Abstract
1-Benzyl-1,2,3,4-tetrahydroisoquinoline (1BnTIQ), an endogenous neurotoxin, is known to cause a parkinsonism-like syndrome in rodents and primates. In this study we evaluated the effects of single and multiple 1BnTIQ (50 mg/kg i.p.) administration on the concentrations of dopamine, serotonin, and respective metabolites (homovanillic acid, HVA; 3,4-dihydroxyphenylacetic acid, DOPAC; 3-methoxytyramine, 3-MT; and 5-hydroxyindolacetic acid, 5-HIAA), in substantia nigra, striatum (STR), and nucleus accumbens of Wistar rats. In addition, the effect of 1BnTIQ on locomotor activity and dopamine release in vivo was also estimated in rat STR. In a behavioral study, acute administration of 1BnTIQ (50 mg/kg i.p.) produced a significant decrease in exploratory locomotor activity. A high-performance liquid chromatography with electrochemical detection ex vivo study showed that a single injection of 1BnTIQ produced a dramatic fall in the dopamine concentration in the noted brain regions (~65%; P < 0.01), but not in striatal serotonin. Moreover, 1BnTIQ reduced the content of the extraneuronal dopamine metabolite 3-MT by 70% (P < 0.01). Conversely, levels of DOPAC, HVA, and 5-HIAA were elevated by 220, 320, and 185%, respectively (P < 0.01). Interestingly, multiple 1BnTIQ treatments (50 mg/kg/day i.p. × 10 days) resulted in development of tolerance to its dopamine depressing effect, while the impairment of dopamine synthesis was persisted. An in vivo microdialysis study demonstrated that 1BnTIQ (50 mg/kg i.p.) produced a profound and long-lasting decrease in extraneuronal striatal dopamine. Concurrently, however, DOPAC and HVA were elevated. This comparison between ex vivo and in vivo effects of 1BnTIQ provides greater insight into the neurotoxic actions of 1BnTIQ specific to dopamine neurons. 1BnTIQ neurotoxicity may be related to an impairment of dopamine storage, leading to a fall in intraneuronal dopamine and enhanced dopamine catabolism through a monoamine oxidize-dependent oxidative pathway that results in free radical production and ultimate cell death. Because 1BnTIQ is an endogenous compound, it may be one of the factors responsible for idiopathic Parkinson’s disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by degeneration of nigro-striatal dopaminergic neurons and a reduction of dopamine levels in these brain regions. The pathogenesis of PD is considered to involve environmental factors and endogenously produced toxins, but the molecular mechanisms initiating dopaminergic cell death remain unknown (Langston et al. 1983; Naoi et al. 2002). 1-Benzyl-1, 2,3,4-tetrahydroisoquinoline (1BnTIQ) and salsolinol are the two endogenous neurotoxins that have been proposed as etiological factors of PD (Moser and Kompf 1992; Kotake et al. 1995; Antkiewicz-Michaluk et al. 2000, 2001). Some evidence suggests that oxidative stress may be a primary cause of degeneration of dopaminergic neurons in PD (Dexter et al. 1989; Alam et al. 1997). Several reports also indicate that dopaminergic neurons undergo apoptosis in the course of this disease (Mochizuki et al. 1996; Mogi et al. 2000). It has been demonstrated that the cerebrospinal fluid (CSF) from PD patients contains toxic substances which produce deleterious effects on dopaminergic neurons (Hao et al. 1995a,b).
Since the discovery that 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) produces parkinsonism in humans, structurally similar 1,2,3,4-tetrahydroisoquinoline (TIQ) derivatives have been regarded as endogenous candidate etiologic factors in PD. Several investigators have found that tetrahydroisoquinoline derivatives, such as TIQ, 1BnTIQ, and salsolinol, exist in the brain of several mammalian species (Ohta et al. 1987; Nagatsu and Yoshida 1988; Yoshida et al. 1990; Moser and Kompf 1992; Kotake et al. 1996).
1BnTIQ, an endogenous tetrahydroisoquinoline, was identified in mouse brain and CSF of normal human subjects. Interestingly, 1BnTIQ was reported to occur in the CSF of PD patients at a threefold higher level than that of non-PD subjects (Kotake et al. 1995).

It is accepted that the metabolites produced by monoamine oxidize type-B (MAO-B), e.g., phenylacetaldehyde and 2-phenylethylamine, facilitate formation and accumulation of 1BnTIQ in brain (Inwang et al. 1973; Kotake et al. 1995). Using a gas chromatography/mass spectrometry method, Yamakawa et al. (1999) observed relatively high levels of 1BnTIQ in the substantia nigra compared to other brain regions of normal monkeys. Acute 1BnTIQ administration induced parkinsonism in both rodents and monkeys (Kotake et al. 1996; Abe et al. 2001). Our earlier study demonstrated that all the investigated tetrahydroisoquinolines had antidopaminergic properties, and we had suggested that differences in the neurotoxicity of various compounds of that group resulted from their diverse actions on dopamine catabolism. 1BnTIQ significantly affected dopamine structures and produced an increase in the rate of dopamine metabolism together with pronounced activation of the oxidative MAO-dependent catabolic pathway (Antkiewicz-Michaluk et al. 2001). The above results provide a biochemical basis for the neurotoxic properties of 1BnTIQ; however, many questions are still unanswered. Oxidative stress is a universal mechanism inducing cell death (Dykens 1999). It is known to be an inherent element of excitotoxic neuronal damage under pathological conditions (Chan 1998). The formation of free radicals (H2O2, •OH) is closely related with excessive dopamine catabolism by MAO-B and an increase in the concentration of its metabolite, 3,4-dihydroxyphenylacetic acid (DOPAC). Cytotoxic reactive oxygen species conceivably may damage complex I of mitochondrial respiratory chain and cause neurodegeneration (Schapira et al. 1990; Adams and Odunze 1991; Miller et al. 1996). However, one must exercise caution while differentiating between extraneuronal and intraneuronal indices of free radical production. In brain tissue the levels of 2,3- and 2,5-DHBA, spin trap products of •OH and salicylate, are altered in neostriatum after dopamine-denervation. Moreover, L-DOPA treatment suppresses •OH formation in neostriatal tissue of rats (Kostrzewa et al. 2000). In contrast, an in vivo microdialysis study has shown that there are no substantial differences in extraneuronal •OH generation in the neostriatum between control and parkinsonian rats (Nowak et al. 2006). Recently, Shavali and Ebadi (2003) suggested that 1BnTIQ activated apoptotic signaling pathways and induced cell death in human dopaminergic cells in culture. It is still unknown whether the endogenous toxin 1BnTIQ, which also causes parkinsonism in rodents, affects dopamine release in rat striatum (STR) in vivo.
