Abstract
Incidence of Parkinson’s disease (PD) is lower in women compared to men (1:1.46), which is reflected in animal models. However, precise mechanisms are unclear. Administration of MPTP (1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine) to female mice does not lead to mitochondrial complex I inhibition as seen in males and the progressive dopaminergic cell loss in substantia nigra (SNpc) is significantly attenuated. Redox driven apoptotic signaling pathways regulated by thiol disulfide oxidoreductase(s) have been implicated in the neurodegeneration seen in PD. Oxidation of thioredoxin leads to activation of apoptosis signal regulating kinase 1 (ASK1; MAPKKK) initiating cell death cascade through MAP kinase(s). Higher constitutive expression of enzymes involved in cellular redox maintenance, such as glutathione reductase, thioredoxin, and thioredoxin reductase is observed in female brain. Exposure to MPTP activates ASK1 in male but not in female mice. Higher expression of Trx in females potentially prevents ASK1 activation. Downstream of ASK1, phosphorylation of p38 MAP kinase is seen in male but not female mice. Expression of DJ-1, the redox sensing protein is higher in females and the loss of nuclear DJ-1, followed by translocation of Daxx (death associated protein) from the nucleus to the cytosol, which promotes ASK1 mediated death cascade is not seen in females. The enzymes involved in redox maintenance potentially could play a crucial role in preventing the activation of redox driven death signaling cascade and offer neuroprotection. Theraupeutic strategies that help maintain redox homeostasis may help prevent the progressive neurodegeneration seen in PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is characterized by selective loss of dopaminergic neurons in substantia nigra pars compacta. Incidence and prevalence of PD has been reported to be more in men as compared to women with an overall incidence ratio of 1.46 in males vs. females (Shulman 2007). Although the neuroprotective actions of estrogen are well acknowledged, the underlying mechanism(s) of protection are unclear.
Estrogen exerts its neuroprotective action via classical/genomic pathway or by non-classical/rapid response. In its action via classical pathway, the steroid hormone binds to estrogen receptors, ERα or ERβ, induces a conformational change in the receptors to facilitate nuclear translocation, and consequently induction of gene transcription. Genes regulated by estrogen signaling include the nerve growth factors, such as brain-derived neurotrophic factors (BDNF; Sasahara et al. 2007; Shalev et al. 2009), neurotrophin 3, their receptors Trk A, Trk C, and p75, insulin-like growth factor 1 (IGF1; Peri and Serio 2008), and transforming growth factor-α (TGF-α). Another group of genes potentially induced by estrogen are anti-apoptotic proteins such as Bcl2 and BclxL, and specific caspase inhibitors (Nilsen and Diaz Brinton 2003). A third group of genes targeted by estrogen are structural proteins, such as neurofilament proteins, microtubulin associated proteins, Tau (Goodenough et al. 2005), GAP43, and proteins involved in neurotransmitter metabolism, such as choline acetyltransferase and tyrosine hydroxylase (Behl 2002). Besides the delayed classical genomic mode of action, estrogen receptors may also act rapidly by interacting with intracellular signaling cascades by associating with membrane structures including G-proteins, caveolins, and receptor tyrosine kinases (Luoma et al. 2008). Estrogen itself induces phosphorylation of ERK1/2 (members of MAPK signaling pathway), activates cyclic-AMP-responsive element binding protein (CREB), Akt/PKB and regulates intracellular Ca2+ levels (Hewitt et al. 2005). Besides this, phenolic structure of estrogen makes it a potent antioxidant and a free radical scavenger. It effectively prevents oxidative stress induced nerve cell death caused by β-amyloid peptide, glutamate, superoxide anions, hydrogen peroxide, and lipid peroxidation in cultured neurons and brain homogenates, however the effective concentration at which it functions as an antioxidant is probably not physiologically relevant (Prokai and Simpkins 2007).
MPTP (1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine), a model neurotoxin causes mitochondral dysfunction and degeneration of dopaminergic neurons in substantia nigra pars compacta (SNpc) of mice and is a commonly used animal model for Parkinsonism. The sex difference seen in human population is reflected in the animal model wherein female mice are protected from MPTP toxicity (Morissette et al. 2008). We examined the molecular mechanisms underlying the neuroprotection seen in females by examining the redox activated MAP kinase signaling cascades that have been implicated in the selective degeneration of SNpc dopaminergic neurons in mice (Karunakaran et al. 2007, 2008).
