Abstract
Ketamine, a common anesthetic used for pediatric patients, has been shown to induce neurotoxicity and alter adolescent behaviors in rats when administered during neonatal period. However, the mechanisms underlying this kind of neurotoxicity remain largely to be determined. Herein, we studied whether the reactive oxygen species (ROS) due to the increased NOX2 mediates loss of phenotype of PV interneurons and thus contributes to long-term cognitive impairments after repeated ketamine exposures. Sprague–Dawley male rat pups received a daily administration of ketamine intraperitoneally (75 mg/kg) from postnatal day 6 (P6) to P8 for three consecutive days. For the interventional study, pups were treated with a NADPH oxidase inhibitor, apocynin (Apo). Learning and memory abilities were tested by the open field, fear conditioning, and Morris water maze on P40, P42–44, and P50–56, respectively. For histological and biochemical assays, a separate cohort of rats was killed on P9 or P60, and the brain tissues were harvested. Our results showed the upregulation of 8-OHdG and gp91/NOX2 and downregulation of PV and glutamic acid decarboxylase 67 (GAD67) after repeated ketamine exposures, which co-occurred with the long-term cognitive impairments as evidenced by the decreased freezing time to context. However, Apo treatment attenuated these abnormalities. Our results suggest that oxidative damage, probably due to the increased NOX2, mediates loss of phenotype of PV interneurons and thus contributes to long-term cognitive impairments after repeated ketamine exposures. Moreover, the inhibition of NADPH oxidase may protect against cognitive dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the development of surgical techniques, more and more babies, infants, and toddlers are subjected to surgery with general anesthesia (Walker and Yaksh 2012). However, there is a growing concern about whether exposure to general anesthetics during the neonatal period could lead to neurotoxic injury and result in alterations of brain function later in life (Paule et al. 2011). Ketamine, a noncompetitive N-methyl-D-aspartate (NMDA) receptor ion channel blocker, is a commonly used anesthetic for pediatric patients (Anis et al. 1983). Preclinical studies have shown that ketamine induces neuroapoptosis in both developing animal brain and primary cultured neurons (Huang et al. 2012; Paule et al. 2011). Human studies also indicate that repeated exposures to anesthetic ketamine can negatively impact neurodevelopment in infants (Yan et al. 2014) as well as lead some learning disabilities or emotional/behavior disorders in children before 2 years of age (Flick et al. 2011), which appears sometimes in clinical practice especially in plastic operations. However, the precise mechanisms underlying this neurotoxic effect of repeated neonatal ketamine exposures remain unclear.
Parvalbumin (PV) interneurons, a subset of inhibitory GABAergic neurons controlling the excitability of postsynaptic pyramidal neurons (Celio 1986), are involved in the generation of gamma oscillations responsible for cognitive performance (He et al. 2014; Stichel et al. 1987). Loss of phenotype of PV interneurons has been implicated in impaired behaviors and cognition associated with a number of major neuropsychiatric disorders, including Alzheimer’s disease (AD) (Verdaguer et al. 2015), schizophrenia (Li et al. 2016), and depression (Smiley et al. 2015). Although the factors triggering dysfunction of PV interneurons are diverse, oxidative stress is regarded as one of the contributing factors because of the high metabolic requirements of these interneurons (Hardingham and Do 2016). Particularly, ROS, as a result of NADPH oxidase 2 (NOX2) activation, can reduce the expression of GABAergic markers and consequently lead to the loss of inhibitory capacity of PV interneurons (Infanger et al. 2006).
Considering that ketamine treatment can induce a persistent increase of superoxide in brain due to activation of NADPH oxidase in vitro (Bedard and Krause 2007), we raised a hypothesis that ROS, probably derived from the upregulation of NOX2, may mediate loss of phenotype of PV interneurons and thus contribute to long-term cognitive impairments after repeated ketamine exposures to newborns. Therefore, we investigated the long-term consequences of neonatal ketamine repeated exposures on behaviors and PV interneuron phenotype in the prefrontal cortex and hippocampus, and determined whether gp91 activation, a catalytic subunit of NOX2, contributes to these consequences.
Methods and Materials
Animals
The experimental protocols and procedures were reviewed and approved by the Animal Investigation Ethics Committee of Jinling Hospital and were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals from the National Institutes of Health (Bethesda, MD, USA). Rat pups with their mothers were purchased from the Animal Center of Jinling Hospital, Nanjing, China. The pups were housed in a room maintained under constant environmental conditions (temperature 22–24 °C, a 12-h light/dark cycle, and 50 ± 10 % humidity) with their mothers till postnatal day 20 (P20). To exclude the influence of estrogen on behavioral data, we used only the male offspring (n = 12 per group) in the behavioral and biochemical tests. At P21, the pups were weaned and housed 4–5 per cage in standard condition. For biochemistry studies, no more than two pups from the same litter were used for each experimental group. The flow chart for the study protocol is summarized in Fig. 1.