In fact, this study was undertaken to determine the effect of 1BnTIQ on dopamine release in rat STR in vivo, as assessed by a microdialysis method in freely moving rats. Additionally, we tested the role of 1BnTIQ on spontaneous locomotor activity, a behavioral test which is closely associated with the activity of dopamine neurons in the extrapyramidal system. Finally, we compared the effects of a single or repeated 1BnTIQ administration on the rate of dopamine and serotonin metabolism in rat brain structures.
Materials and Methods
Animals and Treatment
Experiments were carried out on male Wistar rats having an initial body weight of 220–240 g (behavioral and biochemical ex vivo experiments); or 300–340 g (in vivo microdialysis study). All rats in either behavioral or biochemical studies were group housed, eight per large cage, and maintained under standard laboratory conditions at room temperature (22°C) on a natural day-night cycle. For in vivo microdialysis studies the rats, after stereotaxic surgery (to implant vertically microdialysis guides to the STR), were housed individually for a 6-day recovery period in transparent cages in close proximity to each other (i.e., not in isolation). On day 7, microdialysis probes were placed inside guides, and microdialysis experiments were conducted. All rats had free access to standard laboratory chow and tap water.
1BnTIQ (50 mg/kg i.p.) was administered either once or for ten consecutive days. Previously, we had selected this dose of 1BnTIQ because of its effectiveness in affecting endogenous brain dopamine, as assessed by biochemical (dopamine metabolism) and behavioral (locomotor activity and muscle rigidity) tests (Antkiewicz-Michaluk et al. 2001). Control rats were treated with solvent. Forty-four rats participated in the study. The experiments carried out between 9.00 and 17.00 h.
All procedures were conducted in accordance with the National Institutes of Health Guidelines for Care and Use of Laboratory Animals, and received prior approval from the Bioethics Commission, as compliant with Polish Law (January 21, 2005).
Drugs
1-Benzyl-1,2,3,4-tetrahydroisoquinoline (1BnTIQ hydrochloride) was synthesized according to Cannon and Webster (1958) at the Department of Drug Chemistry of the Institute of Pharmacology, the Polish Academy of Sciences in Krakow. Purity of the compound was verified by measurement of the melting point; and homogeneity was assessed on a chromatographic column. The compound was dissolved in a 0.9% NaCl solution.
Behavioral Testing
Locomotor Activity
Locomotor activity was recorded individually for each animal in Opto-Varimex cages (Columbus Instruments, USA) linked on-line to a compatible IBM-PC. Each cage (43 × 44 × 25 cm3) was surrounded with a 15 × 15 array of photocell beams located 3 cm above the floor surface. Interruptions of those photocell beams resulted in a horizontal activity defined as a distance traveled (in centimeter). Rats were placed in experimental cages 15 min after 1BnTIQ (50 mg/kg i.p.) injection. Horizontal locomotor activity was recorded for 150 min and analyzed using Auto-track software (Columbus Instruments, USA). Six to ten animals per group were used.
Biochemical Determinations
Ex vivo Studies
Two hours after the last 1BnTIQ injection, rats were decapitated and the substantia nigra, STR, and nucleus accumbens were immediately dissected. Tissues were frozen on dry ice (solid CO2, −70°C) until analyzed. Dopamine and its metabolites, DOPAC and 3-methoxytyramine (3-MT), as well as the terminal metabolite, homovanillic acid (HVA), were analyzed by high-performance liquid chromatography (HPLC) with electrochemical detection (ED, HPLC/ED) (Hewlett-Packard 1049A). Tissue samples were weighed frozen, then homogenized in ice-cold 0.1 M perchloroacetic acid containing 0.05 mM ascorbic acid. After centrifugation (10000g, 5 min), supernatants were filtered through RC 58 0.2-im cellulose membranes (Bioanalitycal Systems, West Lafayette, IN, USA). The HP 1050 chromatograph (Hewlett-Packard, Golden, CO, USA) was equipped with C18 columns. The mobile phase consisted of 0.05 M citrate-phosphate buffer (pH 3.5), 0.1 mM EDTA, 1 mM sodium octylsulfonate, and 3.5% methanol. Flow rate was maintained at 1 ml/min and the electrochemical potential was fixed at 800 mV. Dopamine and its metabolites were quantified by peak height comparisons with standards, run on the day of analysis. Six to ten animals per group were used.
