Abstract
Two experiments were performed to investigate the effects of physical exercise upon the hypokinesia induced by two different types of MPTP administration to C57/BL6 mice. In the first, mice were administered either the standard MPTP dose (2 × 20 or 2 × 40 mg/kg, 24-h interval) or vehicle (saline, 5 ml/kg); and over the following 3 weeks were given daily 30-min period of wheel running exercise over five consecutive days/week or placed in a cage in close proximity to the running wheels. Spontaneous motor activity testing in motor activity test chambers indicated that exercise attenuated the hypokinesic effects of both doses of MPTP upon spontaneous activity or subthreshold l-Dopa-induced activity. In the second experiment, mice were either given wheel running activity on four consecutive days (30-min period) or placed in a cage nearby and on the fifth day, following motor activity testing over 60 min, injected with either MPTP (1 × 40 mg/kg) or vehicle. An identical procedure was maintained over the following 4 weeks with the exception that neither MPTP nor vehicle was injected after the fifth week. The animals were left alone (without either exercise or MPTP) and tested after 2- and 4-week intervals. Weekly exercise blocked, almost completely, the progressive development of severe hypokinesia in the MPTP mice and partially restored normal levels of activity after administration of subthreshold l-Dopa, despite the total absence of exercise following the fifth week. In both experiments, MPTP-induced loss of dopamine was attenuated by the respective regime of physical exercise with dopamine integrity more effectively preserved in the first experiment. The present findings are discussed in the context of physical exercise influences upon general plasticity and neuroreparative propensities as well as those specific for the nigrostriatal pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) patients are debilitated, displaying muscular rigidity, impaired movement expressed by akinesia/hypokinesia and tremor at rest. The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model (C57/BL6 mice) of PD requires the repeated (two or more times) administration of the neurotoxin typically at doses ranging from 20 to 40 mg/kg. MPTP induces irreversible Parkinson syndrome in humans and non-human primates (Jackson-Lewis et al. 1995; Langston 1985; Novikova et al. 2006), resulting in the loss of substantia nigra cells in the pars compacta of adult animals. Although humans and non-human primates are most susceptible to the neurotoxin (Decamp and Schneider 2004; Tetrud et al. 1986), the mouse model is applied widely to study neurological and pathophysiological mechanisms underlying the degeneration of nigrostriatal dopamine (DA) neurons. MPTP destroys selectively nigrostriatal neurons, thereby inducing acute, sub-acute and long-term effects resembling certain features of PD, particularly the hypokinesic effect. Systemic administration of MPTP (2 × 40 mg/kg, s.c.) to C57 BL/6 mice caused l-Dopa reversible hypoactivity (Fredriksson et al. 1990; Sundström et al. 1990). A less rigorous dose treatment, e.g. 2 × 20, or 25 or 30 mg/kg of MPTP has been found not to reduce motility in the C57 black mice although DA concentrations may be reduced to 50–80% (Heikkila et al. 1989; Sonsalla and Heikkila 1986). The parameters of MPTP-treatment neurotoxicity are long-lasting (up to and beyond 52 weeks after treatment) with a good correlation between the functional deficits, particularly hypokinesia, the neurochemical concomitant, severe depletions of DA, and a dose- and time-dependent recovery of several parameters of motor behaviour following treatment with the DA precursor, l-Dopa (Archer and Fredriksson 2003; Fredriksson and Archer 1994; Fredriksson et al. 1999, Sundström et al. 1990). Mice treated with moderate doses of the dopaminergic toxin MPTP display deficits in behavioural parameters that are significantly correlated with the loss of striatal DA (Tillerson et al. 2002). Neonatal administration with iron (Fe2+, at doses of 7.5 or 15 mg/kg) potentiated both the functional and neurochemical deficits induced by both a lower (2 × 20 mg/kg) and a higher (2 × 40 mg/kg) dose of MPTP (Fredriksson and Archer 2003; Fredriksson et al. 2001).
Clinical investigations indicate that physical exercise improves motor performance and ambulation of PD patients (Miyai et al. 2000). Morris and Schoo (2004) define exercise to be a planned, structured physical activity which has the purpose of improving one or more aspects of physical fitness and functional capacity. The notion that physical exercise may attenuate the neurodegenerative process in PD has long enjoyed some degree of clinical support (Bilowit 1956; Hurwitz 1989; Palmer et al. 1986). More recently, motor training was shown to facilitate self-repair following unilateral lesions of the striatum in adult rats (Döbrössy and Dunnett 2001, 2003), thereby suggesting that the adult brain is capable of significant neuronal plasticity (cf. Gomez-Pinilla et al. 2002). Unilateral administration of 6-hydroxydopamine (6-OHDA) in adult male rats induces an extreme motor asymmetry due to almost exclusive use of the favoured ipsilateral limb with severe neglect of the contralateral limb. A plaster-of-paris cast, placed on the ipsilateral limb on the 7 days following lesioning, forces the animal to use the contralateral limb during the immediate post-lesioning period, and was found to abolish the motor asymmetry induced by unilateral lesion (Tillerson et al. 2001). It was shown too that both DA and DOPAC were increased markedly in the ‘casted’ 6-OHDA-treated rats in comparison with the ‘non-casted’ 6-OHDA-treated animals (Cohen et al. 2003). Furthermore, glial cell line-derived neurotrophic factor (GDNF), a potent survival factor for DA neurons, markedly enhanced DA levels during the immediate 7-day post-lesion period when the ipsilateral limb was casted (ibid). Yoon et al. (2007) found that treadmill running enhanced the survival of dopaminergic neurons in the substantia nigra of 6-OHDA-induced Parkinsonian rats, and also their fibres projecting into the striatum. In the acute MPTP mouse model, treadmill exercise alleviated both the behavioural and dopaminergic deficits (Tillerson et al. 2003; Fisher et al. 2004). Recently, Kurz et al. (2007) injected male C57/BL mice with 10 doses of MPTP (25 mg/kg) and probenecid (250 mg/kg) over 5 weeks, with control mice receiving probenecid alone. From 15 weeks after the final MPTP injection onwards, MPTP and control mice were videotaped on the sagittal plane, using a digital camera, as they ran on a motorized treadmill at a speed of 10 m/min. They found that MPTP mice showed a significantly more variable stride length and less certain gait pattern than the control mice. However, they made no attempt to compare the effects of motorized treadmill exercise and non-exercise upon subsequent measures of motor function. Petzinger et al. (2007) administered a series of four i.p. injections of MPTP, or saline, at 2-h interval for a total of 80 mg/kg, and treadmill running on an accelerating rotarod was initiated for half the MPTP and saline mice 5 days later. All the exercised mice, MPTP and saline, showed increased latencies to fall off the treadmill (i.e. indicating improved balance) compared with the non-exercised mice. There was no difference in striatal DA levels between MPTP-exercised and MPTP-non-exercised mice. Fast-scan cyclic voltammetry indicated increased stimulus-evoked release and a decrease in decay of dopamine in the dorsal striatum of MPTP plus exercise mice only, whereas the immunohistochemical staining analysis of striatal tyrosine hydroxylase and dopamine transporter proteins showed decreased expression in MPTP plus exercise mice compared with MPTP mice.