Schematic timeline of the experimental procedure. Six-day old rats were treated with Apocynin (Apo) at 5 mg/kg or the equal volume of vehicle 30 min before the ketamine anesthesia from postnatal day 6 (P6) to P8. The behavioral tests include open field (OF), fear conditioning (FC), and Morris water maze (MWM) tests were assessed at P40, P42–44, and P50–55, respectively. The rats used for the biochemical study were not subjected to behavioral tests and were sacrificed 2 h after the last ketamine exposure at P8 and P60
Animal Grouping
The P6 rats were randomly assigned to the following four groups: control + vehicle group; control + Apo group; ketamine + vehicle group; and ketamine + Apo group. Animals in the ketamine groups received ketamine (75 mg/kg) intraperitoneally daily for three consecutive days from P6 to P8 (Huang et al. 2012). After each ketamine exposure, rats were put in the calorstat where the rats were kept warm on a plate heated to 37 ± 1 °C. Pups were returned to their mothers in the cage until return of the righting reflex after each ketamine exposure. The rats in the control groups were placed at calorstat under the same conditions except that they were not exposed to ketamine.
Drug Administration
Apo (A10809, Sigma, St Louise, MO, USA), dissolved in dimethylsulfoxide (DMSO, Sigma, St. Louis, MO, USA) and then diluted in normal saline, was used for the intervention study to determine the effect of pharmacological NADPH oxidase inhibition on ketamine-induced neuropathological alterations in the hippocampus and prefrontal cortex. Rats were intraperitoneally administered with 5 mg/kg of Apo 30 min before each of the ketamine exposure. The dose of Apo was selected on the basis of our previous study, in which we showed that 5 mg/kg is effective in reversing the cognitive impairments in an animal model of sepsis-associated encephalopathy (Ji et al. 2015a).
Behavioral Testing
Behavioral alterations were evaluated by the open-field test, fear conditioning test, and Morris water maze (MWM) test. All behavioral tests were conducted at 14:00–16:00 p.m. in a sound-isolated room with the instruments provided by Shanghai Xinruan Information Technology Co., Ltd., Shanghai, China. All rats after behavioral tests were not used for any biochemistry studies and were sacrificed with deep anesthesia.
Open-Field Test
At P40, rats were gently placed in the center of a black plastic chambers (100 cm × 100 cm × 40 cm) over a period of 10 min while exploratory behavior was automatically recorded by a video tracking system (XR-XZ301, Shanghai Xinruan Information Technology Co., Ltd., Shanghai, China) to measure exploratory behavior and locomotor activity. The total distance and the amount of time traveled in the center area (50 cm × 50 cm) of the maze were measured. After each test, the arena was thoroughly cleaned with 75 % alcohol to avoid the presence of olfactory cues.
Fear Conditioning Test
At P42–44, a computer-controlled fear conditioning system (XRXC404; Shanghai Xinruan Information Technology Co., Ltd., Shanghai, China) was used in the fear conditioning test as we described previously (Ji et al. 2015b). Briefly, it consisted of three phases, training (acquisition), contextual, and cue tests, occurring 24 h apart. In the training phase, rats were placed in the chamber for 3 min for habituation, and then one tone-foot-shock pairing (tone, 30 s, 75 dB, 3 kHz; foot-shock, 2 s, 0.75 mA, foot-shock was delivered during the last 2 s of the tone presentation) was delivered. After that, the rats were left in the chamber for another 30 s and returned to their home cage. In the contextual test (a hippocampus-dependent task), rats were placed in the same chamber without any stimulation for 5 min and scored for the freezing time. In the cue test (a hippocampus-independent task), rats were placed into the altered chamber and allowed to stay for 3 min. Then, the training tone was delivered for another 3 min and the freezing time was scored. Associative learning and memory were assessed by the length of time as “freezing behavior,” which was defined as a completely immobile posture except for respiration. After each test, the conditioning chamber was thoroughly cleaned with 75 % alcohol to avoid the presence of olfactory cues.
Morris Water Maze
At P50–55, the MWM test (XR-XM101; Shanghai Xinruan Information Technology Co., Ltd., Shanghai, China) was performed to investigate spatial learning and memory function. The MWM was a black metal tank (120 cm in diameter, 60 cm in depth) equipped with a platform (10 cm in diameter) 1–2 cm below the surface of the water. The MWM task was performed according to our previous study (Ji et al. 2015b). Briefly, it consisted of two phases, training phase for five consecutive days and probe trial phase on day 6. In the training phase, rats were allowed to face to the pool wall in four random places (N, S, E, W) in the pool to find the fixed platform. Release positions were randomly predetermined. The trial was terminated once the rat reached the platform. If the rat failed to reach the platform within 60 s, it would be guided to the platform and allowed to stay for 15 s, and then the latency was recorded for 60 s. In the probe test, single-probe trial was conducted with the original platform removed 24 h after the last training session. Rats were released at a random start position and allowed to swim for 60 s in the pool.
Western Blot Analysis
Rats were anesthetized with 2 % sodium pentobarbital (50 mg/kg, intraperitoneally; Sigma, St Louise, MO, USA) and then sacrificed to obtain the hippocampus and prefrontal cortex tissues. The harvest tissues were homogenized in ice-cold lysis buffer (10 mM Tris–HCl, pH 7.4, 150 mM NaCl, 2 mM EDTA and 0.5 % NP-40) plus protease inhibitors (Sigma, St. Louis, MO, USA). Homogenates were centrifuged at 12,000 rpm for 10 min at 4 °C, and supernatants were saved. Protein concentrations were determined by Bradford assay. Prepared protein samples (35 μg/well) were separated on 8–12 % SDS-PAGE and transferred to a polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA). After blocking with 5 % nonfat milk, the membranes were incubated with goat anti-NOX2/gp91phox (1:500; Santa Cruz Biotechnology, Dallas, TX, USA), mouse anti-GAD67 (1:1000; Millipore, Billerica, MA, USA), rabbit anti-parvalbumin (1:1000; Abcam, Cambridge, UK), and rabbit anti-GAPDH (1:500; 1:500; Santa Cruz Biotechnology, Dallas, TX, USA), and rabbit anti-Nox4 (1:1000; Abcam, Cambridge, UK) overnight at 4 °C room. After washing, membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit (1:1000; Santa Cruz Biotechnology, Dallas, TX, USA). After washing, the horseradish peroxidase-conjugated secondary antibodies (rabbit anti-goat, goat anti-rabbit and goat anti-mouse [Bioworld Technology, St. Louis Park, MN, USA]) diluted 1:5000 were used to incubate the membranes for 1 h at room temperature. The bands were detected with Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific, Rockford, IL, USA) and semiquantified with J-image software (National Institutes of Health, Bethesda, MD, USA).