In vivo Microdialysis Study
Rats were initially anesthetized with ketamine (75 mg/kg) and xylazine (10 mg/kg), then secured in a stereotaxic frame (Stoelting, USA). Vertical microdialysis guides (Intracerebral Guide Cannula with stylet; cannula length 15 mm; probe volume 2.3 μl; Membrane PAN. 320 μm OD, 4 mm lengths in stock; BAS Bioanalitical, USA) were implanted in the STR using the following stereotaxic coordinates: A/P +1.0, L/M +2.5, and V/D −3.5 mm from bregma and dura, respectively (G. Paxinos and C.H. Watson).
On day 7 after the surgery, microdialysis probes were placed inside guides and then perfused with an artificial CSF consisting of 140 mM NaCL, 2.7 mM KCl, 1.2 mM CaCl2, 1 mM MgCl2, 0.3 mM NaH2PO4, 1.7 mM Na2HPO4 (pH 7.4) at a flow rate of 1.5 μl/min with a microinfusion pump (Stoelting, IL, USA). Samples were collected from freely moving rats at 20 min intervals after a 3-h washout period. 1BnTIQ was injected at a dose of 50 mg/kg i.p., and effluent from the probes was collected throughout 240 min. All samples were immediately frozen on solid CO2 (−70°C) until HPLC analysis.
Six animals per group were used. Immediately after sample collection, brains were examined histologically for correct probe placement, in frozen slices.
Calculations and Statistics
The results of the biochemical and behavioral tests were analyzed by means of one-way ANOVA analysis of variance, followed, when appropriate, by post hoc Fisher’s Least Significant Difference test (LSD test). Total rate of DA catabolism was assessed on the basis of the ratio of HVA to DA, expressed as a catabolism rate index ([HVA]/[DA]) × 100 (see Antkiewicz-Michaluk et al. 2001). The indices were calculated using the concentrations of individual tissue samples (n = 6–10).
Results
The Effect of Acute 1BnTIQ Administration on Locomotor Activity
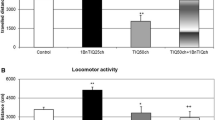
A single dose of 1BnTIQ (50 mg/kg i.p.) produced a significant decrease in locomotor activity in the first period of the experiment but no change from control level in the second phase of the study (e.g., after 30 min). At the end of the experiment we did not observe any differences between the 1BnTIQ and the control group (Fig. 1).
The effect of 1BnTIQ on locomotor activity in rats. Rats received a single injection of saline (control) or 1BnTIQ (50 mg/kg i.p.), and 15 min later were placed into actometers for 150 min. Data are means ± SEM. The number (N) of animals: SAL (control) group, N = 6; 1BnTIQ group, N = 10. **P < 0.01 (difference from control group, SAL), Fisher LSD test
Comparison Between Effects of Acute and Chronic 1BnTIQ Administration on Concentrations of Dopamine, Serotonin, and Their Metabolites in Different Rat Brain Regions
An acute dose of 1BnTIQ (50 mg/kg i.p.) produced a major reduction in the concentrations of dopamine and its metabolites in brain. 1BnTIQ markedly decreased the concentration of dopamine in all brain regions (by ~60%, P < 0.01; Table 1). The level of the intermediary dopamine metabolite DOPAC as well as the concentration of the terminal dopamine metabolite HVA was significantly increased, whereas the level of the extraneuronal dopamine metabolite 3-MT was decreased by acute 1BnTIQ administration. DA metabolism, assessed from the [HVA]/[DA] ratio, was dramatically increased by acute treatment with 1BnTIQ in all brain regions (by 350–700%), with the most pronounced effect being in the STR (Table 1).
The rate of the MAO-dependent oxidative pathway of dopamine catabolism assessed from the ratio of [DOPAC]/[DA] was increased several times for the structures studied (Fig. 2). Conversely, the rate of the COMT-dependent O-methylation pathway of dopamine catabolism assessed from the [3-MT]/[DA] ratio was significantly decreased in both the nucleus accumbens and STR (Fig. 3).
Influence of 1BnTIQ on the ratio of the rate of dopamine catabolism (MAO-dependent oxidation) expressed as [DOPAC]/[DA] × 100 in different dopaminergic structures: nucleus accumbens (NAS); STR, substantia nigra (S. Nigra). 1BnTIQ was administered once (50 mg/kg i.p.) or for ten consecutive days. Rats were killed 120 min after injection of 1BnTIQ. Data are means ± SEM. The number (N) of animals in SAL (control) group, N = 10; 1BnTIQ acute, N = 6; 1BnTIQ chronic, N = 6. **P < 0.01 difference from control group (SAL); ## P < 0.01 difference from 1BnTIQ acute group, Fisher LSD test
Influence of 1BnTIQ on the ratio of the rate of dopamine catabolism (COMT-dependent O-methylation) expressed as [3-MT]/[DA] × 100 in different dopaminergic structures: nucleus accumbens (NAS); STR, substantia nigra (S. Nigra). 1BnTIQ was administered once (50 mg/kg i.p.) or for ten consecutive days. Rats were killed 120 min after injection of 1BnTIQ. Data are means ± SEM. The number (N) of animals in SAL (control) group, N = 10; 1BnTIQ acute group, N = 6; 1BnTIQ chronic group N = 6. *P < 0.05, difference from control group (SAL); # P < 0.05 difference from 1BnTIQ acute group, Fisher LSD test
In contrast to the above findings, 1BnTIQ (50 mg/kg i.p.) administered daily for 10 days produced a considerably weaker biochemical effect compared to the effect after a single injection. Although the concentration of dopamine was reduced, but the magnitude of the changes was considerably less (~30%), compared to a single application. A level of statistical significance was found in the nucleus accumbens only (Table 1). Concentrations of DOPAC and 3-MT returned to the control level after repeated 1BnTIQ administration; however, the level of HVA remained elevated in STR (Table 1).