Measures of wheel-running behaviour have offered important parameters for motor activity and circadian rhythms, at both the individual and group levels, in different rodent species (cf. Cambras et al. 2000; Challet et al. 1996). It is known too that running-wheel activity has rewarding and potentially addictive properties (Belke 1996). It has been shown that in mice wheel-running activity may be performed at the expense of other behaviours (Harri et al. 1999; de Visser et al. 2005). Rodents have been found to display very high levels of motivation in order to gain access to wheel-running activity (Lett et al. 2000; Sherwin 1998a, b). Although the endurance capacity may vary considerably as a function of genetic characteristics (Lightfoot et al. 2004; Meek et al. 2009; Swallow et al. 1998a, b; Turner et al. 2005), and dietary considerations (Challet et al. 1996), the C57BL/6 strain of mice has been studied quite comprehensively with regard to wheel-running and home-cage activities, as well as the interactions between the two situations (de Visser et al. 2005, 2007). Furthermore, it appears that the implication of DA in this activity is reinforced by observations that mutant mice, expressing impaired DA function, display high levels of wheel-running activity (Vargas-Perez et al. 2004; Werme et al. 2000).
The purpose of the present study was to investigate the effects of daily wheel-running exercise, which is comparable to treadmill exercise, upon two different types of MPTP administration. (i) A standard ‘double-dose’, separated by 24 h, administration that comprised either a low-dose (2 × 20 mg/kg) or a high-dose (2 × 40 mg/kg) regime of MPTP administration. Following a 3 week, 5 days/week schedule of wheel-running over 30-min interval, spontaneous motor activity and l-Dopa-induced activity were assessed using the same procedures to those applied previously (Archer and Fredriksson 2003, 2006, 2007; Fredriksson and Archer 2003, 2007; Fredriksson et al. 1994), after which neurochemical analysis of striatal DA levels was performed. (ii) A ‘progressive, repeated weekly single dose’, 40 mg/kg dose following a 60-min motor activity test and interspersed by four consecutive days of running wheel exercise (30 min/day). Progressive (or chronic) MPTP models appear to exert substantial advantages over the standard ‘double-dose’ long-term model (cf. Petroske et al. 2001) from the point-of-view of examining the role of exercise from the aspect of the progressive nature of the disorder. Spontaneous motor activity tests and l-Dopa test were maintained at 2-week interval after the final (fourth) MPTP injection.
Materials and methods
Animals
Male C57 BL/6 mice were purchased from B&K, Sollentuna, Sweden, and were maintained, five-to-a-cage, in plastic cages in a room at temperature of 22 ± 1°C and a 12/12 h constant light/dark cycle (lights on between 06.00 and 18.00 h) in both experiments. They were placed and maintained in groups of 4–6 animals in a room maintained for male mice only following arrival at the laboratory for about 2 weeks in order to acclimatize. Free access to food and water was maintained throughout, except for the day previous to the initiation to wheel-running exercise which occurred at the end of the second week following arrival. They were housed in groups of 4–6 animals and wheel-running exercised and activity chamber tested only during the hours of light (08.00–15.00 h). All exercising and testing was performed in a normally lighted room. Half of the mice in each treatment condition (MPTP-high, MPTP-low and vehicle) were given wheel-running exercise, whereas the other half were placed in a clean laboratory cage for the same period in a room in which the running wheels were placed. Motor activity was tested in a specially arranged test room. This test room, in which all 12 ADEA activity test chambers, each identical to the home cage, were placed, was well-secluded and used only for this purpose. Each test chamber (i.e. motor activity test cage) was placed in a sound-proofed wooden box with 12-cm thick walls and front panels and a small double-glass window to allow observation; each box had dimmed lighting.
Experiment I
Three weeks following arrival, four groups (n = 10) of mice were administered MPTP (either 2 × 20 mg/kg [two groups] or 2 × 40 mg/kg [two groups], s.c., 24 h between injections), and two groups were administered saline (vehicle, 2 ml/kg). Two MPTP groups and one vehicle group were exercised in 30-min session over 5 days each week (Monday–Friday), over the following 3 weeks.
Experiment II
Three weeks following arrival, two groups (n = 10) of mice were administered MPTP (40 mg/kg, s.c.) and two groups administered saline (vehicle, 2 ml/kg) on the Friday of the fourth week following arrival. Similar administrations of MPTP or vehicle were maintained on each Friday on the fifth, sixth and seventh weeks following arrival. In each case, behavioural testing in the activity test chambers was carried out prior to MPTP/vehicle administration (Tests 1–5). Concurrently, during the fourth to seventh weeks and the eighth week one vehicle and one MPTP group were given 30-min exercise sessions over 4 days each week (Monday–Thursday). Following this, exercise sessions were terminated, but all the mice were tested during the 10th and 12th weeks (Tests 6 and 7: Friday).
Experiments were carried out in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) after approval from the local ethical committee (Uppsala University and Agricultural Research Council), and by the Swedish Committee for Ethical Experiments on Laboratory Animals (license S93/92 and S77/94, Stockholm, Sweden).
Drugs
MPTP (Research Biomedical Inc., MA, USA, 2 × 20 or 2 × 40 mg/kg, s.c., with a 24-h interval between injections in each case) was dissolved in saline and administered s.c. in a volume of 2 ml/kg body weight. Saline was used as vehicle in each case.
Behavioural Measurements and Apparatus
Activity Test Chambers
An automated device, consisting of macrolon rodent test cages (40 × 25 × 15 cm) each placed within two series of infra-red beams (at two different heights, one low and one high, 2 and 8 cm, respectively, above the surface of the sawdust, 1-cm deep), was used to measure spontaneous motor activity (RAT-O-MATIC, ADEA Elektronic AB, Uppsala, Sweden). The distance between the infra-red beams was as follows: the low level beams were 73 mm apart lengthwise and 58 mm apart breadthwise in relation to the test chamber; the high level beams, placed only along each long side of the test chamber, were 28 mm apart. According to the procedures described previously (Archer et al. 1986), the following parameters were measured: locomotion was measured by the low grid of infra-red beams. Counts were registered only when the mouse in the horizontal plane, ambulated around the test-cage. Rearing was registered throughout the time when at least one high level beam was interrupted, i.e. the number of counts registered was proportional to the amount of time spent rearing. Total activity was measured by a sensor (a pick-up similar to a gramophone needle, mounted on a lever with a counterweight) with which the test cage was constantly in contact. The sensor registered all types of vibration received from the test cage, such as those produced both by locomotion and rearing as well as shaking, tremors, scratching and grooming. All three behavioural parameters were measured over three consecutive 20-min period. The motor activity test room, in which all 12 ADEA activity test chambers, each identical to the home cage, were placed, was well-secluded and used only for this purpose. Each test chamber (i.e. activity cage) was placed in a sound-proofed wooden box with 12-cm thick walls and front panels, and day-lighting.
Running-wheels
Small rodent running exercise wheels (HamsterWheel ‘DeLuxe’, diameter = 17.5 cm, internal breadth = 7 cm, Article No. 530.0044), purchased from the Pets Department of a large Shopping store (Imazo Ltd) that were adapted and modified for use by mice and placed altogether in a large sound-proofed room within the animal section of the laboratory. All 25 running-wheels were placed equidistant from adjacent wheels in two long rows such that the sounds of the wheels turning by any one wheel could easily be heard by the occupants of all the other wheels. The C57BL/6 mouse strain adapts easily and voluntarily to wheel-running behaviour; mice, running adjacent to each are affected too by the proximity and noise of cages during ‘running’ that induces ‘stimulatory effects reinforcing the behaviour (Sherwin 1998a, b). For the purposes of the present experiment, it was an advantage if the mice in the exercise groups stimulated each other to perform physical exercise. Figure 1 shows a row of the activity running-wheels applied in all the experiments as well as the ‘holding’ cages in which the non-exercise groups remained.
