Abstract
Pretreatment with methamphetamine (METH) can attenuate toxicity due to acute METH challenges. The majority of previous reports have focused mainly on the effects of the drug on the striatal dopaminergic system. In the present study, we used a regimen that involves gradual increases in METH administration to rats in order to mimic progressively larger doses of the drug used by some human METH addicts. We found that this METH preconditioning was associated with complete protection against dopamine depletion caused by a METH challenge (5 mg/kg × 6 injections given 1 h apart) in the striatum and cortex. In contrast, there was no preconditioning-mediated protection against METH-induced serotonin depletion in the striatum and hippocampus, with some protection being observed in the cortex. There was also no protection against METH-induced norepinephrine (NE) depletion in the hippocampus. These results indicate that, in contrast to the present dogmas, there might be differences in the mechanisms involved in METH toxicity on monoaminergic systems in the rodent brain. Thus, chronic injections of METH might activate programs that protect against dopamine toxicity without influencing drug-induced pathological changes in serotoninergic systems. Further studies will need to evaluate the cellular and molecular bases for these differential responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methamphetamine (METH) abuse has reached epidemic proportion in the United States and throughout the world (Rawson et al. 2002). METH addicts suffer from cognitive deficits (Chang et al. 2002; Simon et al. 2002; Sekine et al. 2003; Johanson et al. 2006) and several studies have documented biochemical and structural abnormalities in the brains of chronic abusers (Wilson et al. 1996; Volkow et al. 2001; Jernigan et al. 2005; Chang et al. 2007; reviewed by Cadet and Krasnova 2007). These neuropathological changes have been replicated in mice, rats, and monkeys in which METH causes depletion of monoamines and loss of dopamine and serotonin transporters (Ricaurte et al. 1980, 1982; Cadet et al. 1994; Itzhak and Ali 1996; Friedman et al. 1998; Ladenheim et al. 2000; Jayanthi et al. 2005; reviewed by Cadet and Krasnova 2007; Cadet et al. 2007). The majority of these studies were conducted using model systems where moderate to large doses of METH were injected using short intervals on a single day (see Cadet et al. 2003 for discussion of doses and scheduling of METH injections). These patterns are therefore more compatible with doses taken by addicts during accidental overdoses (Davidson et al. 2001).
Because METH users increase drug usage progressively (Kramer et al. 1967; Cho and Melega 2002), several attempts have been made to replicate patterns of drug abuse by METH addicts (Stephans and Yamamoto 1996; Johnson-Davis et al. 2003, 2004; O’Neil et al. 2006; Danaceau et al. 2007). These experiments have suggested that pretreatment with METH can provide some degree of protection against drug-induced damage to monoaminergic systems (Schmidt et al. 1985; Gygi et al. 1996; Stephans and Yamamoto 1996; Segal et al. 2003; Johnson-Davis et al. 2004; Danaceau et al. 2007). Because most of the studies have reported on the effects of pretreatment on the striatal dopamine (DA) system, we have recently begun to investigate the effects of METH pretreatment on monoaminergic systems in various regions of the rodent brain and have reported that there might be some differential effects of METH pretreatment on DA and serotonin (5-HT) systems in the rat brain (Graham et al. 2008). In order to clarify these issues further, we have modified the pretreatment schedule used in the previous study to now include the administration of larger doses of METH during the second week. We have also eliminated the 3-day holiday that was used before the final drug challenges (Graham et al. 2008). Thus, in the present study, the rats were injected with the challenge METH only after a 1-day holiday (see Table 1). We reasoned that this approach might allow us to better ascertain the differential protective effects of METH preconditioning on the toxic effects of METH challenge (5 mg/kg × 6 given 1 h apart in a single day) on the levels of norepinephrine (NE), DA, 5-HT and their metabolites in specific brain regions.
Materials and Methods
Animals
Male Sprague-Dawley rats (Charles Rivers Laboratories, Raleigh, NC) weighing approximately 350–400 g were used in the present study. Animals were housed in pairs in polyethylene cages containing hardwood bedding in a humidity- and temperature-controlled room. Animals were given rat chow and water ad libitum. All animal use procedures were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the local Animal Care Committee.