The rate of total dopamine catabolism ([HVA]/[DA]) was increased in substantia nigra only (Table 1), and the rates of oxidative and O-methylation catabolic pathways for dopamine were unchanged after chronic 1BnTIQ administration (Figs. 2 and 3).
In contrast to its effect on dopamine, a single dose of 1BnTIQ (50 mg/kg i.p.) did not alter the serotonin level, although the concentration of the 5-HT metabolite 5-hydroxyindolacetic acid (5-HIAA) was elevated (by 80%, P < 0.01). The rate of serotonin metabolism, assessed from the ratio of [5-HIAA]/[5-HT], was increased by approximately 200% (P < 0.01). Following chronic 1BnTIQ administration, there were no alterations in biogenic amines and their metabolites (Table 2).
The Effect of 1BnTIQ Administration on Dopamine Release In vivo: A Microdialysis Study in Rat STR
An in vivo microdialysis study showed that a single dose of 1BnTIQ (50 mg/kg i.p.) produced long-lasting inhibition of dopamine release, reducing the striatal extraneuronal level of dopamine by 20–40% (P < 0.01; Fig. 4). Concentrations of the dopamine metabolites DOPAC and HVA, in contrast, were significantly elevated (Fig. 4).
The effect of 1BnTIQ on dopamine release in rat STR: in vivo microdialysis study. Control samples were collected from “−60” to “0,” while 1BnTIQ (50 mg/kg) was administered i.p., and the dialysate was collected every 20 min. Concentrations of dopamine (DA) and its metabolites (DOPAC and HVA) were assessed. Data are means ± SEM. The number (N) of rats per group (N = 6); *P < 0.05, **P < 0.01 difference from the basal value, Fisher LSD test
Discussion
1BnTIQ is an endogenous neurotoxin which causes parkinsonism-like symptoms. The present in vivo microdialysis studies have shown for the first time that 1BnTIQ significantly reduced dopamine release into the synaptic cleft of rat STR. The ex vivo biochemical data clearly demonstrate that 1BnTIQ increased the rate of dopamine metabolism in dopaminergic structures of the rat brain (Table 1).
In vivo and ex vivo experiments suggest that 1BnTIQ induces a decrease in dopamine both in neurons and in the synaptic cleft, and produces greater oxidation, and thus, causes damage to dopamine cells. Such mechanisms were observed in dopamine-mediated oxidative stress induced in rats by low-dose reserpine (Spina and Cohen 1989), a model substance frequently used to produce parkinsonism in animals (Colpaert 1987; Lorenc-Koci et al. 1995). Reserpine inhibits dopamine storage in synaptic vesicles by interfering with the vesicular monoamine transporter (VMAT) (Henry et al. 1994). Reuptake mechanisms for monoamines utilize unique presynaptic transporters in each different monoamine neuron but the same VMAT. 1BnTIQ inhibits dopamine uptake through the dopamine transporter (DAT) expressed in HEK293 cells (Okada et al. 1998) as well as in rat STR (Patsenka et al. 2004), indicating that 1BnTIQ gains entry to dopamine neurons through the DAT whereby it is able to access the VMAT. As a consequence, oxidative catabolism of cytosolic dopamine by MAO is accelerated, resulting in lowered cytosolic dopamine and simultaneous formation of acidic metabolites (DOPAC), and cellular hydrogen peroxide. This action mimics, to some extent, the increased turnover of dopamine in surviving dopaminergic terminals in PD (Antkiewicz-Michaluk et al. 1999). DAT is a likely candidate for the selective transport of 1BnTIQ into dopaminergic neurons, and its neurotoxicity may be mediated by DAT.
Moreover, it is notable that TIQs derivatives have the potential to inhibit mitochondrial respiration, and that among them, 1BnTIQ is a more potent inhibitor comparable to MPTP (Morikawa et al. 1998), the exogenous neurotoxin able to induce parkinsonism in primates (Langston et al. 1983). In fact, MPTP itself is a pro-toxin, oxidized by MAOB in brain to 1-methyl-4-phenylpyridinium ion (MPP+), the overtly neurotoxic species (Chiba et al. 1984; Fuller et al. 1988). In contrast to MPTP, 1BnTIQ and other derivatives of TIQ (e.g., salsolinol) are not direct substrates for MAOB. Prior to their oxidization by MAOB, the TIQs are N-methylated by N-methyltrasferase to form the neurotoxic N-methylisoquinolinium ion, chemically similar to MPP+ (Naoi et al. 1989). 1BnTIQ and salsolinol act more rapidly than MPTP and produce irreversible neurotoxic changes mainly restricted to the dopaminergic system, even after a single dose (Burns et al. 1985), but they do not produce immediate neurotoxicity (Antkiewicz-Michaluk et al. 2000, 2001; Lorenc-Koci et al. 2004). As so, endogenous derivatives of TIQ (e.g., 1BnTIQ) may offer a better model of PD, exhibiting slowly developing neurodegenerative changes.