Small rodent running exercise wheels, adapted and modified for use by mice, and placed altogether in a large sound-proofed room within the animal section of the laboratory (top shelf). Home cage setting in which MPTP and vehicle mice that did not receive exercise were placed during the period (30 min) when MPTP and vehicle mice receiving exercise were placed in the running wheels
Design and Treatment
Experiment I was designed to examine whether or not the hypokinesic effects of the standard low 2 × 20 mg/kg and high 2 × 40 mg/kg doses of MPTP, separated by 24 h, may be attenuated by a consecutive 5 day/week regime of wheel-running exercise over 3 weeks. Table 1 shows the experimental design and treatment of mice administered either MPTP or vehicle, with or without 3 weeks of running wheel exercise in Experiment I.
Experiment II was designed to examine whether or not a single weekly dose of MPTP (40 mg/kg), instead of the standard 2 × 40 mg/kg dosage separated by 24 h, would provide a progressive hypokinesic increment when activity testing occurred 1 week after MPTP administration. The experiment was designed also to test whether or not a consecutive 4-day regime of wheel-running exercise would attenuate the hypokinesic effects of the neurotoxin. Table 2 shows the experimental design and treatment of mice administered either MPTP or vehicle, with or without 5 weeks of running wheel exercise in Experiment II.
Neurochemical Analysis
Mice were killed by cervical dislocation within 2 weeks of completion of behavioural testing. Determination of DA was performed using an high-performance liquid chromatograph with electrochemical detection (HPLC-EC), according to Björk et al. (1991), as modified by Liu et al. (1995). Striatal regions were rapidly dissected out and stored at −80°C until neurochemical analysis. DA concentration was assessed as follows: frozen tissue samples were weighed and homogenized in 1 ml of 0.1 M perchloric acid and alpha-methyl-5-hydroxytryptophan was added as an internal standard. After centrifugation (12,000 rpm, i.e. 18,600×g, 4°C, 10 min) and filtration, 20 μl of the supernatant was injected into the HPLC-EC to assay DA. The HPLC system consisted of a PM-48 pump (Bioanalytical Systems, BAS) with a CMA/240 autoinjector (injection volume: 20 μl), a precolumn (15 × 3.2 mm, RP-18 Newguard, 7 μm), a column (100 × 4.6 mm, SPHERI-5, RP-18, 5 μm) and an amperometric detector (LC-4B, BAS, equipped with an Ag/AgCl reference electrode and a MF-2000 cell) operating at a potential of +0.85 V. The mobile phase, ph 2.69, consisted of K2HPO4 and citric acid buffer (pH 2.5), 10% methanol, sodium octyl sulphate, 40 mg/l and EDTA. The flow rate was 1 ml/min and the temperature of the mobile phase was 35°C.
Procedure
Experiment I
Following acclimatization to the laboratory, half of the mice (50 mice) were placed in the running wheels (one mouse to each) and allowed to run in the wheel for 30 min after which they were removed and placed in their home cages. The other half of the mice (48 mice) were placed singly in a (25 × 25 × 25 cm) ‘holding’ cage for 30 min. Following treatment with MPTP/saline, group-size was curtailed to N = 10. This procedure was maintained for 5 days. Three days after the final introductory session in the running wheels, two groups of wheel-running mice [MPTP 20 mg/kg + Exer and MPTP 40 mg/kg + Exer] and two groups of non-wheel-running mice [MPTP 40 mg/kg and MPTP 40 mg/kg] were administered MPTP (either 20 or 40 mg/kg, s.c. with a 24-h interval between injections). One group of wheel-running mice [Veh + Exer] and one group of non-wheel-running mice [Veh] were administered saline (0.9% in a volume of 2 ml/kg). Five days later, the three groups of wheel-running mice [MPTP 20 mg/kg + Exer, MPTP 40 mg/kg + Exer and Veh + Exer] were placed in the running wheels (one mouse to each) and allowed to run in the wheel for 30 min after which they were removed and placed in their home cages. Concurrently, the three groups of non-wheel-running mice [MPTP 20 mg/kg, MPTP 40 mg/kg and Veh] were placed in the ‘holding’ cages for 30 min (see Table 2). This procedure was maintained for five consecutive days each week [Monday–Friday] over three consecutive weeks. On the week following the final week of exercise, exercise or non-exercise, behavioural testing was initiated. Here, all the mice were placed singly in the ADEA test chambers and motor activity was measured over a 30-min period. After this, each mouse was injected with a subthreshold dose of l-Dopa (5 mg/kg, s.c.) and placed back in its respective test chamber for over 120 min.
Experiment II
Following acclimatization to the laboratory, half of the mice (24 mice, MPTP + Exer and Veh + Exer groups) were placed in the running wheels (one mouse to each) and allowed to run in the wheel for 30 min, after which they were removed and placed in their home cages. The other half of the mice (24 mice, MPTP and vehicle groups) were placed singly in a small (25 × 25 × 25 cm) ‘holding’ cage for 30 min (n = 12 mice in each case). This procedure was maintained for 4 days [Monday–Thursday] during the first week of the experiment. On the day after this [Friday], all the mice (48 mice in four groups were tested in the activity test chambers over 60 min) (Test 1), placed in their home cages, and about 60–75 min after which half of the mice (MPTP and MPTP-Exer groups) were administered MPTP (40 mg/kg) and the other half (vehicle and Veh-Exer groups) were administered saline; thus, the experimental procedure for the first week was completed, with the mice untouched on Saturday and Sunday. An identical procedure to that of the first week was maintained on the second, third, fourth and fifth weeks with the exception that no MPTP or vehicle administrations were performed on the Friday of that week; thereby providing Tests 2, 3, 4 and 5. After this, the mice were left without exercise or treatment for 2 weeks and then tested, with all the mice placed singly in the ADEA test chambers while motor activity was measured over a 60-min period (Test 6). Following this, each mouse was injected with a subthreshold dose of l-Dopa (5 mg/kg, s.c.) and placed back in its respective test chamber for over 180 min (l-Dopa Test 1). After a further 2 weeks, the mice were left without exercise or treatment and then tested, with all the mice placed singly in the ADEA test chambers while motor activity was measured over a 60-min period (Test 6). Following this, each mouse was injected with a subthreshold dose of l-Dopa (5 mg/kg, s.c.) and placed back in its respective test chamber for over 120 min (l-Dopa Test 2, see Table 2). Following this, the mice were killed and brain regions were dissected for neurochemical analysis.
Statistical Analysis
The locomotion, rearing and total activity data over single 30-min test period (Experiment I) or 60-min test period over Tests 1–7 (Experiment II) in the activity test chambers from the spontaneous motor activity data were submitted to a one-way ANOVA design (Kirk 1995). Brain (striatal) regional levels of dopamine, locomotion, rearing and total activity over the 100-min period following administration of l-Dopa, each were submitted to one-way ANOVA based on a completely randomized design (Kirk 1995). Pairwise testing between the different treatment groups was performed with the Tukey HSD test (Kirk 1995). The 1% level of significance was maintained throughout unless otherwise stated.