Drug Treatment and Tissue Collection
Following habituation, each rat was injected i.p. with either METH-hydrochloride (NIDA, Baltimore, MD) or an equivalent volume of saline as described in Table 1. Following the preconditioning period, the saline-pretreated animals received either saline or METH (5 mg/kg × 6 at 1 h intervals) challenges whereas the METH-pretreated rats were then given the METH challenge. The animals were sacrificed 24 h later by decapitation, their brains were quickly removed, brain regions were microdissected over ice, snap frozen on dry ice, and stored at −80°C until used in the HPLC analysis. One hemisphere of each region frontal cortex, striatum, nucleus accumbens, and hippocampus was used to generate the HPLC data.
HPLC
For monoamine analysis, the brain regions were homogenized in 0.01 M HClO4 and centrifuged at 14, 000g for 15 min. NE, DA, 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), 5-HT and 5-hydroxyindoleacetic acid (5-HIAA) levels were analyzed in the brain extracts using HPLC with electrochemical detection as previously described (Ladenheim et al. 2000; Krasnova et al. 2007). Monoamine levels were calculated as ng/mg of tissue weight and shown as percentages of control concentrations for ease of presentation.
Statistical Analysis
Statistical analysis was performed using analysis of variance (ANOVA) followed by Fisher’s protected least significant difference (StatView 4.02, SAS Institute, Cary, NC). Values were represented as mean ± SEM. The null hypothesis was rejected at P < 0.05.
Results
Striatum
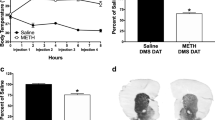
The effects of METH on DA and its metabolites, DOPAC and HVA, in the striatum are shown in Fig. 1a. ANOVA revealed that the drug caused decreases in DA [F (2,23) = 3.22; P = 0.05]. Post-hoc analyses showed that this was due to METH-induced decreases (−33.6%) in DA levels in the striata of rats pretreated with saline. The METH-pretreated animals suffered no drug-induced decreases in striatal DA levels (Fig. 1a). The METH injections caused significant changes [F (2,23) = 3.32, P = 0.05] in the DA metabolite, DOPAC; post-hoc analyses revealed that these were due to differences between the two METH challenged groups in that the METH/METH group had higher levels of DOPAC than the SAL/METH group (Fig. 1a). HVA levels were not significantly affected after the drug challenge [F (2,23) = 2.01, P = 0.15] (Fig. 1a). METH caused no changes in NE levels [F (2,23) = 0.769, P = 0.47] in the striata of SAL/METH and METH/METH groups (data not shown).
Effects of METH pretreatment and challenge on markers of striatal DA (a) and 5-HT (b) terminals. Values were normalized to SAL/SAL controls and expressed as percentages of controls ± SEM. N = 8–10 animals per group. Key to statistics: * P < 0.05 versus SAL/SAL group, # P < 0.05 versus SAL/METH group
The effects of METH on the 5-HT system are shown in Fig. 1b. The drug caused significant changes in the levels of 5-HT in the striatum [F (2,23) = 3.63, P = 0.04]. Post-hoc analyses showed that, in contrast to the observations in the DA system, METH pretreatment did not protect against drug-induced decreases in 5-HT levels in the rat striatum; specifically, the METH challenge caused 15.6% decreases (P = 0.114) in the SAL/METH group but 24.2% decreases (P = 0.013) in 5-HT levels in the METH/METH group (see Fig. 1b). 5-HIAA levels were not significantly affected by the METH challenge [F (2,23) = 1.04, P = 0.36] (Fig. 1b).
Nucleus Accumbens
The effects of METH on the DA system in the nucleus accumbens are shown in Fig. 2a. The METH challenge did not cause significant decreases in DA levels in the nucleus accumbens [F (2,21) = 1.93; P = 0.17]. The METH-induced changes in DOPAC levels almost reached significance by ANOVA [F (2,21) = 3.1; P = 0.06]. These changes were due to METH-induced decreases (−29.7%) in the nucleus accumbens of saline-pretreated rats whereas there were no differences in DOPAC levels in the METH/METH group in comparison to SAL/SAL control (Fig. 2a). HVA levels were not affected by the METH challenge [F (2,21) = 0.812, P = 0.45]. METH treatments caused no significant changes [F (2,21) = 0.492, P = 0.61] in NE levels in the nucleus accumbens (data not shown).