Our present behavioral data demonstrate that 1BnTIQ decreased the exploratory locomotor activity (from 0 to 30 min); however, the basic activity did not differ from control group (Fig. 1). In fact, it should be taken into account that the basic locomotor activity of control group was low, and in such case it would be difficult for any treatment makes it lower. The depression of exploratory locomotor activity evoked by 1BnTIQ may be connected with a prompt and long-lasting fall of an extraneuronal dopamine concentration demonstrated in microdialysis study (Fig. 4). Further we demonstrated that 1BnTIQ decreases motor function in rats (Antkiewicz-Michaluk et al. 2001) and, when given chronically to nonhuman primates, produces symptoms of PD such as akinesia, resting tremor, and rigidity (Kotake et al. 1996). CSF levels of 1BnTIQ are reportedly thrice higher in PD patients versus non-PD subjects (Kotake et al. 1995).
Ex vivo Biochemical Studies
Ex vivo biochemical studies confirmed our earlier findings, demonstrating that 1BnTIQ strongly alters dopamine metabolism and its catabolic pathways (Antkiewicz-Michaluk et al. 2001). A single dose of 1BnTIQ produced a dramatic fall in dopamine level in all brain regions studied (by ~60%, Table 1) and increased the concentration of its metabolites, DOPAC and HVA. Additionally, 1BnTIQ markedly reduced the level of the extraneuronal dopamine metabolite 3-MT in nucleus accumbens and STR, by 80 and 70%, respectively (Table 1). 3-MT is considered to be the most reliable indicator of dopamine release into the synaptic cleft (Egan et al. 1991; Karoum et al. 1994), and its fall after 1BnTIQ administration, observed in this article, indicates the inhibition of dopamine release into the extraneuronal space. However, ex vivo studies were carried out 2 h after 1BnTIQ administration, the fall of 3-MT as the indicator of dopamine release is in agreement with an in vivo microdialysis study demonstrated in this article. The results obtained from this study have showed immediate (during 20 min), and a long-lasting (up to 240 min) inhibitory effect of 1BnTIQ on dopamine release into synaptic cleft within the STR (Fig. 4).
Dopamine is catabolized to HVA, its terminal metabolite, along two pathways: (a) intraneuronal N-oxidation by MAOB to form DOPAC, and (b) extraneuronal O-methylated by COMT to form 3-MT. In addition, a major portion of dopamine released into the synaptic cleft is “taken up” by the DAT into neurons and then catabolized by MAOB, which is located on mitochondrial membranes. Formation of DOPAC through the N-oxidized pathway is accompanied by generation of free radicals (H2O2 and •OH). It is conceivable that a substance may protect dopamine neurons by shifting dopamine metabolism from the N-oxidation pathway to the O-methylation pathway. For example, 1MeTIQ, a structural relative of 1BnTIQ, inhibits MAOB and has neuroprotective properties (Magyar et al. 1998; Stern 1998; Antkiewicz-Michaluk et al. 2003, 2004; Patsenka and Antkiewicz-Michaluk 2004).
As shown in this study, 1BnTIQ, which is potentially neurotoxic, evokes strong (about threefold) activation of the oxidative MAO-dependent catabolic pathway, as expressed by the [DOPAC]/[DA] ratio. In parallel, 1BnTIQ significantly inhibits COMT-dependent O-methylation, as expressed by the [3-MT]/[DA] ratio (Figs. 2 and 3). The rate of total dopamine metabolism, expressed as the [HVA]/[DA] ratio, is strongly accelerated in all the structures studied (from eight to nine times in the nucleus accumbens and STR, and threefold in the substantia nigra). Thus, 1BnTIQ exerts such effects which could produce neurotoxicity.
Summarizing, our biochemical data obtained from ex vivo (brain structures) experiments have shown that 1BnTIQ produced an activation of dopamine metabolism by a strong activation of dopamine N-oxidation (an increase of DOPAC concentration) along an MAO-dependent catabolic pathway. Oxidation of dopamine is directly connected with free radical production (H2O2, •OH), oxidative stress, and finally with cell death and neurodegeneration (Schapira et al. 1990; Adams and Odunze 1991; Miller et al. 1996; Chan 1998; Dykens 1999).
In contrast to dopamine, the concentration of serotonin was not altered, but the level of its metabolite 5-HIAA was significantly increased (Table 2). Thus, the effect of 1BnTIQ was selective for dopaminergic neurons and did not affect serotonin concentrations within the STR. However, the rate of serotonin metabolism was accelerated.
Interestingly, the biochemical effects of chronic administration of 1BnTIQ are considerably weaker, and the fall in dopamine levels, though similar in all the investigated structures (about 30%), reaches the significance level only in the nucleus accumbens. Similarly, the rate of dopamine metabolism after chronic 1BnTIQ administration returns to the control level in the nucleus accumbens and STR, but is still significantly accelerated (about twofold) in the substantia nigra. This pattern of changes suggests that during chronic 1BnTIQ administration some tolerance to its dopamine-depressing effect develops, whereas impairment of dopamine synthesis ensues.
In order to summarize our results, we indicate that (1) the STR and nucleus accumbens represent brain regions in which the depression of dopamine produced by 1BnTIQ is most strongly expressed; and (2) this effect is specific to dopaminergic neurons, as 1BnTIQ does not alter the serotonin concentration in STR. However, the rate of serotonin metabolism is accelerated (Tables 1 and 2).