Results
Experiment I
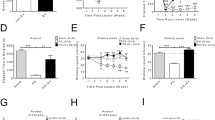
Daily running wheel exercise over 30-min for consecutive 5-day period each week, for 3 weeks significantly attenuated the hypokinesic effects of MPTP on spontaneous motor activity, at both dose regimens of MPTP, although the effect was greater at the 20 mg/kg dose of the neurotoxin. The daily regime of exercise also attenuated the marked DA depletion resulting from MPTP. In the subthreshold l-Dopa-induced (5 mg/kg) motor activity test over 100 min, motor activity was restored partially by daily exercise at the 40 mg/kg dose, and completely restored at the 20 mg/kg dose of MPTP (locomotion and total activity). Thus, for testing of spontaneous motor activity one-way ANOVA indicated significant between-groups effects: locomotion: F(5,54) = 116.27, P < 0.0001; rearing: F(5,54) = 136.15, P < 0.0001 and total activity: F(5,54) = 100.91, P < 0.0001. Figure 2 shows mean and SD values for locomotion, rearing and total activity by MPTP- and vehicle-treated mice given exercise or not.
Locomotion, rearing and total activity counts (mean ± SD) in the spontaneous motor activity test during a 30-min interval by MPTP- and vehicle-treated mice that had been given 30-min physical exercise sessions, or not, 5 days/week, during a 3-week period post-MPTP (either 20 or 40 mg/kg) administration. A versus vehicle; B versus MPTP 20 mg/kg; C versus MPTP 20/40 mg/kg
Pairwise testing using Turkey’s HSD test revealed differences between the MPTP treatment groups and the vehicle groups, as follows:
-
Locomotion:
MPTP40 < MPTP40 + Exer < MPTP20 < MPTP20 + Exer, Vehicle, Vehicle + Exer.
-
Rearing:
MPTP40 < MPTP40 + Exer < MPTP20 < MPTP20 + Exer, Vehicle, Vehicle + Exer.
-
Total activity:
MPTP40 < MPTP40 + Exer < MPTP20 < MPTP20 + Exer, Vehicle, Vehicle + Exer.
The subthreshold dose of l-Dopa (5 mg/kg) did not alter the hypokinesic effects of MPTP. However, the 3-week exercise regime restored partially yet significantly motor activity following acute l-Dopa. Thus, one-way ANOVA indicated significant between-groups effects for: locomotion F(5,54) = 45.56, P < 0.0001; rearing F(5,54) = 19.99, P < 0.0001 and total activity F(5,54) = 28.05, P < 0.0001. Following sub-threshold l-Dopa, there was a complete restoration in the case of the 2 × 20 mg/kg dose of MPTP and a partial restoration in the case of the 2 × 40 mg/kg dose group. Figure 3 shows mean ± SD values for l-Dopa-induced locomotion, rearing and total activity by MPTP- and vehicle-treated mice that were allowed exercise or not.
Locomotion, rearing and total activity counts (mean ± SD) in the subthreshold l-Dopa (5 mg/kg) test during a 120-min interval by MPTP- and vehicle-treated mice that had been given 30-min physical exercise sessions, or not, 5 days/week, during a 3-week period post-MPTP (either 20 or 40 mg/kg) administration. A versus vehicle; B versus MPTP 20/40 mg/kg
Pairwise testing using Tukey’s HSD test revealed differences between the different MPTP treatment groups and the vehicle groups, as follows:
-
Locomotion:
MPTP40 < MPTP40 + Exer, MPTP20 < MPTP20 + Exer, Vehicle, Vehicle + Exer.
-
Rearing:
MPTP40 < MPTP40 + Exer, MPTP20 < MPTP20 + Exer, Vehicle, Vehicle + Exer.
-
Total activity:
MPTP40 < MPTP40 + Exer, MPTP20 < MPTP20 + Exer, Vehicle, Vehicle + Exer.
Neurochemical Analysis
Daily exercise in the running wheels attenuated the loss of DA following administration of MPTP (2 × 40 mg/kg) in Experiment. Table 3 shows the DA concentrations of mice administered either MPTP or vehicle and receiving exercise or not.
One-way ANOVA indicated a significant between-groups effects F(3,16) = 45.23, P < 0.0001. Pairwise testing indicated the following differences:
-
MPTP < MPTP + Exercise < Vehicle, Vehicle + Exercise.
Experiment II
Daily running wheel exercise over 30-min consecutive 4-day period each week [Monday–Thursday] over 5 weeks, interspaced by Test days [Fridays] and MPTP administrations [each Friday after testing, 40 mg/kg, single weekly dose regime], produced a marked blockade of the hypokinesia induced by MPTP administered as a single weekly dose [Fridays]. Thus, for the testing of spontaneous motor activity split-plot ANOVA, with a Groups × Test days design, indicated significant Groups × Test days interactions for locomotion: F(18,252) = 43.54, P < 0.0001; rearing: F(18,252) = 21.76, P < 0.0001 and total activity: F(18,252) = 30.75, P < 0.0001. Figure 4 shows mean ± SD values for locomotion, rearing and total activity.
Locomotion, rearing and total activity counts (mean ± SD) in the successive spontaneous motor activity tests (Tests 1–7) during a 60-min interval by MPTP- and vehicle-treated mice that had been given 30-min physical exercise sessions, or not, 4 days/week from the fourth to eighth week (5 weeks) of the experiment. A versus vehicle; B versus MPTP (no exercise)
Pairwise testing using Turkey’s HSD test revealed differences between the MPTP treatment groups and the vehicle groups, as follows:
-
Locomotion:
MPTP < MPTP + Exercise, Vehicle, Vehicle + Exercise on Tests 1–7.
MPTP + Exercise < Vehicle, Vehicle + Exercise on Tests 5–7.
-
Rearing:
MPTP < MPTP + Exercise, Vehicle, Vehicle + Exercise on Tests 1–6.
MPTP + Exercise < Vehicle, Vehicle + Exercise on Tests 5–7.
-
Total activity:
Same result as for rearing.
The subthreshold dose of l-Dopa (5 mg/kg) did not alter the hypokinesia observed in the MPTP mice that received no exercise. However, the 4 day/week exercise regime maintained significantly more locomotor, rearing and total activity, following acute l-Dopa, in the MPTP + Exer group than in the MPTP alone group, in both Tests 1 [2 weeks without exercise] and 2 [4 weeks without exercise]. There was a significant decrease in locomotor activity in the MPTP + Exer group from Test 1 to Test 2. Thus, one-way ANOVA indicated significant treatment-groups effects for: locomotion F(3,36) = 170.46, P < 0.0001; rearing F(3,36) = 18.06, P < 0.0001 and total activity F(3,36) = 22.43, P < 0.0001. Figure 5 shows mean ± SD values for l-Dopa-induced locomotion, rearing and total activity by MPTP- and vehicle-treated mice that were allowed exercise or not.
Locomotion, rearing and total activity counts (mean ± SD) in the subthreshold l-Dopa (5 mg/kg) test during a 120-min interval by MPTP- and vehicle-treated mice that had been given 30-min physical exercise sessions, or not, from Tests 1 and 2 (10th and 12th weeks). A versus MPTP (no exercise); B versus MPTP + Exercise in Test 1
Pairwise testing using Turkey’s HSD test revealed differences between the different MPTP treatment groups and the vehicle groups, as follows:
-
Locomotion:
MPTP < MPTP + Exercise, Vehicle, Vehicle + Exercise on Tests 1 and 2.
MPTP + Exercise < Vehicle, Vehicle + Exercise on Tests 1 and 2.
For the MPTP + Exercise group, T1 > T2 counts.
-
Rearing:
MPTP < MPTP + Exercise, Vehicle, Vehicle + Exercise on Test 1.
MPTP + Exercise < Vehicle, Vehicle + Exercise on Tests 1 and 2.
-
Total activity:
MPTP < MPTP + Exercise, Vehicle, Vehicle + Exercise on Test 1.