Figure 2b shows the effects of METH on the 5-HT system in the nucleus accumbens. The METH injections did not cause significant changes by ANOVA [F (2,21) = 2.49, P = 0.10]. Further comparisons revealed that 5-HT levels in the SAL/METH group were decreased in comparison to the control group (see Fig. 2b). There were significant METH-induced decreases in 5-HIAA levels in nucleus accumbens [F (2,21) = 4.25, P = 0.02]. These decreases, although small, were observed in both the SAL/METH (−17.5%) and in the METH/METH (−14.2%) groups (Fig. 2b).
Cortex
The effects of METH on DA and its metabolites in the frontal cortex are shown in Fig. 3a. The METH challenge caused significant changes in DA levels in the cortex of rats [F (2,19) = 3.58, P = 0.04]. Post-hoc analyses revealed that there were significant decreases in the saline-pretreated group but not in the METH/METH group (Fig. 3a). The METH challenge did not affect DOPAC [F (2,19) = 0.25; P = 0.77] or HVA [F (2,19) = 0.48, P = 0.61] levels. There were no METH-induced changes [F (2,19) = 0.66, P = 0.52] in NE levels in the cortices of rats (data not shown).
Effects of METH pretreatment and challenge on markers of cortical DA (a) and 5-HT (b) terminals. Values were normalized to SAL/SAL controls and expressed as percentages of controls ± SEM. N = 8–10 animals per group. Key to statistics: * P < 0.05 versus SAL/SAL group, # P < 0.05 versus SAL/METH group
The effects of METH on the cortical 5-HT system are shown in Fig. 3b. ANOVA did not reveal any significant effects of the drug on 5-HT levels [F (2,19) = 2.2, P = 0.13]. Further analyses revealed that 5-HT values were significantly decreased (−24.5%) in the SAL/METH group. METH preconditioned rats did not show significant decreases after the acute drug challenge. 5-HIAA levels were not significantly affected by ANOVA [F (2,19) = 2.6, P = 0.09]. However, further analyses revealed significant decreases in the SAL/METH rats that showed 23.9% decreases (Fig. 3b). There were no differences between the SAL/METH and the METH/METH groups.
Hippocampus
The effects of METH on NE, 5-HT, and 5-HIAA levels in the hippocampus are shown in Fig. 4. The METH challenge caused significant decreases in NE levels in the rat hippocampus [F (2,23) = 8.4, P = 0.001], with the saline- (−27.2%) and the METH-pretreated (−28.9%) groups showing similar changes (Fig. 4). In addition, the METH challenge caused decreases in hippocampal 5-HT levels [F (2,23) = 6.45; P = 0.005]. The SAL/METH (−31.3%) and the METH/METH (−28.4%) groups were similarly affected by the drug challenge. 5-HIAA levels were also significantly affected by the METH challenge [F (2,23) = 7.49, P = 0.003], with the SAL/METH and the METH/METH showing 28.9% and 22.6% decreases, respectively (Fig. 4).
