2 h in 0.01 M PBS containing 3% Triton X-100 and 10% goat serum (Vector S1000) followed by incubation for 24 h at 4 °C with a mouse monoclonal neurofilament 200 kDa antibody (Sigma N0142, 1:200) in 0.01 M PBS containing 0.2% Triton X-100 and 10% goat serum (Vector S1000). Afterwards, the samples were rinsed three times with 0.1 M PBS and then incubated for 4 h at room temperature with Alexa Fluor 568-conjugated goat anti-mouse secondary antibody (Invitrogen A-21124, 1:100) in 0.01 M PBS containing 0.2%Triton X-100 and 10% goat serum (Vector S1000). To label the stereocilia, the tissues were incubated with Alexa Fluor 488-conjugated phalloidin (Sigma P1951, 1:100) in 0.01 M PBS (1:100) for 1 h. Afterwards, the samples were rinsed three times in 0.1 M PBS and then incubated with TO-PRO-3 (Invitrogen T-3605, 1:300) in 0.01 M PBS for 1 h.
To detect the presence of the superoxide radical in P3 rat pups, cultures were incubated in 100 μM dihydroethidium (DHE) (Sigma D-7008) for 30 min after treatment with vehicle only or 1 mM KM (3, 6, 12, or 24 h). Afterwards, half the samples were fixed and labeled with an antibody against neurofilament 200 kDa and TO-PRO-3 as described previously (Deng et al. 2013). To identify cells undergoing caspase-mediated cell death, P3 organotypic cultures were incubated for 1 h with CaspaTag3 (Intergen, 1:30), a cell permeable probe that fluoresces in the presence of activated caspase-3 (Deng et al. 2013).
To differentiate between type I and type II neurons in P3 cochlear organotypic cultures, tissues were fixed in 10% formalin containing 3% Triton X-100. As described previously (Deng et al. 2013), these cultures were then incubated for 48 h at 4 °C with a mouse monoclonal antibody against neurofilament 200 kDa, which is expressed in type I and type II SGN and a rabbit polyclonal antibody against peripherin, an intermediate filament selectively expressed in type II SGN (Barclay et al. 2011; Lang et al. 2005) (Millipore AB1530; 1:200). Afterwards, the cultures were rinsed in 0.1 M PBS and incubated overnight at 4 °C with Alexa Fluor 488-labeled goat anti-mouse secondary antibody (Invitrogen A-21151, 1:100) and Alexa Fluor 568-labeled goat anti-rabbit secondary antibody (Invitrogen A-11008, 1:100) in 0.01 M PBS containing 0.2% Triton X-100 and 10% goat serum (Vector S1000).
Analysis
Tissues were subsequently rinsed and mounted on glass slides in glycerin to assess cellular integrity using a confocal microscope (Zeiss LSM-510 meta, step size 1 μm per slice) with appropriate filters to detect the green fluorescence of Alexa Fluor 488 (excitation 488 nm, emission 525 nm), red fluorescence of Alexa Fluor 568 (excitation 561 nm, emission 595 nm), and blue fluorescence of TO-PRO-3 (excitation 633 nm, emission 672 nm). The resulting confocal images were collected in the same region of different specimens for both experimental and control samples. In some cases, multi-layered images were merged using ZEN Digital Imaging software (Zeiss, 2012 version). For quantitative analysis, either neuronal nuclei or caspase-3-positive/negative cells were counted in every 10th layers of a multi-layered image stack.
The experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University at Buffalo, NY, USA, and conform to the guidelines from the National Institutes of Health, MD, USA.
Results
KM Damage in P3 Cultures
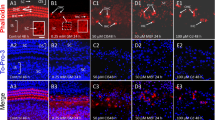
KM was applied to P3 organotypic cultures for 24 h, and the condition of the OHC and IHC was evaluated using Alexa 488 phalloidin to label the actin which is heavily expressed in the stereocilia and cuticular plate of hair cells. The status of the SGN and peripheral auditory nerve fibers (ANFs) which radiate toward the hair cells was evaluated by neurofilament 200 kDa immunolabeling. Representative photomicrographs from the upper basal turn of cochlear cultures maintained for 24 h without KM (0 mM) or with 0.5 and 1 mM KM are presented in Fig. 1. In untreated control cultures, the OHC and IHC were arranged in orderly rows (Fig. 1a). Thick fascicles of ANF projected out radially from the large, round SGN. As the ANFs approache the IHC, they form a dense network. Treatment with 0.5 mM KM for 24 h resulted in a noticeable reduction in the thickness and number of ANF fascicles projecting toward the IHC and a complete loss of the dense network of fiber terminals normally present adjacent to the IHC (Fig. 1b). There was also a slight reduction in the density of SGN and shrinkage in the size of SGN somata. In spite of these neural pathologies, there was little evidence of hair cell loss. Increasing the KM dose to 1 mM led to a further reduction in the number and thickness of ANF fascicles projecting toward the hair cells and a complete loss of the dense network of fiber terminals normally seen adjacent to the IHC (Fig. 1c). The density of SGN was noticeably reduced; many SGN somata were shrunken, and nearly all the IHCs and OHCs were missing. These results indicate that in P3 organotypic cultures, ANF and SGN are more vulnerable to KM toxicity than OHC and IHC.