MPTP + Exercise < Vehicle, Vehicle + Exercise on Tests 1 and 2.
For the MPTP + Exercise group, T1 > T2 counts.
Neurochemical Analysis
Daily exercise in the running wheels attenuated the loss of DA following administration of MPTP (2 × 40 mg/kg) in Experiment II. However, the restorative effects of exercise upon DA loss by MPTP was markedly less than that observed in Experiment I. This is probably not surprising since a much greater dose of the neurotoxin was applied in the former case (4 × 40 mg/kg) versus (2 × 40 or 2 × 20 mg/kg). Figure 6 shows the dopamine concentrations in the striatum of mice in the vehicle, MPTP, Veh + Exer and MPTP + Exer groups.
Thus, one-way ANOVA indicated significant between-groups effects for: F(3,36) = 10.46, P < 0.0001. Figure 6 shows mean ± SD values for DA concentrations in MPTP- and vehicle-treated mice that were allowed exercise or not.
Discussion
The present study examined the propensity for physical exercise (daily wheel-running activity) to restore, albeit partially, the functional, severe or less severe hypokinesic deficits induced by MPTP administration at a lower or a higher dose (2 × 20 or 2 × 40 mg/kg) or at progressive increments due to four single weekly doses of 1 × 40 mg/kg. In addition, in both experiments MPTP-induced DA loss was attenuated. The results may be summarized as follows:
-
The hypokinesic effects of MPTP at both the 20 mg/kg and the 40 mg/kg doses upon (i) spontaneous motor activity and (ii) l-Dopa-induced motor activity were restored almost completely (20 mg/kg) or partially (40 mg/kg) by daily exercise. (iii) For both spontaneous and subthreshold l-Dopa-induced activity, the functional deficits were more markedly severe in the 40 mg/kg dose of MPTP than in the 20 mg/kg dose, as observed previously (Archer and Fredriksson 2003, 2006, 2007; Fredriksson and Archer 2003, 2007; Fredriksson et al. 1994, 2001). (iv) Single weekly administration of MPTP (1 × 40 mg/kg) induced an asymptotic level of hypokinesia from the second administration of the neurotoxin onwards; the 4-day weekly exercise regime abolished this hypokinesia completely with a hypokinesic effect in evidence only after the fourth administration of the neurotoxin (see Fig. 3). (v) The hypokinesic effects of MPTP upon l-Dopa-induced motor activity were restored partially by the exercise regime, although this had been terminated 2 weeks previously. (vi) Exercise termination (following Test 5 and the final MPTP injection) appears to be accompanied by increased hypokinesia; this effect is more pronounced in the l-Dopa Tests 1 and 2 whereby the MPTP-Exer groups displayed less locomotion during the second test. (vii) Neurochemical analysis indicated that wheel running exercise over 6 weeks post-administration of the standard 2 × 40 mg/kg dose of MPTP markedly ameliorated the loss of DA (61% of control in the MPTP-Exer group compared to 17% in the MPTP group). (viii) The restorative effect of exercise upon DA loss by MPTP was markedly less in the Experiment II, the single (40 mg/kg) progressive, incremental regime, than that observed in Experiment I.
In the tests of spontaneous motor activity, 3-week exercise increased locomotion, rearing and total activity for the mice treated with the 2 × 20 mg/kg dose of MPTP during both the 0–20 min and 20–40 test periods in the activity test chambers. For mice treated with the 2 × 40 mg/kg dose of MPTP, exercise increased only locomotor behaviour significantly. In the test of l-Dopa-induced activity, the functional deficit was much more severe in mice treated with the 2 × 40 mg/kg dose of MPTP; the restorative effect of exercise was partial, though significant. The activity deficits accruing to the 2 × 20 mg/kg dose of MPTP did not include rearing behaviour (see Fig. 2, middle panel); nevertheless, locomotor and total activity following the subthreshold dose of l-Dopa were restored completely by the 3-week period of exercise. This observation agrees plausibly with the findings of Muhlack et al. (2007) regarding the effects of exercise upon l-Dopa efficacy in PD patients: they found that, although l-Dopa plasma absorption did not differ between exercise and non-exercise conditions, the motor response was significantly improved 120 and 150 min after l-Dopa intake on the day with exercise than on the day with rest. They concluded that moderate exercise increased the clinical efficacy of l-Dopa in PD patients.
Recently, Ahmad et al. (2009) studied the effects of endurance exercise (motorized rodent treadmill up to 15 m/min, 40 min/day, 5 days/week for 10 and 18 weeks) on ventral tegmental area (VTA) neurons in the chronically MPTP-treated (10 × 12.5 mg/kg combined with probenecid, 250 mg/kg, over 5 weeks with a 3.5-day interval between doses) and probenecid-treated mice (cf. Kurz et al. 2007). This particular MPTP treatment regime results in a 52% loss of VTA neurons, with a decrease in cell volume and irregular or disparaging axonal and dendritic projections (i.e. deficits in arborization morphology), when the mice remained sedentary throughout the study (see also German et al. 1996). However, those MPTP mice given the exercise regime (18 weeks) evidenced a significantly higher total number of VTA cells compared to the sedentary mice. Furthermore, these VTA neurons were densely populated and displayed distinctive axons and dendritic arborizations thereby confirming the neuroprotective propensity of prolonged physical exercise for VTA dopaminergic neurons following MPTP insult. In the present study, the restorative effect of exercise upon DA loss by MPTP was markedly less in the Experiment II, the single (40 mg/kg) progressive, incremental regime, than that observed in Experiment I, where the standard (2 × 40 mg/kg, separated by 24 h), ‘one-off’ regime was maintained. With regard to physical exercise, the major procedural difference (apart from MPTP dose regime) between these studies was that exercise was maintained throughout (until kill and dissection) in the latter case but terminated over 6 weeks prior to kill in the former. Thus, in Experiment I, after the administration of a total of 80 mg/kg MPTP the striatal DA content in the ‘No Exercise’ group was 17% of vehicle control values whereas in the ‘Exercise’ group it was 61%; in contrast, in Experiment II, after the administration of a total of 160 mg/kg MPTP the striatal DA content in the ‘No Exercise’ group was 11% of vehicle control values whereas in the ‘Exercise’ group it was 24%. It is possible that the MPTP dose difference between Experiments I and II may account for the discrepancy, but nevertheless it is possible that the 6-week interval (8th week to 14th week, see Table 2), without physical exercise, produced a detrimental effect upon surviving DA neurons. Ongoing experiments are designed to test this notion.
Pothakos et al. (2009) described the restorative effects of endurance exercise (a six-lane motorized rodent treadmill, 0° inclination, upon which mice were exercised at incremental rates from 6 m/min to 15 m/min, 40 min/day, 5 days/week over 18 weeks from 1 week prior to MPTP, during, and 12 weeks post-administration) in the chronically MPTP-treated (10 × 25 mg/kg combined with probenecid, 250 mg/kg, over 5 weeks) and probenecid-treated mice. The authors did not present any neurochemical data, but however, this type of chronic MPTP treatment induces severe levels of neurodegeneration with neurochemical, histological effects. Functional and pathophysiological characteristics were similar to advanced stages of PD (Lau 2005). Nevertheless, their results indicated that the exercise regime restores convincingly several parameters of the behavioural deficits after chronic MPTP including step length, gait pattern, number of foot slips over a beam, foot slips over steps and cumulative spontaneous ambulatory behaviour in an open-field whereas amphetamine-induced ambulation was partially restored. In comparison with the present Experiment II wherein 160 mg/kg MPTP was administered over 4 weeks, Pothakos et al. (2009) administered 250 mg/kg over 5 weeks. It ought to be noted too that the exercise regime by these authors (ibid) was more intensive (under motorized control compared with free wheel running) with longer exercise periods (40 min compared to 30 min) and was maintained over a much longer duration (18 weeks compared to 5 weeks), yet the restorative properties of exercise, as shown in Experiment II, are favourably comparable with that of the Pothakos et al. (2009) study. It has been shown also that endurance exercise of the type described above promoted cardiovascular rehabilitation in the same chronic mouse MPTP model with severe neurodegeneration (Al-Jarrah et al. 2007), an observation to be borne in mind when considering the above restorative effects.