Discussion
The main findings of the present paper include METH-induced depletion of striatal DA in saline-pretreated rats as reported by us and others (reviewed by Cadet et al. 2003, 2007; Cadet and Krasnova 2007). There were also significant decreases in the levels of DA, 5-HT and 5-HIAA in the frontal cortex and hippocampus and of NE levels in the hippocampus of METH-treated saline-pretreated rats. The decreases in NE in the hippocampus are consistent with those recently reported by Graham et al. (2008). The present data are also the first to report on the effects of METH preconditioning on monoamine levels in the nucleus accumbens of rats. METH preconditioning was associated with almost complete protection against drug-induced depletion of DA in the striatum and in the frontal cortex, findings that are consistent with the reports that various pretreatment METH regimens can protect against METH-induced DA depletion (Schmidt et al. 1985; Gygi et al. 1996; Johnson-Davis et al. 2003; Segal et al. 2003; O’Neil et al. 2006; Danaceau et al. 2007; Graham et al. 2008). The METH pretreatment also provided small degrees of protection against 5-HT depletion in the nucleus accumbens and the frontal cortex but not in the striatum or hippocampus. The present results are consistent with our previous report that a somewhat different pattern of METH pretreatment was only able to afford small protective effects against drug-induced toxicity on striatal and hippocampal 5-HT systems (Graham et al. 2008). This study, which uses a pattern of higher doses of METH pretreatment and includes the elimination of the 3-day holiday used in a previous study (Graham et al. 2008), expands on that report and provides strong evidence of a very clear dissociation between the effects of METH preconditioning on dopaminergic and serotonergic systems in the rat brain.
The present data are consistent with reports that repeated sub-toxic stimuli can provide protection against subsequent exposure to more fulminant stressors, a process referred to as preconditioning (Blanco et al. 2006) or hormesis (Calabrese 2008; Mattson 2008). Hormesis might be a more general and comprehensive term which covers a more varied picture of sub-toxic or sub-clinical manipulations (Calabrese 2008). These phenomena include pre-exposure to mild ischemia (Blanco et al. 2006), endotoxins (Rosenzweig et al. 2007), and thermic conditions (Ren et al. 2004). Multiple mechanisms are involved in preconditioning or hormesis (Mattson 2008). These include activation of cellular defense mechanisms such as antioxidant enzymes and heat shock proteins as well as reduced inflammatory responses (Hoshida et al. 2002; Glantz et al. 2005; Mattson 2008).
In the case of METH toxicity, it has become clear that several pro-toxic events are involved in causing loss of monoaminergic terminals (Cadet et al. 2003, 2007; Segura-Aguilar and Kostrzewa 2004; Cadet and Krasnova 2007). Prominent among these are the production of oxygen-based radicals from DA quinones generated within DA terminals (LaVoie and Hastings 1999), production of reactive species such as superoxide radicals (Cadet et al. 1994) and nitric oxide (Itzhak and Ali 2006), and the involvement of mitochondria-generated toxic proteins (reviewed in Cadet et al. 2007). Because mitochondria plays such an integral part in METH-induced toxicity, drug preconditioning might affect these organelles in such a way that subsequent exposures to toxic doses of METH might not be able to generate enough oxygen-based radicals to cause degeneration of DA terminals. This reprogramming might be dependent on increased production of chaperones such as Hsp60, Hsp70, and others (Saibil 2008) that might participate in the maintenance of homeostasis within DA terminals, given the presence of large number of mitochondria in nerve axons (Ly and Verstreken 2006). Thus, low levels of oxygen-based radicals generated by progressive increases in METH administration might trigger signaling functions that stimulate adaptive responses instead of acute toxic stresses that destroy DA terminals. Protective enzymes such as manganese superoxide dismutase, copper/zinc superoxide dismutase, catalase, and glutathione peroxidase might also be mediators of METH preconditioning because these enzymes would protect against METH-induced generation of reactive oxygen species (ROS) (see Cadet and Brannock 1998 for discussion). In addition to regulating the generation of ROS and increasing enzyme levels, such an adaptation might also lead to tolerance of glutamate-mediated disturbances of calcium homeostasis within these terminals; since excitotoxicity is thought to be involved in METH-induced DA terminal degeneration (see Cadet et al. 2007 for discussion). This suggestion is consistent with the demonstration that sub-toxic levels of activation of the N-methyl-d-aspartate (NMDA) type of glutamate receptors can induce adaptive responses in neurons that become resistant to subsequent severe stressful events (Lin et al. 2008).