In vivo Microdialysis Study
Our in vivo microdialysis study has demonstrated an effect of 1BnTIQ on dopamine release in rat STR. All data from ex vivo studies with 1BnTIQ show such a profound increase in DOPAC and HVA levels, and in the rate of dopamine metabolism, thereby implicating dopamine release from nerve endings. However, the present microdialysis study has yielded interesting, but unexpected results, and clearly demonstrates that systemic 1BnTIQ markedly reduces dopamine release into the synaptic cleft of freely moving rats—producing a long-lasting decrease in extracellular dopamine (Fig. 4). The concentration of the dopamine metabolites DOPAC and HVA was prominently elevated. Experiments of this type provide neurochemical evidence that most probably, 1BnTIQ impairs dopamine storage, leading to enhanced dopamine catabolism along the MAO-dependent oxidative pathway and thus increasing the production of an intraneuronal metabolite of dopamine, DOPAC and free radicals.
Recent findings have led to the proposal that 1BnTIQ activates apoptotic signaling pathways and increases α-synuclein expression, thereby causing nuclear damage to human dopaminergic cells (Shavali and Ebadi 2003; Shavali et al. 2004). Similarly, in organotypic slice co-cultures of mesencephalon and STR, 1BnTIQ reduced dopamine levels and promoted cell death (Kotake et al. 2003). Altered gene expression and abnormal metabolism of α-synuclein may lead to the formation of Lewy bodies in PD, as well as in other neurodegenerative disorders (Spillantini and Goedert 2000). Oxidative stress and glutathione depletion are likely to be factors that contribute to the increase in α-synuclein levels occurring in the pathophysiology of PD (Schulz et al. 2000; Adamczyk et al. 2005).
Summary
In vivo microdialysis studies have shown that administration of 1BnTIQ produces immediate (after 20 min) and a long-lasting (up to 240 min) inhibitory effect on dopamine release into synaptic cleft within the STR. 1BnTIQ affecting dopamine metabolism and its catabolic pathways, fortifying the MAO-dependent oxidation pathway and inhibiting the COMT-dependent O-methylation pathway. The multiple administration of 1BnTIQ leads to the development of tolerance to its dopamine depressing effect while the impairment of dopamine synthesis is still found to persist in brain structures. Since 1BnTIQ is an endogenous compound, it may be one of the factors responsible for idiopathic PD.
References
Abe K, Taguchi K, Wasai T, Ren J, Utsunomiya I, Shinohara T, Miyatake T, Sano T (2001) Biochemical and pathological study of endogenous 1-benzyl-1,2,3,4-tetrahydroisoquinoline-induced parkinsonism in the mouse. Brain Res 907:134–138
Adamczyk A, Solecka J, Strosznajder JB (2005) Expression of α-synuclein in different brain parts of adult and aged rats. J Physiol Pharmacol 56:29–37
Adams JD Jr, Odunze IN (1991) Oxygen free radicals and Parkinson’s disease. Free Radic Biol Med 10:161–169
Alam ZI, Jenner A, Daniel SE, Lees AJ, Cairns N, Marsden CD, Jenner P, Halliwell B (1997) Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8-hydroxyquanine levels in substantia nigra. J Neurochem 69:1196–1203
Antkiewicz-Michaluk L, Krygowska-Wajs A, Michaluk J, Romańska I, Szczudlik A, Vetulani J (1999) Plasticity of extrapyramidal dopamine system in Parkinson`s disease: a postmortem study. Neurosci Res Commun 25:97–109
Antkiewicz-Michaluk L, Romańska I, Papla I, Michaluk J, Bakalarz M, Vetulani J, Krygowska-Wajs A, Szczudlik A (2000) Neurochemical changes induced by acute and chronic administration of 1,2,3,4-tetrahydroisoquinoline and salsolinol in dopaminergic structures of rat brain. Neuroscience 96:59–64
Antkiewicz-Michaluk L, Michaluk J, Mokrosz M, Romańska I, Lorenc-Koci E, Otha S, Vetulani J (2001) Different action on dopamine catabolic pathways of two endogenous 1,2,3,4-tetrahydroisoquinolines with similar antidopaminergic properties. J Neurochem 78:100–108
Antkiewicz-Michaluk L, Karolewicz B, Romańska I, Michaluk J, Bojarski A, Vetulani J (2003) 1-Methyl-1,2,3,4-tetrahydroisoquinoline protects against rotenone-induced mortality and biochemical changes in rat brain. Eur J Pharmacol 466:263–269
Antkiewicz-Michaluk L, Wardas J, Michaluk J, Romańska I, Bojarski A, Vetulani J (2004) Protective effect of 1-methyl-1,2,3,4-tetrahydroisoquinoline against dopaminergic neurodegeneration in the extrapyramidal structures produced by intracerebral injection of rotenone. Int J Neuropsychopharmacol 7:155–163
Burns RS, Lewitt PA, Ebert H, Pakkenberg H, Kopin IJ (1985) The clinical syndrome of striatal dopamine deficiency: parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-terahydropyridine (MPTP). N Engl J Med 312:1418–1421