Measurement of mRNA Levels
Total RNA was extracted from the hippocampus and prefrontal cortex. RNA was isolated using Trizol (Life Technologies, Inc., Grand Island, NY, USA). RNA samples were further purified with CHCl3 and concentrations were determined spectrophotometrically, cDNA was synthesized from 2 μg of total RNA using the First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Loughborough, UK), and PCR was performed in a 20 μL reaction mixture using SYBR Green PCR Master Mix. The reaction mixture consisted of first-strand cDNA, 2 × Master SYBR Green qPCR Master Mix (Applied Biosystems Inc, Grand Island, NY, USA), H2O, and gene-specific PCR primers. Sequences for qPCR primers were as follows: NOX2: Forward, 5′-TGATGTTAGTGGGAGCCGGGATTG-3′, Reverse, 5′-TCTGCAAACCACTCAAAGGCATG-3′; 18S: Forward, 5′-GGCTACCACATCCAAGGAA-3′ Reverse, 5′-GCTGGAATTACCGCGGCT-3′. The following qPCR protocol was used: 95 °C for 3 min followed by 40 cycles of 95 °C for 20 s, 60 °C for 30 s, and 72 °C for 20 s. Serial dilutions (fivefold) of total RNA from one control sample were analyzed for each target gene and used to construct linear standard curves. PCR was performed in triplicate and threshold cycle numbers were averaged. Results were normalized to 18S using the ΔΔCT method.
Immunofluorescence
Rats were anesthetized with 2 % sodium pentobarbital (50 mg/kg, intraperitoneally; Sigma, St Louise, MO, USA) and transcardially perfused with saline, followed by 4 % paraformaldehyde. The harvested brains were postfixed in the same fixative for 2 h and then dehydrated in 30 % sucrose at 4 °C overnight. Frozen coronal sections (10 µm thick) were established for standard immunofluorescence. “Slices were blocked with 3 % bovine serum albumin (BSA) for 1 h at room temperature and followed by incubating primary antibodies: mouse anti-8-hydroxy-2′-deoxyguanosine (8-OHdG; 1:200; Santa Cruz Biotechnology, Dallas, TX, USA), rabbit anti-parvalbumin (1:500; Abcam, Cambridge, UK), and goat anti-NOX2/gp91phox (1:200; Santa Cruz Biotechnology, Dallas, TX, USA) in 1 % BSA at 4 °C overnight. Slices were washed with PBS, and then incubated in a secondary antibody mixture of DyLight 568-conjugated goat anti-mouse (1:1000; Jackson, USA) or mixture of FITC 488-conjugated goat anti-rabbit and DyLight 568-conjugated mouse anti- goat (1:1000; Jackson, USA).” After washing out the secondary antibodies, sections were incubated with 4′, 6-diamidino-2-phenylindole (DAPI) for nuclear staining. Fluorescent images were captured by Olympus FluoView FV10i confocal fluorescence microscope (Japan). The mean value of four random regions across hippocampus or prefrontal cortex in each section was calculated by Image J (National Institutes of Health, Bethesda, MD, USA) for immunofluorescence analysis.
Electron Transport Chain (ETC) Enzymatic Activities and ATP Level Tests
ETC enzymatic activities and ATP level measurements were performed as we described previously (Wu et al. 2015). Briefly, the hippocampal tissues were homogenized in ice-chilled Dounce homogenizers (1:10, w/v) using isolation buffer (Beyotime Institute of Biotechnology, Shanghai, China) and centrifuged at 1000 g for 5 min at 4 °C. Supernatants were transferred into new tubes and centrifuged at 8000 g for 10 min at 4 °C. Then supernatants were removed and centrifuged at 12,000 g to obtain pure cytosol fractions and mitochondria-enriched pellets. The mitochondria-enriched pellets were gently resuspended and washed again with isolation buffer, and then pelleted by centrifugation at 1000 and 8000 g for 5 and 10 min, respectively. Mitochondrial protein was determined by Micro BCA protein assay kit (Beyotime Institute of Biotechnology, Shanghai, China) using bovine serum albumin as standard. The purified mitochondrial samples were used for ETC enzymatic activities test. ETC (complex I, III) enzymatic activities were assayed spectrophotometrically as specific donor–acceptor oxidoreductase activity according to ETC assay kits (Genmed Scientifics Inc., Arlington, MA) following the manufacturer’s protocols. Assessment of relative ATP levels based on the reaction of ATP with recombinant firefly luciferase and its substrate luciferin was performed using the ATP bioluminescence assay kit (Beyotime Institute of Biotechnology, Shanghai, China) following the manufacturer’s instructions.