Representative confocal images from the upper basal turn of the cochlea of P3 organotypic cultures treated with 0 (control), 0.5, or 1 mM KM for 24 h. Specimens double labeled with neurofilament 200 kDa (red) and phalloidin Alexa 488 (green). a In control cultures (0 mM KM), outer hair cells (OHCs, bracket) and inner hair cells (IHCs, white arrowhead) were arranged in orderly rows. Numerous thick fascicles of auditory nerve fibers (ANFs, yellow arrow) project radially from the round spiral ganglion neurons (SGNs, white arrows) toward the hair cells. Nerve fibers terminate as a dense network as they approach the IHC (white star). b After 24-h treatment with 0.5 mM KM, considerable thinning of auditory nerve fiber fascicles (yellow lightning bolt) occurred along with shrinkage of SGN somata (v). Note complete loss of the dense fiber network near the IHC (compare to stars in a). There was little evidence of hair cell loss. c After 24-h treatment with 1 mM KM, there was significant hair cell loss, considerable thinning and loss of auditory nerve fascicles (yellow lightning bolt), complete loss of the dense fiber network near the IHC (compare to star in a), and shrinkage of SGN somata (v)
Kanamycin-Induced Hair Cell Damage in Adult and P3 Cultures
While KM damaged hair cells in both P3 and adult organ cultures, damage occurred at lower concentrations in adults. Figure 2 show representative photomicrographs from the basal turn of adult (Fig. 2, rows 1 and 2) and P3 (row 3) organotypic cultures maintained for 24 h with KM concentrations ranging from 0 (control) to 1 mM KM. In 0 mM control cultures, the OHC and IHC are aligned in orderly rows in both adult (Fig. 2 (A and a)) and P3 cultures (Fig. 2 (F)). The round hair cell nuclei were intensely and uniformly labeled with TO-PRO-3, while the stereocilia and cuticular plate were strongly labeled with phalloidin. Treatment with a low dose (0.1 mM) of KM resulted in considerable hair cell loss in adults (B, b), but not in P3 cultures. In adults, many OHCs were either missing or their nuclei were condensed and irregularly shaped or fragmented, morphological features of apoptosis. The profiles of IHC nuclei were irregularly shaped, and TO-PRO-3 labeling of the nuclei was heterogeneous. Higher doses of KM (0.2–1 mM) resulted in increased and eventual complete loss of IHC and OHC (C, c, D, d, E, e). Hair cells in P3 cultures were more resistant to KM damage. Most OHCs and IHCs were still present after treatment with 0.1 and 0.2 mM KM, but TO-PRO-3 labeling of the nuclei became increasingly heterogeneous, and the profiles of the nuclei shifted from round to a more irregular shape (Fig. 2 (G and H)). Higher concentrations of KM (0.5–1 mM) destroyed nearly all the hair cells (Fig. 2 (I and J)).
Organotypic cultures from basal turn of adult (top two rows) and P3 (bottom row) rats treated for 24 h with different doses of KM. Phalloidin label of stereocilia and cuticular plate shown in red and TO-PRO-3 label of nuclei shown in green. Second row shows representative photomicrographs of surface of organ of Corti from adult rats; first row shows the Z-plane image through a section of the surface preparation. Bottom row shows representative surface preparation view from P3 rat organ cultures. OHC and IHC in adult rats after 24 h in culture without KM (control) (A, a). OHC and IHC arranged in orderly rows separated by tunnel of Corti. Note strong phalloidin labeling of the stereocilia (St) and cuticular plate. Large, round nuclei present in OHC and IHC; note homogeneous labeling of nuclei with To-Pro-3. Treatment with 0.1 mM KM resulted in considerable OHC loss (B, b). Most OHC nuclei condensed and severely shrunken (yellow arrows). Stereocilia present on most IHC, but IHC nuclei slightly shrunken and irregularly labeled with To-Pro-3 (white arrowheads). OHC and IHC losses increased as KM dose increased from 0.2 to 1 mM, nearly all hair cell missing with 0.5 mM KM (C, c, D, d, E, e). Three rows of OHC and single row of IHC in P3 control groups; strong, homogeneous To-Pro-3 labeling evident in IHC (F). OHC and IHC present after 0.1 mM KM treatment, but To-Pro-3 nuclear labeling was irregular (white arrowheads) (G). Most OHC and IHC present after 0.2 mM KM treatment, but To-Pro-3 labeling was heterogeneous, nuclear profiles were irregular and condensed (H). Nearly all OHC and IHC destroyed by 0.5 and 1 mM KM (I, i, J, j)
P3 SGN Vulnerable to KM Damage
Figure 3 shows representative photomicrographs of SGN in the basal turn of adult (upper row) and P3 (lower row) cultures treated with various doses of KM (0 to 1 mM). The cytoplasm of the large, round soma of adult and P3 SGN was homogenously labeled with neurofilament 200 kDa. In control cultures (0 mM KM), intense neurofilament 200 kDa was present in the cytoplasm of the somata of adult (Fig. 3a) and P3 (Fig. 3f) SGN. In these cells, TO-PRO-3 also uniformly labeled the large round nuclei in both adult (Fig. 3a) and P3 (Fig. 3f) SGN. Treatment of adult SGN with 0.1–1 mM KM had minimal effect on neurofilament and TO-PRO-3 labeling (Fig. 3a–e). In contrast, when KM was applied to P3 SGN, there was a dose-dependent loss of neurofilament staining, shrinkage of somata and nuclei, and nuclear fragmentation (Fig. 3f–j), morphological features characteristic of cells undergoing apoptosis. To quantify the changes, we counted the numbers of SGN with apoptotic nuclei (shrunken and fragmented nuclei) and determined the percentage of SGN in each group. As KM concentration increased, the mean (±SEM) percentage of SGN in P3 organotypic cultures increased significantly from ~4% in controls (0 mM) to roughly 80% at 0.5 mM; all groups were significantly different from one another (one-way ANOVA, F = 422.1, p < 0.0001, 4, 22 df, Newman-Keuls post hoc, p < 0.05). Few apoptotic cells were observed in adult organ cultures even at the highest concentration of 1 mM.