The wheel-running physical exercise intervention, during the immediate week prior to MPTP administration and the 3 weeks following treatment, was planned and structured according to prevailing notions (Morris and Schoo 2004). Several animal models have demonstrated the protective and/or restorative benefits of physical exercise against the functional expressions of symptoms of PD (cf. Smith and Zigmond 2003). Fox et al. (2006) have presented five key principles of exercise that enhance neuroplasticity in association with PD, namely: (i) intense activity maximizes synaptic plasticity, (ii) complex activities promote greater structural adaptation, (iii) ‘rewarding’ activities increase DA levels thereby promoting learning/relearning (e.g. motor), (iv) dopaminergic neurons are highly responsive to exercise, on the one hand, and inactivity, on the other (‘use it or lose it’), and, (v) early introduction of exercise retards disease progression. Behaviours dependent upon adequate levels of striatal DA may provide selective targets for therapeutic motor interventions (Ciucci et al. 2008). Foley and Fleshner (2008) showed that habitually physically active animals, compared to sedentary controls, may be better able to increase D2 receptor-mediated inhibition of the indirect pathway of the basal ganglia. Thus, several reviews and/or meta-analyses report physical exercise as being beneficial for physical functioning, strength, balance and gait speed, as well as quality-of-life in individuals with PD (Goodwin et al. 2008; Pohl et al. 2003; Toole et al. 2005). Laboratory studies using animal models confirm these notions: O’Dell et al. (2007) found that unilaterally lesioned 6-OHDA-infused rats that received exercise showed improved motor behaviour outcomes relative to their sedentary lesioned controls, particularly during postoperative days from 17 to 24. It will be noted that in the present study (Experiment I) motor activity tests occurred between posttreatment days 20 and 21, and that the exercised mice performed wheel-running during 15 of those days. However, there were no differences between exercised or sedentary 6-OHDA-lesioned rats with regard to loss of striatal DA transporters and tyrosine-hydroxylase-positive nigral cells (O’Dell et al. 2007). Interestingly, Balthazar et al. (2009) demonstrated recently that increased DA availability in the brain has a performance-enhancing effect, which is mediated by improvements in the tolerance to heat storage and increases in the metabolic rate induced by graded exercise. This observation reinforces further the notion that central activation of dopaminergic pathways plays an important role in exercise performance.
There are several reasons for assuming that the stimulation of an active lifestyle may be of essential benefit to the functional and neurophysiological prospects for PD patients, not only with regard to postponement of depressive symptoms (Dunn et al. 2005) and dementia (Laurin et al. 2001), but also deteriorations in cognitive ability and performance (van Gelder et al. 2004), including associative memory and conditioned fear (Vucković et al. 2008). For example, applying the physical exertions inherent to ‘Nordic walking’, van Eijkeren et al. (2008) showed that this relatively simple form of ambulation, requiring quadrupedal mobilization of motor centres, gave clear improvements both in quality-of-life and motility in PD patients. Another major avenue for functional improvement in the PD patient pertains to motor performance since neurophysiological (Wichmann and DeLong 1996), functional magnetic resonance imaging (Sabatini et al. 2000) and positron emission tomography (Jahanshahi et al. 1995) findings point towards an overall decrease in cortical neural activation contributing to the motor symptoms in these patients. Recently, Ridgel et al. (2009) showed that while both voluntary and forced exercise improved aerobic fitness in PD patients, forced exercise led to improvements in motor functioning and bimanual dexterity. Their biomechanical data indicated that forced exercise induced a shift in motor control strategy, from ‘feedback’ to a greater reliance upon ‘feedforward’ processes, implying that forced exercise may alter central motor control processes.
In summary, the present study provides evidence for an attenuation of hypokinesia and/or a restorative effect of motor activity following either a standard low dose regime (2 × 20 mg/kg) or a standard high dose regime (2 × 40 mg/kg) or a progressive, incremental single weekly dose regime (4 × 40 mg/kg) by different schedules of running wheel physical exercise in mice. The present findings that display significant improvements following regular exercise schedules (in both Experiment I and Experiment II) combined with low/subthreshold doses (e.g. 5 mg/kg) of l-Dopa, the DA precursor that mobilizes surviving DA neurons, suggest that physical exercise co-administered with antiparkinsonian compounds ought to contribute to an enrichment of several aspects of functioning and the quality-of-life of PD patients. These results reinforce the recent observations by Yousefi et al. (2009) that a therapeutic exercise regime induced improvements in daily living activities and perceived health status in patients with PD. Finally, according to the notion by Cotman and Berchtold (2002), a ‘two-step’ view of the effects of physical exercise in mediating the attenuation of hypokinesia may be considered: (a) exercise serves as a ‘gate’ priming the hippocampal responding to environmental stimuli, thereby ensuring neuron-resistance to insults and reinforcing the robustness of brain tissues and (b) exercise-mediated enhancement of the encoding of information and neural resistance may involve factors such as brain-derived neurotrophic factor (BDNF), a prototypical candidate molecule that may facilitate the benefits upon brain structure and function accruing from regular physical exercise. Certainly, recent evidence suggests that by reducing superoxide radical formation chronic exercise exerts a favourable effect of the hippocampus (Aksu et al. 2009). In this regard, the particular benefits of swimming exercise in attenuating the motor disorders related to oxidative stress have been documented (Teixeira et al. 2008).