Materials and Methods
Materials
Antibodies to p38, DJ-1 and Daxx were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antibody to β-tubulin was obtained from Sigma-Aldrich (St. Louis, MO). Antibodies to p-p38 MAPK (Thr180/Tyr182) and p-ASK1 (Thr845) were purchased from Cell Signaling Technology, Inc. (Danvers, MA). Antibody to tyrosine hydroxylase was obtained from Chemicon International (Temecula, CA). Antibodies to thioredoxin and thioredoxin reductase were purchased from Lab Frontiers Life Science Institute (Seoul, Korea). All conjugated antibodies were obtained from Vector Labs (Burlingame, CA). All other chemicals and reagents were of analytical grade and were procured from Sigma Aldrich or Qualigens (India).
Animals
All animal experiments were carried out as per the institutional guidelines for the use and care of animals. All efforts were made to minimize animal suffering, to reduce the number of animals used and to utilize alternatives to in vivo techniques if available. Male and female C57BL6J mice (2–3 months, 25–30 g) were obtained from Central Animal Research Facility of National Brain Research Centre (NBRC). Animals had access to pelleted diet and water ad libitum. Animals were administered MPTP (30 mg/kg body weight in saline; s.c.) whereas control animals received vehicle alone. Animals were treated with a single dose of MPTP and sacrificed 12 or 24 h later. In some experiments animals were administered MPTP daily for 8 or 14 days and sacrificed 24 h after the last dose. Mice were decapitated and ventral midbrain and striatum were dissected as described earlier (Karunakaran et al. 2007) and frozen in liquid nitrogen for immunoblotting and for measurement of enzymatic activity. In some experiments animals were perfused transcardially with buffered paraformaldehyde (4% w/v) and the brain was dissected out and processed for immunohistochemistry.
Processing of Tissue
CNS regions were homogenized in 0.25 M sucrose (Potassium phosphate buffer; pH-7.4) and centrifuged at 1000×g for 10 min to obtain post-nuclear supernatant. The post-nuclear supernatant was used for immunoblotting and for measuring the activity of thioredoxin, thioredoxin reductase, and glutathione reductase. The post-nuclear supernatant was centrifuged again at 14,000×g for 30 min to obtain the mitochondrial pellet. The pellet was resuspended in sucrose (0.25 M) and freeze-thawed for assay of complex I. Protein concentration was estimated by a dye-binding method (Bradford 1976). Nuclear extracts were prepared as described (Korner et al. 1989). Briefly, the nuclear pellet obtained after 1000×g was resuspended in nuclear extraction buffer [20 mM HEPES, (pH 7.9), containing MgCl2 (1.5 mM), NaCl (0.84 M), EDTA (0.4 mM), dithiothreitol (0.5 mM), and protease inhibitors] and homogenized using a Dounce homogenizer. This was incubated at 4°C for 30 min and centrifuged at 14,000×g for 15 min. The supernatant containing the nuclear extract was used for immunoblotting.
Immunoblotting
The post-nuclear supernatants prepared from ventral midbrain and striatum (20 μg protein) of vehicle and MPTP treated mice were resolved on 10 or 15% sodium dodecyl sulfate polyacrylamide gel. Proteins were transferred to polyvinylidene difluoride membranes (Towbin et al. 1979), incubated with primary antibody followed by secondary antibody either conjugated with alkaline phosphatase or with horseradish peroxidase. Immunostained bands were detected using nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate as chromogens or using chemiluminescence kit (ECL, Amersham Pharmacia Biotech, France). All blots were normalized with β-tubulin following densitometric analysis.
Assay of NADH: Ubiquinone Oxido-Reductase (complex I) Activity
Complex I was assayed in mitochondrial preparations as rotenone-sensitive NADH-ubiquinone oxidoreductase activity as described earlier (Kenchappa and Ravindranath 2003).
Assay of Glutathione Reductase Activity
The enzyme reduces glutathione disulfide to reduced glutathione with concomitant oxidation of NADPH to NADP+. The formation of NADP+ was measured by following a change in absorbance at 340 nm. Enzyme activity was expressed as nmol of NADPH oxidized/min/mg protein (Horn 1965).