Cannon JG, Webster GL (1958) Polyphosphoric acid in the Bischler-Napieralski reaction. J Am Pharm Assoc 47:353–358
Chan PH (1998) Oxygen radical mechanisms in cerebral ischemia and reperfusion, in ischemic stroke. In: Hsu CY (ed) Basic mechanisms to new drug development. Monogr clin neurosci, vol 16. Krager, Basel, pp 14–17
Chiba K, Trevor A, Castagnoli N Jr (1984) Metabolism of the neurotoxic tertiary amine, MPTP, by brain monoamine oxidase. Biochem Biophys Res Commun 120:574–578
Colpaert FC (1987) Pharmacological characteristics of tremor, rigidity and hypokinesia induced by reserpine in rat. Neuropharmacology 26:1431–1440
Dexter DT, Carter CJ, Wells FR, Javoy-Agid F, Agid Y, Lees A, Jenner P, Marsden CD (1989) Basal lipid peroxidation in substantia nigra is increased in Parkinson’s disease. J Neurochem 52:381–389
Dykens JA (1999) Free radicals and mitochondria dysfunction in exitotoxicity and neurodegenerative disease. In: Cell death and diseases of the nervous system. Humana Press, Totowa, NJ, pp 45–68
Egan MF, Karoum F, Wyatt RJ (1991) Effects of acute and chronic clozapine and haloperidol administration on 3-methoxytyramine accumulation in the rat prefrontal cortex, nucleus accumbens and striatum. Eur J Pharmacol 199:191–199
Fuller RW, Hemrick-Luecke SK, Perry KW (1988) Deprenyl atagonizes acute lethality of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. J Pharmacol Exp Ther 247:531–535
Hao R, Ebadi M, Pfeiffer RF (1995a) Selegiline protects dopaminergic neurons in culture from toxic factor(s) present in the cerebrospinal fluid of patients with Parkinson’s disease. Neurosci Lett 200:77–80
Hao R, Norgen RB Jr, Lau YS, Pfeiffer RF (1995b) Cerebrospinal fluid of Parkinson’s disease patients inhibits the growth and function of dopaminergic neurons in culture. Neurology 45:138–142
Henry JP, Botton D, Sagne C, Isambert MF, Desnos C, Blanchard V, Raisman-Vozari R, Krejci E, Massoulie J, Gasnier B (1994) Biochemistry and molecular biology of the vesicular monoamine transporter from chromaffin granules. J Exp Biol 196:251–262
Inwang EE, Mosnaim AD, Sabelli HC (1973) Isolation and characterization of phenylethyleamine and phenylethanolamine from human brain. J Neurochem 20:1469–1473
Karoum F, Chrapusta SJ, Egan MF (1994) 3-Methoxytyramine is the major metabolite of released dopamine in the rat frontal cortex: reassessment of the effects of antipsychotics on the dynamics of dopamine release and metabolism in frontal cortex, nucleus accumbens and striatum by a simple two pool model. J Neurochem 63:972–978
Kostrzewa RM, Kostrzewa JP, Brus R (2000) Dopaminergic denervation enhances susceptibility to hydroxyl radicals in rat neostriatum. Amino Acids 19:183–199
Kotake Y, Tasaki Y, Makino Y, Otha S, Hirobe M (1995) 1-Benzyl-1,2,3,4-tetrahydroisoquinoline as a parkinsonism-inducing agent: a novel endogenous amine in mouse brain and parkinsonian CSF. J Neurochem 65:2633–2638
Kotake Y, Yoshida M, Ogawa M, Tasaki Y, Hirobe M, Ohta S (1996) Chronic administration of 1-benzyl-1,2,3,4-tetrahydroisoquinoline, an endogenous amine in mouse brain and parkinsonian CSF. Neurosci Lett 217:69–71
Kotake Y, Ohta S, Kanazawa I, Sakurai M (2003) Neurotoxicity of an endogenous brain amine, 1-benzyl-1,2,3,4-tetrahydroisoquinoline, in organotypic slice co-culture of mesencephalon and striatum. Neurosience 117:63–70
Langston JW, Ballard P, Tetrud JW, Irvin I (1983) Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219:979–980
Lorenc-Koci E, Ossowska K, Wardas J, Wolfarth S (1995) Does reserpine induce parkinsonian rigidity? J Neural Transm Park Dis Dement Sect 9:211–223
Lorenc-Koci E, Antkiewicz-Michaluk L, Wardas J, Zapała M, Wierońska J (2004) Effect of 1,2,3,4-tetrahydroisoquinoline administration under conditions of CYP2D inhibition on dopamine metabolism, level of tyrosine hydroxylase protein and the binding of [3H]GBR 12, 935 to dopamine transporter in the rat nigrostriatal dopaminergic system. Brain Res 1009:67–81
Magyar K, Szende B, Lengyel J, Tarczali J, Szatmary I (1998) The neuroprotective and neuronal rescue effects of (-)-deprenyl. J Neural Transm Suppl 52:109–123
Miller J, Selhub J, Joseph J (1996) Oxidative damage caused by free radicals produced during catecholamine autooxidation: protective effects of O-methylation and melatonin. Free Radic Biol Med 21:241–249
Mochizuki H, Goto K, Mori H, Mizuno Y (1996) Histochemical detection of apoptosis in Parkinson’s disease. J Neurol Sci 137:120–123
Mogi A, Togari A, Kondo T, Mizuno Y, Komure O, Kuno S, Ichinose H, Nagatsu T (2000) Caspase activities and tumor necrosis factor receptor R1 (p55) level are elevated in the substantia nigra from Parkinsonian brain. J Neural Transm 107:335–341