Statistical Analysis
Data were analyzed by the Statistical Product for Social Sciences (Version 16.0; SPSS Inc., Chicago, IL, USA) and presented as mean ± S.E.M. Data of open-field test, fear conditioning test, probe trial of MWM, and Western Blot for PV and GAD67 were analyzed by one-way analysis of variance (ANOVA) followed by Tukey test. Western Blot for gp91 and NOX4 was analyzed using independent-samples t test. Comparisons for the spatial training sessions of MWM were performed by repeated two-way ANOVA followed by LSD test. Significant difference was defined as P < 0.05.
Results
Increased NOX2 Expression in Neonatal Brain After Repeated Ketamine Exposures
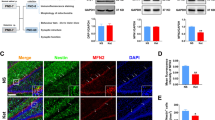
To assess the effects of ketamine on gp91-phox/NOX2 and NOX4 expressions in the hippocampus and prefrontal cortex, Western Blot and qPCR were performed. Compared with the control group, repeated ketamine exposures significantly increased the expression of gp91/NOX2 in the hippocampus (for Western Blot, 1.00 ± 0.06 vs. 1.35 ± 0.04, Fig. 2a; P = 0.0022; for qPCR, 1.00 ± 0.09 vs. 1.70 ± 0.06, Fig. 3a; P < 0.0001) and prefrontal cortex (for Western Blot, 1.00 ± 0.07 vs. 1.91 ± 0.14, Fig. 2b; P = 0.0011; for qPCR, 1.00 ± 0.09 vs. 1.48 ± 0.10, Fig. 3b; P < 0.05) at P8. However, there was no difference in the expression of NOX4 in the hippocampus and prefrontal cortex at P8 between the two groups (Fig. 2c, d; P > 0.05). The results suggested that ketamine-induced alteration of NADPH oxidase is selective to NOX2.
Western blot results of the hippocampus and prefrontal cortex. Representative blots of gp91/NOX2 of the two groups in the hippocampus (a) and prefrontal cortex (b). Representative blots of Nox4 of the two groups in the hippocampus (c) and prefrontal cortex (d). GAPDH was included as loading control. Data are presented as mean ± SEM (n = 4). **P < 0.01 vs. the control + vehicle group
mRNA expression of NOX2 in the hippocampus and prefrontal cortex. Fold increase in the mRNA expression relative to 18S and control subjects for NOX2 after the last ketamine exposure in the hippocampus (a) and prefrontal cortex (b). Data are presented as mean ± SEM; n = 3 for each group; * P < 0.05, **** P < 0.0001 vs. the Con + Vehicle group; # P < 0.05 vs. the Ket + Vehicle group
Increased Oxidative Stress in Neonatal Brain After Repeated Ketamine Exposures was Generated from NOX2 but Not from Mitochondria
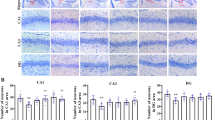
To check whether the increase of NOX2 was expression would lead to oxidative stress after repeated ketamine exposures, immunofluorescence was performed to detect 8-OHdG (a marker of oxidative stress to DNA) (Di Minno et al. 2015). An increase of 8-OHdG staining in the prefrontal cortex (Fig. 4a) and hippocampus (Fig. 5) was observed in the Ket + vehicle group compared with the control + vehicle group, whereas administration of Apo attenuated DNA oxidation in neonatal rats at P8, suggesting a possible role of overproduction of ROS possibly due to increased NOX2 induced by repeated ketamine exposures in neonatal rats at P8. However, there was no significant difference in 8-OHdG staining among the four groups in the prefrontal cortex in adolescent rats at P60 (Fig. 4b).
Immunofluorescence staining to detect 8-OHdG in the prefrontal cortex after repeated ketamine exposures. a Representative images of 8-OHdG (red) in the prefrontal cortex in neonatal rats at P8. b Representative images of 8-OHdG (red) in the prefrontal cortex in adolescent rats at P60. Data are presented as mean ± SEM (n = 4). DAPI staining is shown in blue. Scale bar = 50 µm (Color figure online)
Ketamine-induced overproduction of ROS in the brain in neonatal rats in the hippocampus was attenuated by administration of Apo. Representative images of 8-OHdG (red) in the hippocampus in neonatal rats at P8. Data are presented as mean ± SEM (n = 4). DAPI staining is shown in blue. Scale bar = 50 µm (Color figure online)
ETC is a crucial structure with organized protein complexes located in the mitochondrial inner membrane and thought to be a possible target of anesthetics in rodents and humans (Bains et al. 2006; Hirata et al. 2011; Kayser et al. 2011). To exclude the mitochondria-derived oxidative stress, we measured the activities of complexes I and III, and ATP levels. No significant difference among the four groups was observed in the hippocampus (Fig. 6a–c; P > 0.05) and prefrontal cortex (Fig. 6d–f; P > 0.05), suggesting that ketamine-induced oxidative stress, at least mainly, resulted from the upregulation of NOX2.