Assay for Measuring Thioredoxin (Trx) Activity
Trx activity was measured by insulin disulfide reduction assay (Holmgren and Bjornstedt 1995). Briefly, samples (postnuclear supernatant, containing 20 μg of protein) were incubated in dithiothrietol (DTT) activation buffer (50 mM HEPES pH 7.6, 1 mM EDTA, 1 mg/ml BSA, 2 mM DTT) to reduce thioredoxin. Reaction mixture (40 μl) consisting of HEPES buffer (250 mM; pH. 7.6), EDTA (10 mM), NADPH (2 mg/ml), and insulin (6.5 mg/ml) was added to the preincubated samples followed by addition of thioredoxin reductase (1.6 A412 units/ml) and the samples were incubated at 37°C for 20 min. Reaction was terminated by addition of 0.3 mg/ml 5,5′ dithio (bis)-2-nitrobenzoic acid in 6 M guanidine hydrochloride and the absorbance at 412 nm was measured. Blank reactions omitting either postnuclear supernatant or thioredoxin reductase were run in parallel.
Assay for Measuring Thioredoxin Reductase (TR) Activity
Activity for TR was measured as described earlier (Holmgren and Bjornstedt 1995). Briefly, the reaction mixture consisted of HEPES buffer (250 mM; pH. 7.6), EDTA (10 mM), NADPH (2 mg/ml), and insulin (6.5 mg/ml). Postnuclear supernatant (20 μg protein) was added to the reaction mixture (40 μl) followed by the addition of thioredoxin (1 μM; E. coli Trx) and the mixture was incubated at 37°C in a water bath for 20 min. Reaction was stopped by addition of 0.3 mg/ml of 5,5′ dithio (bis)-2-nitrobenzoic acid in 6 M guanidine hydrochloride and the absorbance at 412 nm was recorded and compared to the standard curve.
Immunohistochemistry and Stereological Analysis
Coronal sections (30 μm thick) were cut throughout the entire midbrain of paraformaldehyde fixed brain using a cryostat. Immunostaining for tyrosine hydroxylase (TH) and pp38 was performed using respective primary antibodies followed by incubation with secondary antibodies conjugated to FITC or Texas red and counterstained with DAPI. Stereological analysis was carried out by cutting coronal sections (30 μm) throughout the entire midbrain from a random start point and every fifth section was processed for tyrosine hydroxylase immunostaining. Sections passing through rostral, middle, and caudal regions of the SN were examined. The pars compacta region was delineated for stereological counting. This delineation excluded pars reticulata (SNpr), ventral tegmental area, and the retrorubral area. Persons blind to the experiment counted the number of tyrosine hydroxylase positive neurons in the substantia nigra pars compacta (SNpc). Immunostaining for Daxx was performed by exposing the sections to hydrogen peroxide (3% v/v) and incubating sections with antibody against Daxx, followed by treatment with biotinylated anti-rabbit IgG for 1 h and incubation with VECTASTAIN-Elite ABC reagent. The color was developed using diaminobenzidine and hydrogen peroxide.
Statistical Analysis
Statistical analysis of the data was performed using one way analysis of variance followed by Student-Newman-Keuls or Dunnet’s test. Student’s ‘t’ test was also used where appropriate.
Results
MPTP Induced Loss of Dopaminergic Neurons is Attenuated in Female Mice
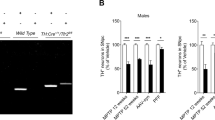
We examined the loss of tyrosine hydroxylase positive neurons in SNpc following administration of MPTP for 8 and 14 days (Fig. 1a). MPTP administration caused 21% loss of dopaminergic neurons in male mice as compared to 12.5% loss in female mice after 8 days while the corresponding loss was 47 and 22.5% after 14 days of treatment (Fig. 1b). When we looked at the cell loss in SNpc after 8 days, we found that the difference between males and females was small (21% vs. 12.5%), albeit significant. However, between 8 and 14 days, while the number of neurons declined sharply in males (47%), the females showed less decline (22.5%; Fig. 1c). The number of TH positive neurons in the saline treated groups in male and female SNpc was not significantly different. They were 1885 ± 96 and 1918 ± 28 neurons respectively, in males and females. We validated the above by counting Nissl positive neurons in SNpc and observed that MPTP treatment for 8 days caused 18% and 12.5% loss in male and female mice, respectively (Fig. 1d). Although the cell loss was only attenuated but not eliminated in female mice, the mitochondrial dysfunction seen as inhibition of complex I activity was completely abolished in female mice after 8 days of MPTP treatment (Fig. 1e). This indicates that in addition to mitochondrial complex I dysfunction, other cell death pathways may operate in MPTP mediated degeneration.