It is important to note that the METH-induced inaugural events that led to protection against METH-induced striatal and cortical DA depletion did not have similar protective effects against 5-HT depletion in the striatum and the hippocampus, with only small attenuating effects being observed in the cortex and the nucleus accumbens. These findings suggest that the pattern of METH preconditioning used in the present study was not able to generate similar adaptive changes in 5-HT terminals. These observations are important because it is often assumed that identical mechanisms are involved in METH-induced destruction of DA and 5-HT terminals since various manipulations that protect against damage to DA systems also protect against loss of 5-HT terminals (Cadet et al. 2003, 2007). The present observations are more in accord with gene expression studies that suggest that diverse stimuli might trigger protection through unique though overlapping processes that generate molecular reprogramming. This reprogramming would, in turn, activate the transcription of protective factors such as chaperones, trophic factors and antioxidants (see Dirnagl et al. 2003 and Stenzel-Poore et al. 2003, for further discussion). Because our observations are different from those of Danaceau et al. (2007) who reported METH preconditioning-induced protection against brain 5-HT systems using a different METH pre-treatment paradigm, the present data suggest that a given stimulus might cause differential adaptive responses depending on the patterns of drug administration and the neurotransmitter systems under observation. This suggestion is consistent with the fact that the present study was able to document different patterns of protection (or lack thereof) from those that we previously published (Graham et al. 2008) after increasing the doses of METH used during the pretreatment period and eliminating the long interval before challenging the rats with toxic doses of METH. Thus, this discussion suggests that future studies will need to identify specific patterns of drug preconditioning that provide protection against specific aspects of METH-induced neurotoxicity and use these paradigms to clarify the cellular and molecular bases of the observed protection.
Conclusion
In summary, chronic injections of METH in a pattern that is reminiscent of that used by METH addicts caused almost complete protection against drug-induced DA depletion in the striatum and cortex. This preconditioning paradigm was not protective against the toxic effects of the drug on 5-HT systems in the striatum and hippocampus. METH-induced NE depletion in the hippocampus was also not prevented. These observations suggest that there might be substantial differences between the mechanisms involved in the METH-induced degeneration of these three monoaminergic systems. More in-depth studies will be needed to clarify similarities and differences between the responses of these monoaminergic systems to METH assaults on their structural integrity. Finally, studies focusing on the dissection of the endogenous mechanisms involved in protecting DA terminals should advance our approaches to the treatment of METH addicted individuals and of Parkinsonian patients who suffer from pathologies in their dopaminergic systems.
References
Blanco M, Lizasoain I, Sobrino T, Vivancos J, Castillo J (2006) Ischemic preconditioning: a novel target for neuroprotective therapy. Cerebrovasc Dis 21(Suppl. 2):38–47
Cadet JL, Brannock C (1998) Free radicals and the pathobiology of brain dopamine systems. Neurochem Int 32:117–131
Cadet JL, Krasnova IN (2007) Interactions of HIV and methamphetamine: cellular and molecular mechanisms of toxicity potentiation. Neurotox Res 12:181–204
Cadet JL, Ali S, Epstein C (1994) Involvement of oxygen-based radicals in methamphetamine-induced neurotoxicity: evidence from the use of CuZnSOD transgenic mice. Ann NY Acad Sci 738:388–391
Cadet JL, Jayanthi S, Deng X (2003) Speed kills: cellular and molecular bases of methamphetamine-induced nerve terminal degeneration and neuronal apoptosis. FASEB J 17:1775–1788