Photomicrographs of SGN from basal turn of adult (top row) and P3 rats treated for 24 h with 0 (control) to 1 mM KM. Specimens stained with neurofilament 200 kDa (red) and To-Pro-3 (green). a Adult SGN in 0 mM control group characterized by large round somas labeled by neurofilament stain and round, centrally located To-Pro-3-labeled nucleus. b–e Soma and nuclear morphology of adult SGN treated with 0.1 to 1 mM KM were similar to 0 mM control. f P3 SGN in 0 mM control group characterized by large, round To-Pro-3-labeled nucleus located in the center of the SGN; cytoplasm of SGN soma heavily labeled with neurofilament. g–j As KM dose increased, soma size decreased and many SGN had condensed and/or fragmented nuclei (white arrowhead). Mean percentage (±SEM) of apoptotic SGN versus KM dose (H); control = 0 mM KM. (Number sign indicates significant differences between doses in the P3 group)
Superoxide in P3 SGN and Hair Cells
Because aminoglycosides reportedly increase ROS levels in the cochlea in vitro (Hirose et al. 1997), we used DHE to detect the expression of the superoxide radical in cultures treated for 3, 6, 12, or 24 h with 1 mM KM. Representative photomicrographs illustrating DHE labeling in the basal turn of P3 cochlear cultures and P3 SGN are shown in Fig. 4. In the organ of Corti, DHE labeling was first observed after 12 and 24 h of KM treatment in regions where there was significant hair cell loss (Fig. 4d, e, yellow arrows). DHE labeling was largely absent in controls and cultures treated with KM for 3 and 6 h (Fig. 4a–c); in these conditions, most hair cells were present. In P3 SGN, little of no DHE labeling was observed in control cultures (Fig. 4a) and only a few DHE-positive cells were observed after 3 h of KM treatment (Fig. 4g). However, as the duration of KM treatment increased from 6 to 24 h, DHE labeling progressively increased. These results indicate that KM-induced superoxide generation is much stronger in SGN than the organ of Corti.
Representative photomicrographs from organ of Corti (a–e) and SGN (f–j) in the basal turn of P3 rats treated with 0 (control) or 1 mM KM for 3, 6, 12, or 24 h. Dihydroethidium (DHE) used to detect superoxide (red). TO-PRO-3 used to label nuclei (blue) in the organ of Corti (a–e) and neurofilament 200 kDa (green) used to label the SGN (f–j)
Caspase-3 Activated Earlier in P3 SGNs than in HCs
To explore the mechanisms and time course of cell death, P3 cochlear and SGN cultures were labeled a fluorogenic probe that detects activated caspase-3. Figure 5 shows confocal micrographs from cultures treated for 3, 6, 12, and 24 h with 1 mM KM. Caspase-3 was largely absent from the hair cell region of the organ of Corti of control cultures and cultures treated with KM for 3 and 6 h (Fig. 5 (A–C)). However, many caspase-3-positive cells were evident in the organ of Corti of cultures treated with KM for 12 and 24 h (Fig. 5 (D and E)); caspase-3 labeling was evident in regions with extensive hair cell damage. In contrast to the organ of Corti, caspase-3 labeling was already evident in SGN after just 3 h of KM treatment (Fig. 5 (G)); labeling progressively increased as treatment duration increased (Fig. 5 (H–J)). Caspase-3 labeling was strongest in the nucleus of SGN with a shrunken soma (Fig. 5 (G and H)) (Kamada et al. 2005), consistent with cells undergoing apoptosis. To quantify the expression of caspase-3, we counted the numbers of caspase-3-positive hair cells and SGN in cultures treated for 3, 6, 12, and 24 h with 1 mM KM and determined the percentages of caspase-3-positive hair cells and SGN at each time point. Few caspase-3-positive hair cells were observed at 0, 3, and 6 h post-treatment (Fig. 5 (L)), but the mean (SEM) percentages of caspase-3-positive hair cells increased to 85–90% at 12 and 24 h post-treatment. There was a significant effect of treatment duration on caspase-3 expression in hair cells (one-way ANOVA, F = 753.8, p < 0.0001, 4, 15 df). There were significant differences between 0 h vs 12 and 24 h, 3 h vs 12 and 24 h, 6 h vs 12 and 24 h, and 12 vs 24 h (Newman-Keuls post hoc, p < 0.05). Few caspase-3-positive SGNs were observed at 0 h, but the percentage progressively increased from 3 to 24 h reaching a maximum of around 93% at 24 h (Fig. 5 (L)). There was a significant effect of treatment duration on caspae-3 expression (one-way ANOVA, F = 754.9, p < 0.0001, 4, 15 df). There were significant differences between 0 h vs 3, 6, 12, and 24 h, 3 h vs 6, 12, and 24 h, 6 h vs 12 and 24 h, and 12 vs 24 h (Newman-Keuls post hoc, p < 0.05).