Loss of dopaminergic neurons and inhibition of complex I activity is more pronounced in MPTP treated male mice than female. Animals were treated with a single dose of vehicle or MPTP daily, for 8 or 14 days following which they were sacrificed on 9th or 15th day. a Tyrosine hydroxylase (TH) immunostaining in male and female mice treated with vehicle (saline; control), and MPTP for 8 days and 14 days. Bar represents 50 μm. b Quantitative stereological analysis of tyrosine hydroxylase (TH) positive neurons in male and female mice treated with vehicle or MPTP for 8 or 14 days, showing significant loss of TH positive neurons in males, after 14 days of MPTP administration. Values are mean ± SD (n = 3). c The data was analyzed to determine the rate of degeneration, and is represented as a solid line (____) for male mice, while for females it is depicted as a dotted line (……). d Quantitative stereological analysis of Nissl stained cells present in SNpc, in male and female mice treated with vehicle or MPTP for 8 days. Values are mean ± SD (n = 3). e Complex I activity is inhibited in midbrain and striatum of male mice treated with MPTP for 8 days whereas females and vehicle treated controls were unaffected. Activity is expressed as nmoles of NADH oxidized/min/mg protein. Values are mean ± SD (n = 3). Asterisks indicate values significantly different from vehicle treated controls
Activation of Apoptotic Signal-Regulating Kinase I (ASK1) does not Occur in Female Mice Treated with MPTP
ASK1, a MAPKKK is activated in the ventral midbrain after MPTP treatment in male but not female mice (Fig. 2a). Under normal conditions, ASK1 is bound to reduced thioredoxin (Trx), a protein disulfide oxidoreductase preventing its autophosphorylation at serine 845. Oxidation of the cysteine thiols in Trx results in its dissociation from ASK1 triggering its autophosphorylation, which in turn propagates the downstream death cascade (Saitoh et al. 1998). Thus, the levels of thioredoxin and thioredoxin reductase are critical determinants of ASK1 activation (Fig. 2b).
Activation of apoptotic signal regulating kinase 1 (ASK1) is suppressed in females but not in male mice following MPTP administration. Animals were treated with a single dose of MPTP or vehicle and sacrificed 12 h later. a Representative immunoblot from midbrain of male and female mice treated with vehicle (Control, C) and MPTP (12 h) depicting the protein levels of phosphorylated-ASK1 (Thr 845) and ASK1. β-tubulin levels were measured as loading control. Densitometric analysis of the immunoblots representing the relative intensity of the immunoreactive bands, showing upregulation of pASK only in MPTP treated males. Values are mean ± SD (n = 6). Asterisks indicate values significantly different from vehicle treated controls. b Schematic representation of mechanism of autophosphorylation of ASK1 is depicted. ROS generated during oxidative stress causes oxidative modification of Trx, which in its reduced state remains bound to ASK1 but dissociates once it is oxidized thus releasing ASK1 and triggering its autophosphorylation. Higher levels of reduced Trx prevent autophosphorylation of ASK1
Enhanced Expression of Thioredoxin, Thioredoxin Reductase, and Glutathione Reductase in Midbrain of Female Mice
Higher expression of thioredoxin measured as total protein level and activity was seen in midbrain and striatum of female mice compared to males (Fig. 3a and b). Thioredoxin activity was significantly reduced in ovariectomized females compared to sham operated animals (Fig. 3b). Thioredoxin reductase maintains thioredoxin in its reduced form. Higher constitutive expression of thioredoxin reductase (TR; Fig. 3c) and enzyme activity (Fig. 3d) was observed in the midbrain of female mice, which was significantly reduced in ovariectomized animals (Fig. 3c and d). We also observed that the activity of glutathione reductase (GR), a key enzyme involved in reduction of GSSG to GSH, was higher in the midbrain and striatum of female mice (Fig. 3e), which could be reversed by ovariectomy indicating the critical role of estrogen in the expression of these enzymes.