Enzymatic activities of complexes I and III, and ATP levels did not change after repeated ketamine exposure. Quantitative analysis of complexes I (a) and III (b) activities in the neonatal hippocampus. c Quantitative analysis of ATP levels in the neonatal hippocampus. Quantitative analysis of complexes I (d) and III (e) activities in the neonatal prefrontal cortex. f Quantitative analysis of ATP levels in the neonatal prefrontal cortex. Data are presented as mean ± SEM (n = 6)
Loss of Phenotype of PV Interneurons After Repeated Ketamine Exposures was Prevented by Apo
Considering PV interneurons particularly sensitive to oxidative stress, we checked the expression of PV and GAD67. The downregulation of PV and GAD67 in the hippocampus (for PV, F (3,20) = 97.73, P < 0.0001; for GAD67, F (3,20) = 7.54, P = 0.0014, Fig. 7a, c) and prefrontal cortex (for PV, F (3,20) = 9.42, P = 0.0004; for GAD67, F (3,20) = 4.517, P = 0.0135, Fig. 7b, d) of neonatal rats at P8 was observed in the Ket + vehicle group compared with the control + vehicle group. However, Apo administration protected PV and GAD67 from downregulation in the Ket + Apo group compared with the Ket + Vehicle group (Fig. 7), confirming the role of ROS possibly mediated by NADPH oxidase in the ketamine-induced abnormality of PV interneurons.
Western blot results of the hippocampus and prefrontal cortex in neonatal rats at P8 as well as in adolescent rats at P60. A representative blot of PV and GAD67 of the four groups in the hippocampus at P8 (a) and P60 (f), GAPDH was included as loading control. b A representative blot of PV and GAD67 of the four groups in the prefrontal cortex at P8 (c) and P60 (g), GAPDH was included as loading control. Quantitative analysis of PV and GAD67 in the hippocampus at P8 (c) and P60 (g) and in the prefrontal cortex at P8 (d) and P60 (h). Data are presented as mean ± SEM (n = 6). *P < 0.05, **P < 0.01, *** P < 0.001 vs. the control + vehicle group; # P < 0.05, ## P < 0.01, ### P < 0.01 vs. the Ket + vehicle group
Unexpectedly, the long-term examination showed that the downregulation of PV and GAD67 in the hippocampus (for PV, F (3,20) = 82.46, P < 0.0001; for GAD67, F (3,20) = 15.73, P = 0.0014, Fig. 7e, g) and prefrontal cortex (For PV, F (3,20) = 10.19, P = 0.0042; for GAD67, F (3,20) = 8.249, P = 0.0079, Fig. 7f, h) of adolescent rats at P60 was still significant in the Ket + vehicle group compared with the control + vehicle group. Moreover, Apo treatment did not only prevent the downregulation but even enhanced the expression of PV and GAD67 in the Ket + Apo group compared with the Ket + vehicle group (Fig. 7). These data strongly, suggest that repeated exposures to ketamine in newborn potentially have long-term adverse impact and Apo does provide the protective effect on PV interneurons.
In addition, double-immunofluorescence staining was performed for the PV and gp91-phox/NOX2 (n = 4). Most PV immunoreactive interneurons were stained for gp91-phox/NOX2 in the ketamine-exposed rats (Fig. 8). These findings suggested a role of ROS possibly mediated by NADPH oxidase in the ketamine-induced loss of PV immunoreactivity.
Repeated Ketamine Exposures Did Not Induce Anxiety-Like Behavior and Locomotor Activity in Adolescent Rats
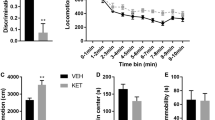
To assess the influence of repeated exposures of neonatal rats to ketamine on the spontaneous explorative activity, rats underwent the open field test. No difference was observed in the spontaneous locomotor activity as reflected by the time spent in the center (F (3,44) = 1.633, P = 0.1954; Fig. 9a) or the total distance (F (3,44) = 0.7520, P = 0.5271; Fig. 9b) during the open field test among the four groups, excluding the possibility that the locomotor activity per se contributed to the changes in the fear conditioning and MWM tests.
Apo alleviated the neonatal ketamine exposure-induced cognitive impairments. There was no significant difference at time spent in the center a and ambulatory distance b in the open field test among the four groups. c The freezing time to context was significantly decreased in the Ket + vehicle group than control + vehicle group, while Apo evidently increased the freezing time. d No significant difference was detected in the cued fear conditioning among the four groups. e A representative trajectory of rats in the probe trial of MWM. f Escape latency recorded every day during the spatial training sessions of MWM. g Time spent in the target quadrant in the probe trial of MWM. Data are presented as mean ± SEM (n = 12); *P < 0.05, **P < 0.01, *** P < 0.001 vs. the control group. ### P < 0.001 vs. the Ket + vehicle group
Repeated Ketamine Exposures Induced Cognitive Impairments in the Contextual Fear Conditioning in Adolescent Rats
To assess the influence of repeated ketamine exposures on the long-term memory, rats underwent contextual/cued fear conditioning tests. In the contextual test, the freezing time was decreased after repeated ketamine exposures in the Ket + vehicle group compared with the control + vehicle group, whereas the freezing time was increased in the Ket + Apo group compared with the Ket + vehicle group (F (3,44) = 60.80, P < 0.0001; Fig. 9c), suggesting that repeated ketamine exposures induced hippocampus-dependent memory impairments and ROS possibly mediated by NADPH oxidase might contribute to this kind of cognitive impairments in rats.