References
Ahmad AO, Park J-H, Stenho-Bittel L, Lau YS (2009) Effects of endurance exercise on ventral tegmental area neurons in the chronic 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine and probenecid-treated mice. Neurosci Lett 450:102–105
Aksu I, Topcu A, Camsari UM, Acikgoz O (2009) Effect of acute and chronic exercise on oxidant-antioxidant equilibrium in rat hippocampus, prefrontal cortex and striatum. Neurosci Lett 452:281–285
Al-Jarrah M, Pothakos K, Novikova L, Smirnova IV, Kurz MJ, Stenho-Bittel L, Lau YS (2007) Endurance exercise promotes cardiorespiratory rehabilitation without neurorestoration in the chronic mouse model of Parkinsonism with severe neurodegeneration. Neuroscience 149:28–37
Archer T, Fredriksson A (2003) An antihypokinesic action of α2-adrenoceptors upon MPTP-induced behavior deficits in mice. J Neural Transm 110:183–200
Archer T, Fredriksson A (2006) Influence of noradrenaline denervation upon MPTP-induced deficts in mice. J Neural Transm 113:1119–1129
Archer T, Fredriksson A (2007) Functional consequences of iron overload in catecholaminergic interactions: the Youdim factor. Neurochem Res 32:1625–1639
Archer T, Fredriksson A, Jonsson G, Lewander T, Mohammed AK, Ross SB, Söderberg U (1986) Central noradrenaline depletion antagonizes aspects of d-amphetamineinduced hyperactivity in the rat. Psychopharmacology 88:141–146
Balthazar CH, Leite LH, Rodrigues AG, Coimbra CC (2009) Performance-enhancing and thermoregulatory effects of intracerebroventricular dopamine in running rats. Pharmacol Biochem Behav 93:465–469
Belke W (1996) Investigating the reinforcing properties of running. In: Epling WF, Pierce WD (eds) Activity anorexia: theory, research, and treatment. Erlbaum, Mahwah, pp 45–55
Bilowit DS (1956) Establishing physical objectives in rehabilitation of patients with Parkinson’s disease. Phys Ther Rev 36:176–178
Björk L, Lindgren S, Hacksell U, Lewander T (1991) (S)-UH-301 antagonizes (R)-8-OH-DPAT-induced cardiovascular effects in the rat. Eur J Pharmacol 199:367–370
Cambras T, Vilaplana J, Campuzano A, Canal-Corretger MM, Carulla M, Diez-Noguera A (2000) Entrainment of the rat motor activity rhythm: effects of light-dark cycle and physical exercise. Physiol Behav 70:227–232
Challet E, Malan A, Pévet P (1996) Daily hypocaloric feeding entrains circadian rhythms of wheel-running and body temperature in rats kept at constant darkness. Neurosci Lett 211:1–4
Ciucci MR, Ma ST, Kane JR, Ahrens AM, Schallert T (2008) Limb use and complex ultrasonic vocalization in a rat model of Parkinson’s disease: deficit-targeted training. Parkinsonism Relat Disord 14(Suppl. 2):S172–S175
Cohen AD, Tillerson JL, Smith AD, Schallert T, Zigmond MJ (2003) Neuroprotective effects of prior limb use in 6-hydroxydopamine-treated rats: possible role of GDNF. J Neurochem 85:299–305
Cotman CW, Berchtold NC (2002) Exercise: a behavioural intervention to enhance brain health and plasticity. Trends Neurosci 25:295–301
de Visser L, van der Bos R, Spruijt BM (2005) Automated home cage observations as a tool to measure the effects of wheel running on cage floor locomotion. Behav Brain Res 160:382–388
de Visser L, van der Bos R, Stoker AK, Kas MJH, Spruijt BM (2007) Effects of genetic background and environmental novelty on wheel running as a rewarding behaviour in mice. Behav Brain Res 177:290–297
Decamp E, Schneider JS (2004) Attention and executive function deficits in chronic low-dose MPTP-treated non-human primates. Eur J Neurosci 20:1371–1378
Döbrössy MD, Dunnett SB (2001) The influence of environment and experience on neural grafts. Nat Rev Neurosci 2:871–879
Döbrössy MD, Dunnett SB (2003) Motor training effects on recovery of function after striatal lesions and striatal grafts. Exp Neurol 184:274–284
Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO (2005) Exercise treatment for depression: efficacy and dose response. Am J Prev Med 28:1–8
Fisher BE, Petzinger GM, Nixon K, Hogg E, Bremmer S, Meshul CK, Jakowec MW (2004) Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-lesioned mouse basal ganglia. J Neurosci Res 77:378–390
Foley TE, Fleshner M (2008) Neuroplasticity of dopamine circuits after exercise: implications for central fatigue. Neuromol Med 10:67–80
Fox CM, Ramig LO, Ciucci MR, Sapir S, McFarland DH, Farley BG (2006) The science and practice of LSVT/LOUD: neural plasticity approach to treating individuals with Parkinson’s disease and other neurological disorders. Semin Speech Lang 27:283–299
Fredriksson A, Archer T (1994) MPTP-induced behavioural and biochemical deficits: a parametric analysis. J Neural Transm Dis Dement Sect 7:123–132
Fredriksson A, Archer T (2003) Effect of postnatal iron administration on MPTP-induced behavioural deficits and neurotoxicity: behavioural enhancement by L-Dopa-MK-801 co-administration. Behav Brain Res 139:31–46
Fredriksson A, Archer T (2007) Postnatal iron overload destroys NA-DA functional interactions. J Neural Transm 114:195–203
Fredriksson A, Gentsch C, Archer T (1994) Synergistic interactions between NMDA-antagonists and L-Dopa on activity in MPTP-treated mice. J Neural Transm [Gen Sect] 97:197–209
Fredriksson A, Plaznik A, Sundström E, Jonsson G, Archer T (1990) MPTP-induced hypoactivity in mice: reversal by L-Dopa. Pharmacol Toxicol 67:295–301
Fredriksson A, Palomo T, Chase TN, Archer T (1999) Tolerance to a suprathreshold dose of L-Dopa in MPTP mice: effects of glutamate antagonists. J Neural Transm 106:283–300
Fredriksson A, Schröder N, Eriksson P, Izquierdo I, Archer T (2001) Neonatal iron potentiates adult MPTP-induced neurodegenerative and functional deficits. Parkinsonism Relat Dis 7:97–105
German DC, Nelson EL, Liang CL, Speciale SG, Sinton CM, Sonsalla PK (1996) The neurotoxin MPTP causes degeneration of specific nucleus A8, A9 and A10 dopaminergic neurons in the mouse. Neurodegeneration 5:299–312
Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR (2002) Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol 88:2187–2195
Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL (2008) The effectiveness of exercise interventions for people with Parkinson’s disease: a systematic review and meta-analysis. Mov Dis 23:631–640
Harri M, Lindblom J, Malinen H, Hyttinen M, Lapvetelainen T, Escola S, Helminen HJ (1999) Effect of access to a running wheel on behaviour of C57Bl/6 J mice. Lab Anim Sci 49:401–405
Heikkila RE, Sieber B-A, Manzino L, Sonsalla PK (1989) Some features of the nigrostriatal dopaminergic neurotoxin 1-methyl-4-phenyl-1, 2, 3, 6-tetrohydropyridine (MPTP) in the mouse. Mol Chem Neuropathol 10:171–183
Hurwitz A (1989) The benefit of a home exercise regime for ambulatory Parkinson’s disease patients. J Neurosci Nurs 21:180–184
Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S (1995) Time course and morphology of dopaminergic neuronal death cauesed by the neurotoxin 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine. Neurodegeneration 4:257–269
Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ (1995) Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain 118:913–933
Kirk R (1995) Experimental design: procedures for the behavioral sciences. Brooks/Cole, Belmont
Kurz MJ, Pothakos K, Jamaluddon S, Scott-Pandorf M, Arellano C, Lau Y-S (2007) A chronic mouse model of Parkinson’s disease has a reduced gait pattern certainty. Neurosci Lett 429:39–42