Higher constitutive expression and enzyme activity of redox modulating enzymes in female mice. Constitutive expression and activity of enzymes involved in thioredoxin system and glutathione reductase were quantitated by immunoblotting and enzymatic assay. a Representative immunoblot from midbrain and striatum of male (M) and female (F) animals depicting the protein levels of thioredoxin. β-tubulin levels were measured as loading control. Densitometric analysis of the immunoblots representing the relative intensity of the immunoreactive bands, showing higher expression of thioredoxin in females. Values are mean ± SD (n = 3 animals). b Enzymatic activity of thioredoxin estimated from midbrain (MB) and striatum (ST) of male and female animals was found to be higher in females, the activity decreased in ovariectomized animals as compared to the sham operated females. Activity is expressed as total units/mg of protein. Values are mean ± SD (n = 3). c Representative immunoblots for thioredoxin reductase (TR) from midbrain of male and female mice as well as ovariectomized (O) and sham operated (S) females. β-tubulin levels were measured as loading control. Densitometric analysis of the immunoblots shows higher expression of TR in females, which decreased after ovariectomy, is shown below. d Measurement of enzymatic activity of TR in midbrain of male and female animals showing higher activity in females. Less activity was observed in ovariectomized females as compared to sham operated mice. Activity is expressed as total units/mg of protein. Values are mean ± SD (n = 3). e Glutathione reductase activity measured in midbrain (MB) and striatum (ST) of male and female mice. Activity was found to be higher in females, which consequently decreased in ovariectomized females as compared to sham operated controls. Activity is expressed as nmoles of NADPH oxidized/min/mg protein. Values are mean ± SD (n = 3). Asterisks indicate values significantly different from vehicle treated controls
Downstream of ASK1 Activation, Phosphorylation of p38 MAP Kinase Occurs in Males but not Female Mice SNpc
Activation of ASK1 leads to phosphorylation of downstream targets, such as p38 MAP kinase. Increased phosphorylation of p38 was observed 12 h following MPTP treatment only in ventral midbrains of male (Fig. 4a) but not female mice. Immunohistochemical analysis co-localizing pp38 and TH clearly demonstrated the increased phosphorylation of p38 in TH positive neurons in the SNpc of male mice (Fig. 4b), which is clearly discernible at low and high magnification. In female mice, p38 activation was not observed in the TH positive neurons in SNpc (Fig. 4c).
Activation of p38 in male but not in female mice after MPTP administration. Animals were treated with a single dose of MPTP or vehicle and sacrificed 12 or 24 h later. a Representative immunoblot from midbrain of male and female mice treated with vehicle (control; C) and MPTP (12 h) depicting the protein levels of phosphorylated p38 and p38. β-tubulin levels were measured as loading control. Densitometric analysis of the immunoblots representing the relative intensity of the immunoreactive bands is shown below, higher levels of phosphorylated-p38 found in MPTP treated males only. Values are mean ± SD (n = 7). Asterisks indicate values significantly different from vehicle treated controls. b Immunohistochemical co-localization revealed the presence of phosphorylated p38 (pp38; FITC) in the soma of tyrosine hydroxylase positive (TH; Texas red) neurons of SNpc, in the ventral midbrain of male mice, after 24 h of exposure to MPTP, whereas control animals show low levels of pp38. Bar represents 200 μm (B; upper panel). Lower panel shows corresponding magnified images of the neurons in upper panel. Bar represents 20 μm (B; lower panel). c Co-immunostaining for pp38 and tyrosine hydroxylase revealed negligible presence of pp38 in the soma of tyrosine hydroxylase positive neurons of SNpc and ventral midbrain of female mice 24 h after exposure to MPTP or vehicle. Bar represents 200 μm (C; upper panel). Lower panel shows corresponding magnified images of the neurons in upper panel. Bar represents 20 μm (C; lower panel)
Loss of Nuclear DJ-1 and Translocation of Daxx to the Cytosol was not seen in Females
DJ-1, a redox sensing protein sequesters Daxx (a transcriptional repressor and death associated protein) within the nucleus and prevents its association with ASK1 and subsequent propagation of death cascade (Junn et al. 2005). MPTP exposure led to significant decrease in nuclear DJ-1 levels in the ventral midbrain of male mice, whereas females were unaffected (Fig. 5a). Further, the constitutive expression of DJ-1 was higher in the ventral midbrain of female mice indicating its possible regulation by estrogen (Fig. 5b). In female mice, translocation of Daxx from the nucleus to the cytosol also did not occur either in SNpc or VTA neurons, unlike in males where Daxx clearly translocated in SNpc neurons but not in VTA (Fig. 5c and d). Thus, decrease in nuclear DJ-1 levels triggers cytosolic translocation of Daxx which could potentially associate with ASK1 in the cytoplasm and propagate cell death cascade (Fig. 5e).