P3 cultures of organ of Corti (surface view (A–E), Z-plane section (a–e)) from basal turn treated with 0 (control) or 1 mM KM for 3, 6, 12, or 24 h. Organ of Corti stained with caspase-3 (Cas3, red), TO-PRO-3 (blue), and phalloidin Alexa 488 (green). Many hair cells missing after 12 and 24 h KM treatment; many caspase-3 labeled cells (yellow arrows) present at this time (d–e). P3 SGN cultures (f–j) from the basal turn treated with 0 (control) or 1 mM KM for 3, 6, 12, or 24 h. Specimens labeled with neurofilament 200 kDa (green) and caspase-3 (Cas3, red). Note increase in caspace-3 SGN between 3 and 24 h of kanamycin treatment. Histograms showing percentages of casapse-3-positive hair cells (K) and caspase-3-positive SGN (L). Horizontal lines indicate significant (p < 0.05) between group differences (see text for details)
Type II SGNs Resistant to KM Treatment
Adult type II SGN and auditory nerve fibers are typically more resistant to ototraumatic insult than type I neurons (Spoendlin 1982; Spoendlin and Schrott 1989). To determine if postnatal type II SGN were more resistant than type I neurons to KM treatment, cochlear cultures containing SGN were stained with peripherin which is only present in type II neurons as well as neurofilament 200 kDa which is expressed in both type I and type II neurons (Barclay et al. 2011; Froud et al. 2015). Figure 6 shows representative photomicrographs of cochlear cultures double-labeled with neurofilament 200 kDa and peripherin and treated with 1 mM KM for 24 or 48 h. In control cultures, neurofilament labeling of SGN and their processes was more extensive and robust than that for peripherin, consistent with the fact that the former labels both type I and type II neurons, whereas the later labels only type II neurons (Fig. 6a, c, g). Robust peripherin labeling was expressed in a smaller subpopulation of type II SGN somas; the peripherin-labeled fibers projected radially and then formed three longitudinal fiber bundles near the location of the OHC (Fig. 6b). In the merged image (Fig. 6g), red peripherin labeling was exclusively seen in the three parallel longitudinal fiber bundles corresponding to the location of the three rows of OHC. The yellow labeling representing the overlap of neurofilament and peripherin only projected radially and stopped in the region occupied by IHC. After 48-h KM treatment, there was a dramatic decline in the extent and intensity of neurofilament staining (Fig. 6a–c), consistent with overall loss of SGN noted above. In contrast, there was only a modest loss of peripherin labeling of fibers and somata of type II SGN. There was little evidence of soma shrinkage in the peripherin-labeled type II SGN after KM treatment (Fig. 6a vs f), and the three longitudinal fiber bundles were still present.
Basal turn P3 organotypic control cultures (a, d, g) and cultures treated with 1 mM KM for 24 h (b, e, h) or 48 h (c, f, i). Specimens labeled with neurofilament 200 kDa (green), expressed in both type I and type II SGN, and peripherin (red), expressed only in type II SGN. Note large loss of neurofilament-positive SGN soma and radiating fibers (a, b, c), but major retention of peripherin-positive type II SGN and fibers (d, e, f). Panels in the left and the middle column merged (yellow) in the right column
Discussion
Many studies report that SGN degeneration begins weeks or months following aminoglycoside treatment, long after the hair cells have disappeared (Landry et al. 2011; McFadden et al. 2004; Shepherd et al. 2008); however, some find that SGN and afferent nerve terminals begin to degenerate soon after the onset of aminoglycoside treatment and hair cell loss (Dodson and Mohuiddin 2000; Kong et al. 2010; Xu et al. 1993). To explore this issue, we assessed the time course of hair cell loss and SGN degeneration in vitro by treating rat P3 cochlear organotypic cultures and adult cochlear cultures with 0.1 to 1 mM KM. In our in vitro assays, KM destroyed both OHC and IHC; however, adult hair cells were slightly more vulnerable to KM damage than P3 hair cells. Treatment for 24 h with 0.1 mM KM caused significant hair cell damage in adult cultures, whereas 0.2 to 0.5 mM of KM was required to produce comparable hair cell damage in P3 cultures (Fig. 2). In contrast, P3 SGN and nerve fibers were much more vulnerable to KM than adult SGN. KM doses as low as 0.1 mM resulted in significant somata shrinkage and nuclear condensation in P3 SGN, whereas adult SGN were largely unaffected by KM doses as high as 1 mM (Fig. 3). KM also caused considerable thinning and loss of P3 auditory nerve fibers projecting to the hair cells (Fig. 1b, c) and significant loss of the dense network of nerve fibers adjacent to the IHC. Damage to P3 SGN was associated with increased expression of the toxic superoxide radical and executioner caspase-3; these changes were observed as early as 3 h post-treatment (Figs. 4 and 5). A comparison of neurofilament and peripherin labeling of auditory nerve fibers and SGN in P3 cochlear cultures revealed much greater loss of neurofilament than peripherin staining (Fig. 6). These results suggest that peripherin-positive type II fibers are more resistant to KM damage than type I fibers, consistent with other forms of trauma (Spoendlin 1969, 1971).