Repeated Ketamine Exposures Induced Cognitive Impairments in the MWM in Adolescent Rats
To evaluate whether repeated neonatal ketamine exposures induced spatial learning and memory deficits, the MWM test was performed. In the MWM training test, a longer escape latency was observed after repeated ketamine exposures from 3 to 5 days in the Ket + vehicle group than in the control + vehicle group (Fig. 9f; P = 0.043 for day 3; P = 0.006 for day 4; P = 0.007 for day 5), whereas the escape latency was shorter from 3 to 5 days (Fig. 9f; P = 0.012 for day 3; P = 0.011 for day 4; P = 0.008 for day 5) in the Ket + Apo group than in the Ket + vehicle group. In the MWM probe trial, the target quadrant time in the Ket + vehicle group was shorter than the control + vehicle group (F (3,44) = 6.708, P < 0.001; Fig. 9g). Unexpectedly, there was no significant difference in the target quadrant time between the Ket + Apo group and Ket + vehicle group, suggesting that repeated ketamine exposures induced long-term memory impairments in rats could not be ameliorated by the Apo treatment.
Discussion
In the present study, we showed that repeated ketamine exposures resulted in an increase in NOX2 and subsequent enhanced oxidative stress, which in turn caused loss of phenotype of PV interneurons in the hippocampus and prefrontal cortex and ultimately led to the neurobehavioral impairments later in life. On the basis of these results, we propose a working model (Fig. 10) that ketamine-induced oxidative stress produces a specific effect on PV interneurons, leading to disinhibition of glutamatergic activity which might result in the imbalance of excitatory and inhibitory circuits and consequent neurobehavioral impairments.
Proposed model of ketamine-induced parvalbumin interneuron loss and long-term cognitive impairments in neonatal rats. Ketamine-induced oxidative stress produce a specific effect on PV interneurons that lead to disinhibition of glutamatergic activity, which further results in the imbalance of excitatory and inhibitory circuits and consequent neurobehavioral impairments
The pathogenesis underlying ketamine-induced neurobehavioral impairments is not completely understood. An extensive body of evidence indicates that oxidative stress is closely associated with the pathophysiology of mental disorders such as schizophrenia (Bahceci et al. 2015) and AD (Vedagiri and Thangarajan 2016). Recent studies also show that treatment with antioxidants can improve working memory deficits in a model of schizophrenia (Rapado-Castro et al. 2015). Consistently, our results revealed that the oxidative damage in the hippocampus and prefrontal cortex was critically involved in the development of neurobehavioral impairments after repeated neonatal ketamine exposures, as reflected by the decreased freezing time to context in the fear conditioning test. Notably, treatment with Apo abolished the increased oxidative stress and attenuated the long-term neurobehavioral impairments. It has been shown that ROS can be generated by various subcellular compartments, including the mitochondria, cellular membrane, lysosomes, peroxisomes, and endoplasmic reticulum (McGuire et al. 2011; Shadel and Horvath 2015). However, we showed that repeated ketamine exposures did not affect the complexes I and III activities, and ATP levels. Given that complex I and III are crucial structures of ETC located in the mitochondrial inner membrane, the contribution of ROS from mitochondria might be excluded. Apart from mitochondria, accumulating evidence has suggested that various subunits of NADPH oxidase in the plasma membrane are highly expressed in the cortical neurons and can also generate ROS (Behrens et al. 2007). Activation of NADPH oxidase leads to the generation of the superoxide ion, which can be converted to the highly reactive hydroxyl radical and peroxynitrite (Behrens et al. 2007; Hernandes et al. 2014). In the central nervous system, NOX2 and NOX4 are the main isoforms of NADPH oxidase (Infanger et al. 2006). Specifically, NOX2 is thought to be involved in cell fate and modulation of neuronal activity (Hernandes et al. 2014). In line with this notion, we found that only NOX2 was involved in ketamine-induced oxidative stress because NOX4 level was not altered. Our results are supported by the finding that IL-6 mediates ketamine-induced upregulation of NOX2 and subsequent cognitive impairments in a ketamine-induced schizophrenia model (Behrens et al. 2008). However, the mechanism by which enhanced oxidative stress elicited long-term neurobehavioral impairments after repeated neonatal ketamine exposures remains elusive.
PV interneurons comprise about 40–50 % of the total GABAergic populations, which regulate the activity of neural networks through perisomatic inhibition onto the pyramidal cells (Hu et al. 2014). It has been suggested that PV expression during the developmental period is important for neuronal maturation and neural plasticity (Sohal et al. 2009). More important, the fast-spiking properties make PV interneurons present high metabolic demand, which renders PV interneurons particularly sensitive to oxidative stress (Behrens et al. 2007). By contrast, abnormities in PV interneurons have been proposed to play a causal role in the development of cognitive impairments associated with major psychiatric disorders (Hu et al. 2014). Here we reported that PV interneuron phenotype loss, as indicated by the decreased PV expression in the prefrontal cortex and hippocampus, co-occurred with the cognitive impairments after repeated ketamine exposures. Notably, most of these abnormalities were precluded in rats treated by Apo, providing evidence that PV interneuron phenotype loss and cognitive impairments may be causally linked. PV interneurons control the excitability of postsynaptic pyramidal neurons, and downregulation of PV therefore may result in diminished cortical inhibitory drive, leading to the abnormity in the balance of excitation and inhibition in the hippocampus and prefrontal cortex, which ultimately leads to consequent cognitive impairments. Surprisingly, our data also indicated that GAD67, a key enzyme for GABA synthesis, was overly reversed by Apo treatment. Indeed, higher GAD67 is previously reported to impair spike probability, synaptic plasticity, and learning and memory in a mouse model of AD (Wu et al. 2014). This might partly explain why Apo treatment was not sufficient to reverse the spatial learning memory in the MWM test at P55. Thus, we speculate that other mechanisms except for oxidative stress are also responsible for the long-term cognitive impairments after repeated neonatal ketamine exposures.