Cadet JL, Krasnova IN, Jayanthi S, Lyles J (2007) Neurotoxicity of substituted amphetamines: molecular and cellular mechanisms. Neurotox Res 11:183–202
Calabrese EJ (2008) Converging concepts: adaptive response, preconditioning, and the Yerkes-Dodson Law are manifestations of hormesis. Ageing Res Rev 7:8–20
Chang L, Ernst T, Speck O, Patel H, DeSilva M, Leonido-Yee M, Miller EN (2002) Perfusion MRI and computerized cognitive test abnormalities in abstinent methamphetamine users. Psychiatry Res 114:65–79
Chang L, Alicata D, Ernst T, Volkow N (2007) Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction 102(Suppl. 1):16–32
Cho AK, Melega WP (2002) Patterns of methamphetamine abuse and their consequences. J Addict Dis 21:21–34
Danaceau JP, Deering CE, Day JE, Smeal SJ, Johnson-Davis KL, Fleckenstein AE, Wilkins DG (2007) Persistence of tolerance to methamphetamine-induced monoamine deficits. Eur J Pharmacol 559:46–54
Davidson C, Gow AJ, Lee TH, Ellinwood EH (2001) Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res Brain Res Rev 36:1–22
Dirnagl U, Simon RP, Hallenbeck JM (2003) Ischemic tolerance and endogenous neuroprotection. Trends Neurosci 26:248–254
Friedman SD, Castaneda E, Hodge GK (1998) Long-term monoamine depletion, differential recovery, and subtle behavioral impairment following methamphetamine-induced neurotoxicity. Pharmacol Biochem Behav 61:35–44
Glantz L, Avramovich A, Trembovler V, Gurvitz V, Kohen R, Eidelman LA, Shohami E (2005) Ischemic preconditioning increases antioxidants in the brain and peripheral organs after cerebral ischemia. Exp Neurol 192:117–124
Graham DL, Noailles PA, Cadet JL (2008) Differential neurochemical consequences of an escalating dose-binge regimen followed by single-day multiple-dose methamphetamine challenges. J Neurochem 105:1873–1885
Gygi MP, Gygi SP, Johnson M, Wilkins DG, Gibb JW, Hanson GR (1996) Mechanisms for tolerance to methamphetamine effects. Neuropharmacology 35:751–757
Hoshida S, Yamashita N, Otsu K, Hori M (2002) The importance of manganese superoxide dismutase in delayed preconditioning: involvement of reactive oxygen species and cytokines. Cardiovasc Res 55:495–505
Itzhak Y, Ali SF (1996) The neuronal nitric oxide synthase inhibitor, 7-nitroindazole, protects against methamphetamine-induced neurotoxicity in vivo. J Neurochem 67:1770–1773
Itzhak Y, Ali SF (2006) Role of nitrergic system in behavioral and neurotoxic effects of amphetamine analogs. Pharmacol Ther 109:246–262
Jayanthi S, Deng X, Ladenheim B, McCoy MT, Cluster A, Cai NS, Cadet JL (2005) Calcineurin/NFAT-induced up-regulation of the Fas ligand/Fas death pathway is involved in methamphetamine-induced neuronal apoptosis. Proc Natl Acad Sci USA 102:868–873
Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, Heaton RK, Ellis RJ, Grant I (2005) Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry 162:1461–1472
Johanson CE, Frey KA, Lundahl LH, Keenan P, Lockhart N, Roll J, Galloway GP, Koeppe RA, Kilbourn MR, Robbins T, Schuster CR (2006) Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology (Berl.) 185:327–338
Johnson-Davis KL, Fleckenstein AE, Wilkins DG (2003) The role of hyperthermia and metabolism as mechanisms of tolerance to methamphetamine neurotoxicity. Eur J Pharmacol 482:151–154
Johnson-Davis KL, Truong JG, Fleckenstein AE, Wilkins DG (2004) Alterations in vesicular dopamine uptake contribute to tolerance to the neurotoxic effects of methamphetamine. J Pharmacol Exp Ther 309:578–586
Kramer JC, Fischman VS, Littlefield DC (1967) Amphetamine abuse. Pattern and effects of high doses taken intravenously. JAMA 201:305–309
Krasnova IN, Betts ES, Dada A, Jefferson A, Ladenheim B, Becker KG, Cadet JL, Hohmann CF (2007) Neonatal dopamine depletion induces changes in morphogenesis and gene expression in the developing cortex. Neurotox Res 11:107–130
Ladenheim B, Krasnova IN, Deng X, Oyler JM, Polettini A, Moran TH, Huestis MA, Cadet JL (2000) Methamphetamine-induced neurotoxicity is attenuated in transgenic mice with a null mutation for interleukin-6. Mol Pharmacol 58:1247–1256