Critical Period
In altricial species such as cats, rats, and mice, cochlear function and structure develop rapidly after birth. During development, the cochlea is especially vulnerable to aminoglycoside antibiotics during a critical period which begins around the onset of cochlear functions (~8 days in postnatal rat) and subsides once the cochlea matures (~28 days in postnatal rat). The greatest hearing loss and hair cell loss occur when aminoglycosides are administered in vivo during this critical period and substantially less if administered before or after this critical period (Carlier and Pujol 1980; Henley et al. 1996; Shepherd and Martin 1995). The critical period for aminoglycoside ototoxicity is thought to be related to mechanisms associated with cochlear development. One factor that could conceivably contribute to the critical period observed in vivo is maturation of the hair cell transduction channels in the stereocilia (Hashino and Salvi 1997; Hashino et al. 1999). Stereocilia damage and manipulations that limit uptake through the transduction channels suppress the entry of aminoglycosides into the hair cell (Gale et al. 2001; Richardson et al. 1997). According to this view, the hair cell lesions at age P15 and beyond should be more severe than at P3. Our data show that hair cell lesions in adults were more severe than in P3 rats, consistent with the hypothesis that aminoglycoside entry through the transduction channel may be an important factor in ototoxicity. However, aminoglycoside uptake in vivo is likely regulated by other factors such as the blood-labyrinth barrier, tight junctions, channels, and transporters which regulate the movement of aminoglycosides from the stria vascularis into the endolymph (Dai et al. 2006). Aminoglycosides are readily taken up into neonatal hair cells prior to the establishment of a mature blood-labyrinth barrier or endolymphatic potential, but uptake slows considerably after the blood-labyrinth barrier matures (Dai et al. 2006). However, disrupting the blood-labyrinth barrier in adult animals promotes entry of aminoglycoside into the cochlea; this results in rapid hair cell loss followed by a slow progressive degeneration of auditory nerve fiber and spiral ganglion neurons over weeks and months (Ding et al. 2003; McFadden et al. 2004).
P3 KM Neurotoxicity
The most unexpected finding of the current study was that KM, but not gentamycin, caused considerable damage to ANF and SGN in P3 cultures, but KM failed to damage ANF and SGN in adult cultures. One possible explanation for this is that KM induced greater expression of superoxide in SGN than hair cells in P3 explants; the strong expression of superoxide may be related to the paucity of myelination on P3 neurons. Conversely, adult SGN may be more resistant to KM neurotoxicity due to extensive myelination which provides neurotrophic support (Agterberg et al. 2008; Leake et al. 2011). Neither of these explanations, however, can account for the fact the gentamicin does not damage postnatal SGN, whereas KM does. Given that P3 SGN are damaged by KM but adult SGN are not, an important issue that needs to be addressed in future studies is the age at which SGN becomes resistant to KM damage.
We were initially suspect of our finding that KM damaged P3 SGN and ANF because we had previously reported that another aminoglycoside, gentamicin, failed to damage P3 ANF or SGN with concentrations that caused massive hair cell loss (Ding et al. 2002). To verify our earlier gentamicin results, we repeated our earlier study with P3 cochlear cultures treated with 0.5 mM gentamicin for 24 h. Consistent with our earlier studies, we confirmed that gentamicin caused massive hair cell damage, but failed to damage ANF or SGN (Fig. 7). To further confirm that our KM studies were correct, we performed further replication studies with KM and again found that KM damaged P3 ANF and SGN as well as hair cells as noted above.
We carried out an extensive literature search to determine if KM or other aminoglycosides damaged ANF or SGN and found one report showing that local drug application to the adult guinea pig cochlea resulted in weak to moderate gentamicin immunolabeling in SGN and ANF from 6 h to 7 days post-treatment (Imamura and Adams 2003). However, our in vitro results with adult cochlear cultures revealed no damage to ANF or SGN. On the other hand, in vivo application of a high concentration of gentamicin into the perilymph of the guinea pig cochlea resulted in severe damage not only to hair cells but also to ANF, SGN, the organ of Corti, and lateral wall of the cochlea (Dodson and Mohuiddin 2000). In this case, the massive damage seen when gentamicin was applied to the cochlea may be the result of nonspecific damage caused by the high drug concentration.