Reduced nuclear DJ-1 levels and cytosolic translocation of Daxx in male but not female mice treated with MPTP. Animals were treated with a single dose of MPTP or vehicle and sacrificed 12 or 24 h later. a Representative immunoblot for nuclear DJ-1 from ventral midbrain of male and female mice treated with saline (S) and MPTP (12 h) is depicted. Densitometric analysis of the immunoblots representing the relative intensity of the immunoreactive bands shows significant decrease in DJ-1 levels in MPTP treated males. b Representative immunoblot for extranuclear DJ-1 from ventral midbrain of male and female mice. Densitometric analysis of the immunoblots shows higher constitutive expression of DJ-1 in females. Values are mean ± SD (n = 7). Asterisks indicate values significantly different from vehicle treated controls. c Immunohistochemical localization of Daxx in SNpc, revealed its translocation to the cytosol in male mice after 24 h of MPTP administration whereas it was retained within the nucleus in female mice and controls. Bar represents 50 μm (C; upper panel). Lower panel shows corresponding magnified images of the neurons in upper panel. Bar represents 10 μm (C; lower panel). d Immunohistochemical localization of Daxx in VTA reveals its retention within the nucleus after MPTP administration in both male and female mice. Bar represents 50 μm (D; upper panel). Lower panel shows corresponding magnified images of the neurons in upper panel. Bar represents 10 μm (D; lower panel). e Schematic representation showing nuclear translocation of Daxx triggered by decreased nuclear DJ-1 levels following which Daxx associates with ASK1 in the cytosol and propagates the death cascade
Discussion
In the current study, we demonstrate that the degeneration of dopaminergic neurons in SNpc following MPTP is attenuated significantly in female mice as compared to males. Earlier studies on sex difference in MPTP toxicity have shown that striatal dopamine levels as well as the SNpc dopaminergic neurons and their terminals are protected in female mice, which are abolished by ovariectomy (Freyaldenhoven et al. 1996; Morissette et al. 2008; Liu et al. 2008). Thus, the sex difference in response to MPTP toxicity is not restricted to striatum alone but also extends to the preservation of the dopaminergic neurons in SNpc as shown in the present study. It is unlikely that the observed sex difference is due to altered metabolism of MPTP or uptake of MPP+ since C57 black female mice are known to metabolize MPTP in a manner similar to males and further, since DAT (dopamine transporter) and VMAT2 (vesicular monoamine transporter) expressions are higher, the uptake of MPP+ may in fact be greater in females (Dluzen and McDermott 2008).
Another interesting observation is the fact that mitochondrial dysfunction seen as complex I inhibition is completely absent in females but some degree of cell loss is seen after 8 days of MPTP (Fig. 1b). This indicates that in addition to the mitochondrial dysfunction, a parallel pathway of cell loss is operative in MPTP treated animals. This is similar to the observation made in male mice pretreated with α-lipoic acid (ALA) prior to MPTP (Karunakaran et al. 2007), wherein, the mitochondrial dysfunction was abolished by ALA but the cell loss was only attenuated. Earlier studies from our laboratory have shown that redox modification of critical thiols can lead to aberrant signaling and initiation of the death signaling cascade by the MAPKKK, ASK1 (Karunakaran et al. 2007). We, therefore examined if such a cascade was operative in females after MPTP treatment.
In order to examine the early effects after MPTP treatment, we chose to study the MAPK signaling cascade at early time points after a single dose of MPTP. We found that ASK1 was not activated in ventral midbrain of female mice unlike males (Fig. 2b). Since the levels of reduced thioredoxin are critical for ASK1 activation, we examined the constitutive expression of Trx in female midbrain and found that Trx, TR, and GR were constitutively expressed in high amounts in females and the difference was abolished following ovariectomy (Fig. 3). Trx is oxidized during oxidative stress and TR helps in reducing oxidized Trx thus maintaining the homeostasis. Thus, higher levels of Trx in female mice could potentially prevent the autophosphorylation of ASK1 and the downstream activation of MAP kinase, such as p38. Further, we also observed the lack of p38 activation in the TH positive neurons in SNpc of female mice quite unlike that seen in males (Fig. 4b and c). Our earlier studies have documented that p38 is activated selectively in the dopaminergic neurons of SNpc following MPTP and this leads to phosphorylation and transactivation of p53 and enhanced expression of pro-apoptotic genes, such as Bax and Puma (Karunakaran et al. 2008). This cascade is not propagated in female mice SNpc potentially due to the high levels of Trx.