By abolishing oxidative stress, Apo can act as a ROS scavenger to protect PV interneurons from the deleterious effects of ketamine (Behrens et al. 2007). The beneficial effects of Apo might be related to its ability to block the activity of NADPH oxidase, possibly by interfering with the assembly of the cytosolic NOX components with the membrane (Jaquet et al. 2009). It has been demonstrated that Apo can penetrate the intact blood–brain barrier (Wang et al. 2008), conferring neuroprotective effects against neuronal damage, particularly in global and focal cerebral ischemia animal model (Tang et al. 2007; Wang et al. 2006). In addition, Apo has anti-inflammatory properties, which could minimize the inflammation-mediated impairment of PV interneurons. Indeed, there is a positive feedback between oxidative stress and inflammatory processes. Even if Apo cannot be regarded a specific NOX inhibitor, one previous study has already shown that it is efficacious in reducing NOX-dependent superoxide and oxidative stress in rodents (Qiu et al. 2016). To provide more definite evidence on whether the activation of NOX2 can influence the ROS level in the brain, NOX2/gp91phox-knocked out mice are needed in our future study.
In summary, our results suggest that oxidative stress mediated-NOX2, at least partly, provides a causal link between repeated neonatal ketamine exposures and long-term neurobehavioral impairments. Thus, NADPH oxidase inhibitors may protect against cognitive dysfunction resulting from repeated neonatal ketamine exposures.
References
Anis NA, Berry SC, Burton NR, Lodge D (1983) The dissociative anesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurons by N-methyl-aspartate. Br J Pharmacol 79:565–575
Bahceci B, Kokacya MH, Copoglu US, Bahceci I, Sahin K, Bagcioglu E, Dokuyucu R (2015) Elevated nucleosome level and oxidative stress in schizophrenia patients. Bratisl Lek Listy 116:587–590
Bains R, Moe MC, Larsen GA, Berg-Johnsen J, Vinje ML (2006) Volatile anaesthetics depolarize neural mitochondria by inhibiton of the electron transport chain. Acta Anaesthesiol Scand 50:572–579
Bedard K, Krause KH (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87:245–313
Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL (2007) Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science 318:1645–1647
Behrens MM, Ali SS, Dugan LL (2008) Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J Neurosci 28:13957–13966
Celio MR (1986) Parvalbumin in most gamma-aminobutyric-acid containing neurons of the rat cerebral-cortex. Science 231:995–997
Di Minno A, Turnu L, Porro B, Squellerio I, Cavalca V, Tremoli E, Di Minno MN (2015) 8-hydroxy-2-deoxyguanosine levels and cardiovascular disease: a systematic review and meta-analysis of the literature. Antioxid Redox Signal 24:548–555
Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, Olson MD, Sprung J, Weaver AL, Schroeder DR, Warner DO (2011) Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics 128:E1053–E1061
Hardingham GE, Do KQ (2016) Linking early-life NMDAR hypofunction and oxidative stress in schizophrenia pathogenesis. Nat Rev Neurosci 17:125–134
He LJ, Liu N, Cheng TL, Chen XJ, Li YD, Shu YS, Qiu ZL, Zhang XH (2014) Conditional deletion of Mecp2 in parvalbumin-expressing GABAergic cells results in the absence of critical period plasticity. Nat Commun 5:5036
Hernandes MS, D’Avila JC, Trevelin SC, Reis PA, Kinjo ER, Lopes LR, Castro-Faria-Neto HC, Cunha FQ, Britto LR, Bozza FA (2014) The role of Nox2-derived ROS in the development of cognitive impairment after sepsis. J Neuroinflamm 11:36
Hirata N, Shim YH, Pravdic D, Lohr NL, Pratt PF Jr, Weihrauch D, Kersten JR, Warltier DC, Bosnjak ZJ, Bienengraeber M (2011) Isoflurane differentially modulates mitochondrial reactive oxygen species production via forward versus reverse electron transport flow: implications for preconditioning. Anesthesiology 115:531–540
Hu H, Gan J, Jonas P (2014) Interneurons. fast-spiking, parvalbumin(+) GABAergic interneurons: from cellular design to microcircuit function. Science 345:1255263
Huang L, Liu Y, Jin W, Ji X, Dong Z (2012) Ketamine potentiates hippocampal neurodegeneration and persistent learning and memory impairment through the PKCgamma-ERK signaling pathway in the developing brain. Brain Res 1476:164–171
Infanger DW, Sharma RV, Davisson RL (2006) NADPH oxidases of the brain: distribution, regulation, and function. Antioxid Redox Signal 8:1583–1596
Jaquet V, Scapozza L, Clark RA, Krause KH, Lambeth JD (2009) Small-molecule NOX inhibitors: ROS-generating NADPH oxidases as therapeutic targets. Antioxid Redox Signal 11:2535–2552
Ji MH, Qiu LL, Tang H, Ju LS, Sun XR, Zhang H, Jia M, Zuo ZY, Shen JC, Yang JJ (2015a) Sepsis-induced selective parvalbumin interneuron phenotype loss and cognitive impairments may be mediated by NADPH oxidase 2 activation in mice. J Neuroinflamm 12:182