There is considerable evidence that aminoglycoside antibiotics rapidly enter hair cells through nonselective cation channels located in the stereocilia at the apical pole of the hair cell (Corey and Hudspeth 1979). However, uptake has also been observed in many other cell types including the dorsal root ganglion neurons, trigeminal ganglion neurons, tongue papillae, vibrissae, and proximal tubule kidney cells (Dai et al. 2006; Imamura and Adams 2005; Raisinghani and Premkumar 2005). In these cases, drug entry is believed to occur through TRP channels and P2X receptors (Bongartz et al. 2010; Corey et al. 2004; Garcia-Anoveros and Duggan 2007; Raisinghani and Premkumar 2005; Xu et al. 2011). TRPV1 channels are expressed in adult rat SGN (Zheng et al. 2003). TRPM4 immunolabeling was absent in murine embryonic SGN, but immunolabeling increased from P0 to 2 weeks old followed by decreased expression in adult SGN (Sakuraba et al. 2014). P2X2 receptor expression was observed in SGN and ANF from embryonic day 19 until early postnatal life followed by a decline in expression as the rat cochlea matured (Jarlebark et al. 2000). One interpretation of these results is that KM-induced damage to P3 SGN and ANF could be mediated through aminoglycoside uptake through TRP or P2X2 channels; however, this explanation fails to account for lack of neuronal damage caused by gentamicin which should permeate these channels. At this time, we are left with paradoxical and unexplained finding that KM is neurotoxic to postnatal SGN and ANF, whereas gentamicin is not. Some clues regarding its neurotoxic potential may be gleaned from a recent study showing that KM caused considerable mitochondrial swelling, vacuole formation, and endoplasmic reticulum dilation in adult dorsal cochlear nucleus neurons; however, these effects were largely reversible (Fan et al. 2013). Further work is needed to more fully elucidate the mechanism underlying KM neurotoxicity in P3 SGN and ANF. From a clinical perspective, our results suggest that KM could be especially toxic to human SGN which begin to develop and mature in utero around the first trimester (Bibas et al. 2006).
References
Agterberg MJ, Versnel H, de Groot JC, Smoorenburg GF, Albers FW, Klis SF (2008) Morphological changes in spiral ganglion cells after intracochlear application of brain-derived neurotrophic factor in deafened guinea pigs. Hear Res 244:25–34
Barclay M, Ryan AF, Housley GD (2011) Type I vs type II spiral ganglion neurons exhibit differential survival and neuritogenesis during cochlear development. Neural Dev 6:33
Bibas A, Hornigold R, Liang J, Michaels L, Anagnostopoulou S, Wright A (2006) The development of the spiral ganglion in the human foetus. Folia Morphol (Warsz) 65:140–144
Bongartz EV, Rettinger J, Hausmann R (2010) Aminoglycoside block of P2X2 receptors heterologously expressed in Xenopus laevis oocytes. Purinergic Signal 6:393–403
Carlier E, Pujol R (1980) Supra-normal sensitivity to ototoxic antibiotic of the developing rat cochlea. Arch Otorhinolaryngol 226:129–133
Corey DP, Hudspeth AJ (1979) Ionic basis of the receptor potential in a vertebrate hair cell. Nature 281:675–677
Corey DP, Garcia-Anoveros J, Holt JR, Kwan KY, Lin SY, Vollrath MA et al (2004) TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature 432:723–730
Dai CF, Mangiardi D, Cotanche DA, Steyger PS (2006) Uptake of fluorescent gentamicin by vertebrate sensory cells in vivo. Hear Res 213:64–78
Dallos P, Harris D (1978) Properties of auditory nerve responses in absence of outer hair cells. J Neurophysiol 41:365–383
Deng L, Ding D, Su J, Manohar S, Salvi R (2013) Salicylate selectively kills cochlear spiral ganglion neurons by paradoxically up-regulating superoxide. Neurotox Res
Ding D, Salvi R (2005) Review of cellular changes in the cochlea due to aminoglycoside antibiotics. The Volta Review 105:407–438
Ding D, Stracher A, Salvi RJ (2002) Leupeptin protects cochlear and vestibular hair cells from gentamicin ototoxicity. Hear Res 164:115–126
Ding D, McFadden SL, Browne RW, Salvi RJ (2003) Late dosing with ethacrynic acid can reduce gentamicin concentration in perilymph and protect cochlear hair cells. Hear Res 185:90–96
Ding D, Jiang H, Salvi RJ (2010a) Mechanisms of rapid sensory hair-cell death following co-administration of gentamicin and ethacrynic acid. Hear Res 259:16–23
Ding D, Tao J, Qu Y, Qi W, Salvi R (2010b) Science of the inner ear. Beijing Chinese Science and Technology Publishing Co.