ASK1 can also propagate the death cascade by interacting with Daxx, a transcriptional repressor that is normally present in the nucleus (Song and Lee 2003). Daxx, a death associated protein associates with DJ-1, a redox sensing protein within the PML (promyelocytic leukemia) bodies in the nucleus preventing the translocation of Daxx to the cytosol (Junn et al. 2005). Following MPTP there is loss of DJ-1 in the nucleus leading to translocation of Daxx and its association with ASK1 in the cytosol (Karunakaran et al. 2007). Interestingly, in female mice nuclear DJ-1 levels are unaffected after MPTP administration and Daxx translocation to the cytosol does not take place (Fig. 5c). Further, we also observed that the constitutive levels of DJ-1 are higher in female mice compared to males (Fig. 5b). This is a significant observation since mutations in DJ-1 have been implicated in familial PD cases (Djarmati et al. 2004).
While the incidence of PD is known to be lower in women and significant neuroprotection is seen in female rodents in animal models, the underlying mechanisms are unclear. It is unlikely that estrogen has a direct anti-oxidant effect since the concentrations required for such action are several fold higher than the physiological levels (Santanam et al. 1998). It has been proposed that estrogen mediates its action through its receptors, α and β by a variety of mechanisms including transcription of critical genes which are neuroprotective, such as BDNF, IGF1, and Bcl2 (Nilsen and Diaz Brinton 2003). Here we demonstrate that constitutively the levels of Trx, TR, and GR are maintained at higher levels in the female brain, which in turn prevent aberrant signaling mediated through ASK1 (Fig. 6). Once the activation of ASK1 is prevented, the downstream cascades mediated through p38 do not take place. Further, we also demonstrate that DJ-1 is expressed at higher levels in the midbrain of female mice, which prevents the translocation of Daxx and its interaction with ASK1. Thus, small molecules targeted at increasing the transcription of critical genes such as Trx, TR, and DJ-1 may help protect the SNpc dopaminergic neurons and slow down the progression of the disease.
Schematic representation of differential activation of apoptotic pathways in male and female mice in response to MPTP. 1-methyl-4-phenylpyridinium (MPP+), the toxic metabolite of 1-methyl-4-phenyl-1, 2, 3, 6 tetrahydropyridine (MPTP) causes increased production of reactive oxygen species (ROS) and mitochondrial dysfunction in dopaminergic neurons by inhibiting complex I of the electron transport chain. In male mice treated with MPTP, ROS activates apoptosis signal regulating kinase (ASK1) through the oxidation of thioredoxin (Trx), which results in its dissociation from ASK1. As a consequence, the downstream kinase p38 is phosphorylated. ROS also triggers decrease in levels of nuclear DJ-1 and subsequent translocation of Daxx from the nucleus to the cytosol. Interaction of Daxx with ASK1 in the cytosol further propagates the death cascade. Activation of MAP kinase (ASK1 and p38) cascade and translocation of Daxx is suppressed in females due to enhanced thioredoxin system and retention of DJ-1 and sequestration of Daxx within the nucleus
References
Behl C (2002) Oestrogen as a neuroprotective hormone. Nat Rev Neurosci 3:433–442
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Djarmati A, Hedrich K, Svetel M, Schafer N, Juric V, Vukosavic S, Hering R, Riess O, Romac S, Klein C, Kostic V (2004) Detection of Parkin (PARK2) and DJ1 (PARK7) mutations in early-onset Parkinson disease: Parkin mutation frequency depends on ethnic origin of patients. Hum Mutat 23:525
Dluzen DE, McDermott JL (2008) Sex differences in dopamine- and vesicular monoamine-transporter functions. Ann NY Acad Sci 1139:140–150
Freyaldenhoven TE, Cadet JL, Ali SF (1996) The dopamine-depleting effects of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine in CD-1 mice are gender-dependent. Brain Res 735:232–238