Ji MH, Qiu LL, Yang JJ, Zhang H, Sun XR, Zhu SH, Li WY, Yang JJ (2015b) Pre-administration of curcumin prevents neonatal sevoflurane exposure-induced neurobehavioral abnormalities in mice. Neurotoxicology 46:155–164
Kayser EB, Suthammarak W, Morgan PG, Sedensky MM (2011) Isoflurane selectively inhibits distal mitochondrial complex I in Caenorhabditis elegans. Anesth Analg 112:1321–1329
Li JT, Su YA, Wang HL, Zhao YY, Liao XM, Wang XD, Si TM (2016) Repeated blockade of NMDA receptors during adolescence impairs reversal learning and disrupts GABAergic interneurons in rat medial prefrontal cortex. Front Mol Neurosci 9:17
McGuire KA, Barlan AU, Griffin TM, Wiethoff CM (2011) Adenovirus type 5 rupture of lysosomes leads to cathepsin B-dependent mitochondrial stress and production of reactive oxygen species. J Virol 85:10806–10813
Paule MG, Li M, Allen RR, Liu F, Zou X, Hotchkiss C, Hanig JP, Patterson TA, Slikker W, Wang C (2011) Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol 33:220–230
Qiu LL, Ji MH, Zhang H, Yang JJ, Sun XR, Tang H, Wang J, Liu WX, Yang JJ (2016) NADPH oxidase 2-derived reactive oxygen species in the hippocampus might contribute to microglial activation in postoperative cognitive dysfunction in aged mice. Brain Behav Immun 51:109–118
Rapado-Castro M, Berk M, Venugopal K, Bush AI, Dodd S, Dean OM (2015) Towards stage specific treatments: effects of duration of illness on therapeutic response to adjunctive treatment with N-acetyl cysteine in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 57:69–75
Shadel GS, Horvath TL (2015) Mitochondrial ROS signaling in organismal homeostasis. Cell 163:560–569
Smiley JF, Hackett TA, Bleiwas C, Petkova E, Stankov A, Mann JJ, Rosoklija G, Dwork AJ (2015) Reduced GABA neuron density in auditory cerebral cortex of subjects with major depressive disorder. J Chem Neuroanat. doi:10.1016/j.jchemneu.2015.10.008
Sohal VS, Zhang F, Yizhar O, Deisseroth K (2009) Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459:698–702
Stichel CC, Singer W, Heizmann CW, Norman AW (1987) Immunohistochemical localization of calcium-binding proteins, parvalbumin and calbindin-D 28k, in the adult and developing visual-cortex of cats—a light and electron-microscopic study. J Comp Neurol 262:563–577
Tang LL, Ye K, Yang XF, Zheng JS (2007) Apocynin attenuates cerebral infarction after transient focal ischaemia in rats. J Int Med Res 35:517–522
Vedagiri A, Thangarajan S (2016). Mitigating effect of chrysin loaded solid lipid nanoparticles against Amyloid beta induced oxidative stress in rat hippocampal region: an efficient formulation approach for Alzheimer’s disease. Neuropeptides
Verdaguer E, Brox S, Petrov D, Olloquequi J, Romero R, de Lemos ML, Camins A, Auladell C (2015) Vulnerability of calbindin, calretinin and parvalbumin in a transgenic/knock-in APPswe/PS1dE9 mouse model of Alzheimer disease together with disruption of hippocampal neurogenesis. Exp Gerontol 69:176–188
Walker SM, Yaksh TL (2012) Neuraxial analgesia in neonates and infants: a review of clinical and preclinical strategies for the development of safety and efficacy data. Anesth Analg 115:638–662
Wang Q, Tompkins KD, Simonyi A, Korthuis RJ, Sun AY, Sun GY (2006) Apocynin protects against global cerebral ischemia-reperfusion-induced oxidative stress and injury in the gerbil hippocampus. Brain Res 1090:182–189
Wang Q, Smith RE, Luchtefeld R, Sun AY, Simonyi A, Luo R, Sun GY (2008) Bioavailability of apocynin through its conversion to glycoconjugate but not to diapocynin. Phytomedicine 15:496–503
Wu Z, Guo Z, Gearing M, Chen G (2014) Tonic inhibition in dentate gyrus impairs long-term potentiation and memory in an Alzheimer’s [corrected] disease model. Nat Commun 5:4159
Wu J, Zhang M, Hao S, Jia M, Ji M, Qiu L, Sun X, Yang J, Li K (2015) Mitochondria-targeted peptide reverses mitochondrial dysfunction and cognitive deficits in sepsis-associated encephalopathy. Mol Neurobiol 52:783–791
Yan J, Li YR, Zhang Y, Lu Y, Jiang H (2014) Repeated exposure to anesthetic ketamine can negatively impact neurodevelopment in infants: a prospective preliminary clinical study. J Child Neurol 29:1333–1338
Acknowledgments
This work was supported by the grants from the National Natural Science Foundation of China (Nos. 81300946 and 81471105).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, H., Sun, Xr., Wang, J. et al. Reactive Oxygen Species-mediated Loss of Phenotype of Parvalbumin Interneurons Contributes to Long-term Cognitive Impairments After Repeated Neonatal Ketamine Exposures. Neurotox Res 30, 593–605 (2016). https://doi.org/10.1007/s12640-016-9653-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-016-9653-1