Ding D, Jiang H, Fu Y, Salvi R, Someya S, Tanokura M (2012) Ototoxic effects of carboplatin in organotypic cultures in chinchillas and rats. J Otol 7:92–101
Ding D, Qi W, Yu D, Jiang H, Han C, Kim MJ et al (2013a) Addition of exogenous NAD+ prevents mefloquine-induced neuroaxonal and hair cell degeneration through reduction of caspase-3-mediated apoptosis in cochlear organotypic cultures. PLoS One 8:e79817
Ding D, Jiang H, Chen GD, Longo-Guess C, Muthaiah VP, Tian C et al (2016) N-acetyl-cysteine prevents age-related hearing loss and the progressive loss of inner hair cells in γ-glutamyl transferase 1 deficient mice. Aging (Albany NY) 8:730–750
Dodson HC, Mohuiddin A (2000) Response of spiral ganglion neurones to cochlear hair cell destruction in the guinea pig. J Neurocytol 29:525–537
Esterberg R, Hailey DW, Coffin AB, Raible DW, Rubel EW (2013) Disruption of intracellular calcium regulation is integral to aminoglycoside-induced hair cell death. J Neurosci 33:7513–7525
Fan GR, Yin ZD, Sun Y, Chen S, Zhang WJ, Huang X et al (2013) Reversible neurotoxicity of kanamycin on dorsal cochlear nucleus. Brain Res 1502:30–46
Fischel-Ghodsian N (1998) Mitochondrial mutations and hearing loss: paradigm for mitochondrial genetics. Am J Hum Genet 62:15–19
Froud KE, Wong AC, Cederholm JM, Klugmann M, Sandow SL, Julien JP et al (2015) Type II spiral ganglion afferent neurons drive medial olivocochlear reflex suppression of the cochlear amplifier. Nat Commun 6:7115
Fu Y, Ding D, Wei L, Jiang H, Salvi R (2013) Ouabain-induced apoptosis in cochlear hair cells and spiral ganglion neurons in vitro. Biomed Res Int 2013:628064
Gale JE, Marcotti W, Kennedy HJ, Kros CJ, Richardson GP (2001) FM1-43 dye behaves as a permeant blocker of the hair-cell mechanotransducer channel. J Neurosci 21:7013–7025
Garcia-Anoveros J, Duggan A (2007) TRPA1 in auditory and nociceptive organs
Hashino E, Salvi RJ (1997) Regenerated hair cells exhibit a transient resistance to aminoglycoside toxicity. Brain Res 720:172–182
Hashino E, Shero M, Salvi RJ. (1999). Lyosomal targeting and accumulation of aminoglycoside antibiotics in cochlear hair cells. Am Soc Cell Biol
Henley CM, Weatherly RA, Martin GK, Lonsbury-Martin B (1996) Sensitive developmental periods for kanamycin ototoxic effects on distortion-product otoacoustic emissions. Hear Res 98:93–103
Hirose K, Hockenbery DM, Rubel EW (1997) Reactive oxygen species in chick hair cells after gentamicin exposure in vitro. Hearing Res 104:1–14
Imamura S, Adams JC (2003) Distribution of gentamicin in the guinea pig inner ear after local or systemic application. J Assoc Res Otolaryngol 4:176–195
Imamura S, Adams JC (2005) Selective gentamicin uptake by cytochemical subpopulations of guinea-pig geniculate ganglion cells. Neuroscience 131:125–133
Izumikawa M, Batts SA, Miyazawa T, Swiderski DL, Raphael Y (2008) Response of the flat cochlear epithelium to forced expression of Atoh1. Hear Res 240:52–56
Jarlebark LE, Housley GD, Thorne PR (2000) Immunohistochemical localization of adenosine 5′-triphosphate-gated ion channel P2X(2) receptor subunits in adult and developing rat cochlea. J Comp Neurol 421:289–301
Jeong SW, Kim LS, Hur D, Bae WY, Kim JR, Lee JH (2010) Gentamicin-induced spiral ganglion cell death: apoptosis mediated by ROS and the JNK signaling pathway. Acta Otolaryngol 130:670–678
Kamada S, Kikkawa U, Tsujimoto Y, Hunter T (2005) Nuclear translocation of caspase-3 is dependent on its proteolytic activation and recognition of a substrate-like protein(s). J Biol Chem 280:857–860
Kong WJ, Yin ZD, Fan GR, Li D, Huang X (2010) Time sequence of auditory nerve and spiral ganglion cell degeneration following chronic kanamycin-induced deafness in the guinea pig. Brain Res 1331:28–38
Kopelovich JC, Cagaanan AP, Miller CA, Abbas PJ, Green SH (2013) Intracochlear electrical stimulation suppresses apoptotic signaling in rat spiral ganglion neurons after deafening in vivo. Otolaryngol Head Neck Surg 149:745–752
Kujawa SG, Liberman MC (2006) Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci 26:2115–2123
Kujawa SG, Liberman MC (2015) Synaptopathy in the noise-exposed and aging cochlea: primary neural degeneration in acquired sensorineural hearing loss. Hear Res
Lalwani AK, Han JJ, Castelein CM, Carvalho GJ, Mhatre AN (2002) In vitro and in vivo assessment of the ability of adeno-associated virus-brain-derived neurotrophic factor to enhance spiral ganglion cell survival following ototoxic insult. Laryngoscope 112:1325–1334
Landry TG, Wise AK, Fallon JB, Shepherd RK (2011) Spiral ganglion neuron survival and function in the deafened cochlea following chronic neurotrophic treatment. Hear Res 282:303–313
Lang H, Schulte BA, Schmiedt RA (2005) Ouabain induces apoptotic cell death in type I spiral ganglion neurons, but not type II neurons. J Assoc Res Otolaryngol 6:63–74
Lautermann J, Crann SA, McLaren J, Schacht J (1997) Glutathione-dependent antioxidant systems in the mammalian inner ear: effects of aging, ototoxic drugs and noise. Hear Res 114:75–82
Leake PA, Hradek GT, Hetherington AM, Stakhovskaya O (2011) Brain-derived neurotrophic factor promotes cochlear spiral ganglion cell survival and function in deafened, developing cats. J Comp Neurol 519:1526–1545