Langston JW (1985) MPTP neurotoxicity: an overview and characterization of phases of toxicity. Life Sci 36:201–206
Lau YS (2005) Progressive neurodegeneration in the chronic MPTP/probenecid model of Parkinson’s disease. In: Ebadi M, Pfeiffer R (eds) Parkinson’s disease. CRC Press, Boca Raton, pp 109–115
Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K (2001) Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol 58:498–504
Lett BT, Grant VL, Byrne MJ, Koh MT (2000) Pairings of a distinctive chamber with the after effect of wheel running produce conditioned place preference. Appetite 34:87–94
Lightfoot JT, Turner MJ, Daves M, Vordermark A, Kleeberger SR (2004) Genetic influence on daily wheel running activity level. Physiol Genomics 19:270–276
Liu Y, Yu H, Mohell N, Nordvall G, Lewander T, Hacksell U (1995) Derivatives of cis-2-amino-8-hydroxy-1-methyltetralin: mixed 5-HT1A-receptor agonists and dopamine D2-receptor antagonists. J Med Chem 38:150–160
Meek TH, Lonquich BP, Hannon RM, Garland T (2009) Endurance capacity of mice selectively bred for high voluntary wheel running. J Exp Biol 212:2908–2917
Miyai I, Fujimoto Y, Ueda Y, Yamamoto H, Nozaki S, Saito T, Kang J (2000) Treadmill training with body weight support: its effect on Parkinson’s disease. Arch Phys Med Rehabil 81:849–852
Morris M, Schoo A (2004) Optimizing exercise and physical activity in older adults. Butterworth Heinemann, Edinburgh
Muhlack S, Welnic J, Woitalla D, Müller T (2007) Exercise improves efficacy of levodopa in patients with Parkinson’s disease. Mov Dis 22:427–430
Novikova L, Garris BL, Garris DR, Lau YS (2006) Early signs of neuronal apoptosis in the substantia nigra pars compacta of the progressive neurodegenerative mouse 1.methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine/probenecid model of Parkinson’s disease. Neuroscience 140:67–76
O’Dell SJ, Gross NB, Fricks AN, Casiano BD, Nguyen TB, Marshall JF (2007) Running wheel exercise enhances recovery from nigrostriatal dopamine injury without inducing neuroprotection. Neuroscience 144:1141–1151
Palmer SS, Mortimer JA, Webster DD, Bistevins R, Dickinson GL (1986) Exercise therapy for Parkinson’s disease. Arch Phys Med Rehab 67:741–745
Petroske E, Meredith GE, Callen S, Totterdell S, Lau YS (2001) Mouse model of Parkinsonism: a comparison between subacute MPTP and chronic MPTP/probenecid treatment. Neuroscience 106:589–601
Petzinger GM, Walsh JP, Akopian G, Hogg E, Abernathy A, Arevalo P, Turnquist P, Vuckovic M, Fisher BE, Togasaki DM, Jakowec MW (2007) Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci 27:52291–52300
Pohl M, Rockstroh G, Ruckreim S, Mrass G, Mehrholz J (2003) Immediate effects of speed-dependent treadmill training on gait parameters in early Parkinson’s disease. Arch Phys Med Rehabil 84:1760–1766
Pothakos K, Kurz MJ, Lau YS (2009) Restorative effect of endurance exercise on behavioral deficits in the chronic mouse model of Parkinson’s disease with severe neurodegeneration. BMC Neurosci 10:1–14
Ridgel AL, Vitek JL, Alberts JL (2009) Forced, not voluntary, exercise improves motor function in Parkinson’s disease patients. Neurorehabil Neural Repair 23:600–608
Sabatini U, Boulanouar K, Fabre N et al (2000) Cortical motor reorganisation in akinetic patients with Parkinson’s disease: a functional MRI study. Brain 123:394–403
Sherwin CM (1998a) The use and perceived importance of three resources which provide caged laboratory mice the opportunity for extended locomotion. Appl Anim Behav Sci 55:353–367
Sherwin CM (1998b) Voluntary wheel running: a review and novel interpretation. Anim Behav 56:11–27
Smith AD, Zigmond MJ (2003) Can the brain be protected through exercise? Lessons from an animal model of parkinsonism. Exp Neurol 184:31–39
Sonsalla PK, Heikkila RE (1986) The influence of dose and dosing interval on MPTP-induced dopaminergic neurotoxicity in mice. Eur J Pharmacol 129:339–345
Sundström E, Fredriksson A, Archer T (1990) Chronic neurochemical and behavioural changes in MPTP-lesioned C57 BL/6 mice: a model for Parkinson’s disease. Brain Res 528:181–188
Swallow JG, Carter PA, Garland T (1998a) Artificial selection for increased wheel-running behavior in house mice. Behav Genet 28:227–237
Swallow JG, Carter PA, Zhan WZ, Sieck GC (1998b) Effects of voluntary activity and genetic selection on aerobic capacity in house mice (Mus domesticus). J Appl Physiol 84:69–76
Teixeira AM, Trevizol F, Colpo G, Garcia SC, Charão M, Pereira RP, Fachinetto R, Rocha JB, Bürger ME (2008) Influence of chronic exercise on reserpine-induced oxidative stress in rats: behavioral and antioxidant evaluations. Pharmacol Biochem Behav 88:465–472
Tetrud JW, Langston JW, Redmond DE, Roth RH, Sladek JR, Angel RW (1986) MPTP-induced tremor in human and non-human primates. Neurology 36(Suppl. 1):308
Tillerson JL, Cohen AD, Philpower J, Miller GW, Zigmond MJ, Schallert T (2001) Forced limb-use effects on the behavioral and neurochemical effects of 6-hydroxydopamine. J Neurosci 21:4427–4435
Tillerson JL, Caudle WM, Reveron ME, Miller GW (2002) Detection of behavioral impairments correlated to neurochemical deficits in mice treated with moderate doses of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine. Exp Neurol 178:80–90
Tillerson JL, Caudle WM, Reveron ME, Miller GW (2003) Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson’s disease. Neuroscience 119:899–911
Toole T, Maitland CG, Warren E, Hubmann MF, Panton L (2005) The effects of loading and unloading treadmill walking on balance, gait, fall risk, and daily function in Parkinsonism. NeuroRehabilitation 20:307–322
Turner MJ, Kleeberger SR, Lightfoot JT (2005) Influence of genetic background on daily running-wheel activity differs with aging. Physiol Genomics 22:76–85
Van Eijkeren FJM, Reijmers RSJ, Kleinveld MJ, Minten A, ter Bruggen JP, Bloem BR (2008) Nordic walking improves mobility in Parkinson’s disease. Mov Dis 23:2239–2243
Van Gelder BM, Tijhuis MA, Kalmijn S, Giampeoli S, Nissinen A, Kromhout D (2004) Physical activity in relation to cognitive decline in elderly men: the FINE study. Neurology 63:2316–2321
Vargas-Perez H, Borelli E, Diaz J-L (2004) Wheel running use in dopamine D2L receptor knockout mice. Neurosci Lett 366:172–175
Vucković MG, Wood RI, Holschneider DP, Abernathy A, Togasaki DM, Smith A, Petzinger GM, Jakowec MW (2008) Memory, mood, dopamine, and serotonin in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. Neurobiol Dis 32:319–327
Werme M, Thoren P, Olson L, Brene S (2000) Running and cocaine both upregulate dynorphin mRNA in medial caudate putamen. Eur J Neurosci 12:2967–2974
Wichmann T, DeLong MR (1996) Functional and pathophysiological models of the basal ganglia. Curr Opin Neurobiol 6:751–758
Yoon MC, Shin MS, Kim TS, Kim BK, Ko IG, Sung YH, Kim SE, Lee HH, Kim YP, Kim CJ (2007) Treadmill exercise suppresses nigrostriatal dopaminergic neuronal loss in 6-hydroxydopamine-induced Parkinson’s rats. Neurosci Lett 423:12–17
Yousefi B, Tadibi V, Khoei AF and Montazeri A (2009) Exercise therapy, quality of life, and activities of daily living in patients with Parkinson disease: a small scale quasi-randomised trial. Trials 10:67
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Archer, T., Fredriksson, A. Physical Exercise Attenuates MPTP-Induced Deficits in Mice. Neurotox Res 18, 313–327 (2010). https://doi.org/10.1007/s12640-010-9168-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-010-9168-0