Goodenough S, Schleusner D, Pietrzik C, Skutella T, Behl C (2005) Glycogen synthase kinase 3beta links neuroprotection by 17beta-estradiol to key Alzheimer processes. Neuroscience 132:581–589
Hewitt SC, Deroo BJ, Korach KS (2005) Signal transduction. A new mediator for an old hormone? Science 307:1572–1573
Holmgren A, Bjornstedt M (1995) Thioredoxin and thioredoxin reductase. Methods Enzymol 252:199–208
Horn H (1965) Glutathione reductase. In: Bergmayer H (ed) Methods of enzymatic analysis. Academic Press, New York, pp 875–879
Junn E, Taniguchi H, Jeong BS, Zhao X, Ichijo H, Mouradian MM (2005) Interaction of DJ-1 with Daxx inhibits apoptosis signal-regulating kinase 1 activity and cell death. Proc Natl Acad Sci USA 102:9691–9696
Karunakaran S, Diwakar L, Saeed U, Agarwal V, Ramakrishnan S, Iyengar S, Ravindranath V (2007) Activation of apoptosis signal regulating kinase 1 (ASK1) and translocation of death-associated protein, Daxx, in substantia nigra pars compacta in a mouse model of Parkinson’s disease: protection by alpha-lipoic acid. FASEB J 21:2226–2236
Karunakaran S, Saeed U, Mishra M, Valli RK, Joshi SD, Meka DP, Seth P, Ravindranath V (2008) Selective activation of p38 mitogen-activated protein kinase in dopaminergic neurons of substantia nigra leads to nuclear translocation of p53 in 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-treated mice. J Neurosci 28:12500–12509
Kenchappa RS, Ravindranath V (2003) Glutaredoxin is essential for maintenance of brain mitochondrial complex I: studies with MPTP. FASEB J 17:717–719
Korner M, Rattner A, Mauxion F, Sen R, Citri Y (1989) A brain-specific transcription activator. Neuron 3:563–572
Liu L, Chen W, Xie J, Wong M (2008) Neuroprotective effects of genistein on dopaminergic neurons in the mice model of Parkinson’s disease. Neurosci Res 60:156–161
Luoma JI, Boulware MI, Mermelstein PG (2008) Caveolin proteins and estrogen signaling in the brain. Mol Cell Endocrinol 290:8–13
Morissette M, Al Sweidi S, Callier S, Di Paolo T (2008) Estrogen and SERM neuroprotection in animal models of Parkinson’s disease. Mol Cell Endocrinol 290:60–69
Nilsen J, Diaz Brinton R (2003) Mechanism of estrogen-mediated neuroprotection: regulation of mitochondrial calcium and Bcl-2 expression. Proc Natl Acad Sci USA 100:2842–2847
Peri A, Serio M (2008) Neuroprotective effects of the Alzheimer’s disease-related gene seladin-1. J Mol Endocrinol 41:251–261
Prokai L, Simpkins JW (2007) Structure-nongenomic neuroprotection relationship of estrogens and estrogen-derived compounds. Pharmacol Ther 114:1–12
Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H (1998) Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J 17:2596–2606
Santanam N, Shern-Brewer R, McClatchey R, Castellano PZ, Murphy AA, Voelkel S, Parthasarathy S (1998) Estradiol as an antioxidant: incompatible with its physiological concentrations and function. J Lipid Res 39:2111–2118
Sasahara K, Shikimi H, Haraguchi S, Sakamoto H, Honda S, Harada N, Tsutsui K (2007) Mode of action and functional significance of estrogen-inducing dendritic growth, spinogenesis, and synaptogenesis in the developing Purkinje cell. J Neurosci 27:7408–7417
Shalev I, Lerer E, Israel S, Uzefovsky F, Gritsenko I, Mankuta D, Ebstein RP, Kaitz M (2009) BDNF Val66Met polymorphism is associated with HPA axis reactivity to psychological stress characterized by genotype and gender interactions. Psychoneuroendocrinology 34:382–388
Shulman LM (2007) Gender differences in Parkinson’s disease. Gend Med 4:8–18
Song JJ, Lee YJ (2003) Role of the ASK1-SEK1-JNK1-HIPK1 signal in Daxx trafficking and ASK1 oligomerization. J Biol Chem 278:47245–47252
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saeed, U., Karunakaran, S., Meka, D.P. et al. Redox Activated MAP Kinase Death Signaling Cascade Initiated by ASK1 is not Activated in Female Mice Following MPTP: Novel Mechanism of Neuroprotection. Neurotox Res 16, 116–126 (2009). https://doi.org/10.1007/s12640-009-9058-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-009-9058-5