Morikawa N, Naoi M, Maruyama W, Ohta S, Kotake Y, Kawai H, Niwa T, Dostert P, Mizuno Y (1998) Effects of various tetrahydroisoquinoline derivates on mitochondrial respiration and the electron transfer complexes. J Neural Transm 105:677–688
Moser A, Kompf D (1992) Presence of methyl-6, 7-dihydroxy-1,2,3,6-tetrahydroisoquinolines, derivatives of the neurotoxin isoquinoline, in parkinsonian lumbar CSF. Life Sci 50:1885–1891
Nagatsu T, Yoshida M (1988) An endogenous substance of the brain, tetrahydroisoquinoline, produces parkinsonism in primates with decreased dopamine, tyrosine hydroxilase and biopterin in the nigrostriatal regions. Neurosci Lett 87:178–182
Naoi M, Matsuura S, Parvez H, Takahashi T, Hirata Y, Minami M, Nagatsu T (1989) Oxidation of N-methyl-1,2,3,4-tetrahydroisoquinoline into the N-methyl-isoquinolinium ion by monoamine oxidase. J Neurochem 52:653–655
Naoi M, Maruyama W, Akao Y, Yi H (2002) Dopamine-derived endogenous N-methyl-(R)-salsolinol: its role in Parkinson’s disease. Neurotoxicol Teratol 24:579–591
Nowak P, Szczerbak G, Biedka I, Drosik M, Kostrzewa RM, Brus R (2006) Efect of ketanserin and amphetamine on nigrostriatal neurotransmission and reactive oxygen species in parkinsonian rats. In vivo microdialysis study. J Physiol Pharmacol 57:583–597
Ohta S, Kohno M, Makino Y, Tachikawa O, Hirobe M (1987) Tetrahydroisoquinoline and 1-methyltetrahydroisoquinoline are present in the human brain: relation to Parkinson’s disease. Biomed Res 8:453–456
Okada T, Shimada S, Sato K, Kotake Y, Kawai H, Ohta S, Tohyama M, Nishimura T (1998) Tetrahydropapaveroline and its derivatives inhibit dopamine uptake through dopamine transporter expressed in HEK293 cells. Neurosci Res 30:87–90
Patsenka A, Antkiewicz-Michaluk L (2004) Inhibition of rodent brain monoamine oxidase and tyrosine hydroxylase by endogenous compounds 1,2,3,4-tetrahydroisoquinoline alkaloids. Pol J Pharmacol 56:727–734
Patsenka A, Michaluk J, Antkiewicz-Michaluk L (2004) 1,2,3,4-Tetrahydroisoquinoline alkaloids as endogenous inhibitors of brain monoamine oxidase, tyrosine hydroxylase and uptake of monoamines: in vitro study. In 13th international symposium on molecular and physiological aspects of regulatory processes of the organism, Krakow, Poland, Materials 2004, p 344
Schapira AH, Mann VM, Cooper JM, Dexter D, Daniel SE, Jenner P, Clark JB, Marsden CD (1990) Anatomic and disease specificity of NADH CoQ1 reductase (complex I) deficiency in Parkinson’s disease. J Neurochem 55:2142–2145
Schulz JB, Lindenau J, Seyfried J, Dichgans J (2000) Glutathione, oxidative stress and neurodegeneration. Eur J Biochem 267:4909–4911
Shavali S, Ebadi M (2003) 1-Benzyl-1,2,3,4-tetrahydroisoquinoline (1BnTIQ), an endogenous neurotoxin, induces dopaminergic cell death through apoptosis. Neuro Toxicol 24:417–424
Shavali S, Carlson EC, Swinscoe JC, Ebadi M (2004) 1-Benzyl-1,2,3,4-tetrahydroisoquinoline, a Parkinsonism-inducing endogenous toxin, increases α-synuclein expression and causes nuclear damage in human dopaminergic cells. J Neurosci Res 76:563–572
Spillantini MG, Goedert M (2000) The alpha-synucleinopathies: Parkinson’s disease, dementia with Lewy bodies, and multiple system atrophy. Ann N Y Acad Sci 920:16–27
Spina MB, Cohen G (1989) Dopamine turnover and glutathione oxidation: implications for Parkinson disease. Proc Natl Acad Sci U S A 86:1389–1400
Stern G (1998) Neuroprotection by selegiline and other MAO inhibitors. J Neural Transm Suppl 52:99–107
Yamakawa T, Kotake Y, Fuijtani M, Shintani H, Makino Y, Otha S (1999) Regional distribution of parkinsonism-preventing endogenous tetrahydroisoquinoline derivatives and an endogenous parkinsonism-preventing substance-synthesizing enzyme in monkey brain. Neurosci Lett 276:68–70
Yoshida M, Niwa T, Nagatsu T (1990) Parkinsonism in monkeys produced by chronic administration of an endogenous substance of the brain, tetrahydroisoquinoline: the behavioral and biochemical changes. Neurosci Lett 119:109–113
Acknowledgments
Thanks are due to Dr Jan Boksa (Department of Medicinal Chemistry, Institute of Pharmacology PAS, Krakow, Poland) for the synthesis of 1BnTIQ. Gratefully acknowledge the technical assistance of Maria Kafel and Krzysztof Michalski. This study was supported by Polish Committee of Scientific Research, Grant No. 2 PO5A 030 29 and by the statutory funds of the Institute of Pharmacology, Polish Academy of Sciences, Krakow, Poland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wąsik, A., Romańska, I. & Antkiewicz-Michaluk, L. 1-Benzyl-1,2,3,4-Tetrahydroisoquinoline, an Endogenous Parkinsonism-Inducing Toxin, Strongly Potentiates MAO-Dependent Dopamine Oxidation and Impairs Dopamine Release: Ex vivo and In vivo Neurochemical Studies. Neurotox Res 15, 15–23 (2009). https://doi.org/10.1007/s12640-009-9001-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-009-9001-9