LaVoie MJ, Hastings TG (1999) Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: evidence against a role for extracellular dopamine. J Neurosci 19:1484–1491
Lin CH, Chen PS, Gean PW (2008) Glutamate preconditioning prevents neuronal death induced by combined oxygen-glucose deprivation in cultured cortical neurons. Eur J Pharmacol 589:85–93
Ly CV, Verstreken P (2006) Mitochondria at the synapse. Neuroscientist 12:291–299
Mattson MP (2008) Hormesis defined. Ageing Res Rev 7:1–7
O’Neil ML, Kuczenski R, Segal DS, Cho AK, Lacan G, Melega WP (2006) Escalating dose pretreatment induces pharmacodynamic and not pharmacokinetic tolerance to a subsequent high-dose methamphetamine binge. Synapse 60:465–473
Rawson RA, Simon SL, Ling W (2002) If a US drug abuse epidemic fails to include a major east coast city, can it be called an epidemic? J Addict Dis 21:1–4
Ren Y, Hashimoto M, Pulsinelli WA, Nowak TS Jr (2004) Hypothermic protection in rat focal ischemia models: strain differences and relevance to “reperfusion injury”. J Cereb Blood Flow Metab 24:42–53
Ricaurte GA, Schuster CR, Seiden LS (1980) Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res 193:153–163
Ricaurte GA, Guillery RW, Seiden LS, Schuster CR, Moore RY (1982) Dopamine nerve terminal degeneration produced by high doses of methylamphetamine in the rat brain. Brain Res 235:93–103
Rosenzweig HL, Minami M, Lessov NS, Coste SC, Stevens SL, Henshall DC, Meller R, Simon RP, Stenzel-Poore MP (2007) Endotoxin preconditioning protects against the cytotoxic effects of TNFalpha after stroke: a novel role for TNFalpha in LPS-ischemic tolerance. J Cereb Blood Flow Metab 27:1663–1674
Saibil HR (2008) Chaperone machines in action. Curr Opin Struct Biol 18:35–42
Schmidt CJ, Sonsalla PK, Hanson GR, Peat MA, Gibb JW (1985) Methamphetamine-induced depression of monoamine synthesis in the rat: development of tolerance. J Neurochem 44:852–855
Segal DS, Kuczenski R, O’Neil ML, Melega WP, Cho AK (2003) Escalating dose methamphetamine pretreatment alters the behavioral and neurochemical profiles associated with exposure to a high-dose methamphetamine binge. Neuropsychopharmacology 28:1730–1740
Segura-Aguilar J, Kostrzewa RM (2004) Neurotoxins and neurotoxic species implicated in neurodegeneration. Neurotox Res 6:615–630
Sekine Y, Minabe Y, Ouchi Y, Takei N, Iyo M, Nakamura K, Suzuki K, Tsukada H, Okada H, Yoshikawa E, Futatsubashi M, Mori N (2003) Association of dopamine transporter loss in the orbitofrontal and dorsolateral prefrontal cortices with methamphetamine-related psychiatric symptoms. Am J Psychiatry 160:1699–1701
Simon SL, Domier CP, Sim T, Richardson K, Rawson RA, Ling W (2002) Cognitive performance of current methamphetamine and cocaine abusers. J Addict Dis 21:61–74
Stenzel-Poore MP, Stevens SL, Xiong Z, Lessov NS, Harrington CA, Mori M, Meller R, Rosenzweig HL, Tobar E, Shaw TE, Chu X, Simon RP (2003) Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxia-tolerant states. Lancet 362:1028–1037
Stephans S, Yamamoto B (1996) Methamphetamines pretreatment and the vulnerability of the striatum to methamphetamine neurotoxicity. Neuroscience 72:593–600
Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J (2001) Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci 21:9414–9418
Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ (1996) Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med 2:699–703
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Institute on Drug Abuse, NIH/DHHS. The authors thank the staff of the NIDA/IRP animal facility for the excellent care provided to the animals used in our studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cadet, J.L., Krasnova, I.N., Ladenheim, B. et al. Methamphetamine Preconditioning: Differential Protective Effects on Monoaminergic Systems in the Rat Brain. Neurotox Res 15, 252–259 (2009). https://doi.org/10.1007/s12640-009-9026-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-009-9026-0