Matsuda K, Ueda Y, Doi T, Tono T, Haruta A, Toyama K et al (1999) Increase in glutamate-aspartate transporter (GLAST) mRNA during kanamycin-induced cochlear insult in rats. Hear Res 133:10–16
McFadden SL, Ding D, Jiang H, Woo JM, Salvi RJ (2002) Chinchilla models of selective cochlear hair cell loss. Hear Res 174:230–238
McFadden SL, Ding D, Jiang H, Salvi RJ (2004) Time course of efferent fiber and spiral ganglion cell degeneration following complete hair cell loss in the chinchilla. Brain Res 997:40–51
Pittinger C, Adamson R (1972) Antibiotic blockade of neuromuscular function. Annu Rev Pharmacol 12:169–184
Raisinghani M, Premkumar LS (2005) Block of native and cloned vanilloid receptor 1 (TRPV1) by aminoglycoside antibiotics. Pain 113:123–133
Richardson GP, Forge A, Kros CJ, Fleming J, Brown SD, Steel KP (1997) Myosin VIIA is required for aminoglycoside accumulation in cochlear hair cells. J Neurosci 17:9506–9519
Sakuraba M, Murata J, Teruyama R, Kamiya K, Yamaguchi J, Okano H et al (2014) Spatiotemporal expression of TRPM4 in the mouse cochlea. J Neurosci Res 92:1409–1418
Sergeyenko Y, Lall K, Liberman MC, Kujawa SG (2013) Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci 33:13686–13694
Sha SH, Schacht J (2000) Antioxidants attenuate gentamicin-induced free radical formation in vitro and ototoxicity in vivo: D-methionine is a potential protectant. Hearing Res 142:34–40
Sha SH, Taylor R, Forge A, Schacht J (2001a) Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear Res 155:1–8
Sha SH, Zajic G, Epstein CJ, Schacht J (2001b) Overexpression of copper/zinc-superoxide dismutase protects from kanamycin-induced hearing loss. Audiol Neurootol 6:117–123
Shepherd RK, Martin RL (1995) Onset of ototoxicity in the cat is related to onset of auditory function. Hear Res 92:131–142
Shepherd RK, Coco A, Epp SB (2008) Neurotrophins and electrical stimulation for protection and repair of spiral ganglion neurons following sensorineural hearing loss. Hear Res 242:100–109
Spoendlin H (1969) Innervation patterns in the organ of corti of the cat. Acta Otolaryngol 67:239–254
Spoendlin H (1971) Degeneration behaviour of the cochlear nerve. Archiv Klinische Exper Ohren Nasen Kehlkopfheilkunde 200:275–291
Spoendlin H (1982) The innervation of the outer hair cell system. Am J Otol 3:274–278
Spoendlin H, Schrott A (1989) Analysis of the human auditory nerve. Hear Res 43:25–38
Staecker H, Galinovic-Schwartz V, Liu W, Lefebvre P, Kopke R, Malgrange B et al (1996) The role of the neurotrophins in maturation and maintenance of postnatal auditory innervation. Am J Otol 17:486–492
Sugawara M, Corfas G, Liberman MC (2005) Influence of supporting cells on neuronal degeneration after hair cell loss. J Assoc Res Otolaryngol 6:136–147
Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA et al (2014) Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 14:742–750
Wei L, Ding D, Salvi R (2010) Salicylate-induced degeneration of cochlea spiral ganglion neurons-apoptosis signaling. Neuroscience 168:288–299
Xu SA, Shepherd RK, Chen Y, Clark GM (1993) Profound hearing loss in the cat following the single co-administration of kanamycin and ethacrynic acid. Hear Res 70:205–215
Xu GY, Li G, Liu N, Huang LY (2011) Mechanisms underlying purinergic P2X3 receptor-mediated mechanical allodynia induced in diabetic rats. Mol Pain 7:60
Yu J, Ding D, Wang F, Jiang H, Sun H, Salvi R (2014) Pattern of hair cell loss and delayed peripheral neuron degeneration in inner ear by a high-dose intratympanic gentamicin. Journal of Otology 9:126–135
Zheng J, Dai C, Steyger PS, Kim Y, Vass Z, Ren T et al (2003) Vanilloid receptors in hearing: altered cochlear sensitivity by vanilloids and expression of TRPV1 in the organ of corti. J Neurophysiol 90:444–455
Zilberstein Y, Liberman MC, Corfas G (2012) Inner hair cells are not required for survival of spiral ganglion neurons in the adult cochlea. J Neurosci 32:405–410
Zucca G, Vega R, Botta L, Perez ME, Valli P, Soto E (1992) Streptomycin blocks the afferent synapse of the isolated semicircular canals of the frog. Hearing Res 59:70–74
Acknowledgements
Work supported in part by grant from National Institutes of Occupational Safety and Health (R01OH010235)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Author RS received a grant from Anida Inc., is a paid consultant for CilCare, and serves as unpaid member of the scientific advisory board of Hyperacusis Research, a nonprofit organization. None of these activities are related to the results contained in the manuscript. The authors declare that they do not have any financial conflict of interest with any of the funding agencies supporting this research.
Research Involving Animals
The experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University at Buffalo, NY, USA, and conform to the guidelines from the National Institutes of Health, MD, USA.
Rights and permissions
About this article
Cite this article
Gao, K., Ding, D., Sun, H. et al. Kanamycin Damages Early Postnatal, but Not Adult Spiral Ganglion Neurons. Neurotox Res 32, 603–613 (2017). https://doi.org/10.1007/s12640-017-9773-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-017-9773-2