Abstract
Bulinus are intermediate snail hosts of Schistosoma haematobium. Despite their vectorial role, the transmission dynamics and infectivity of these intermediate snail hosts remain understudied in the Ase River. This longitudinal study evaluated the geospatial and seasonal transmission patterns and infectivity of three S. haematobium vectors between November 2020 and October 2022 in the Ase River catchment, Delta State, Nigeria. Eleven (11) geospatial water contact coordinates were mapped for monthly spatiotemporal collection of Bulinus species along the Ase River and its catchment, for two years. Snail sampling was performed for 45 min at each study site using scooping/hand-picking techniques and subsequently counted, identified and recorded. Snails of the Bulinus genus were individually placed in a beaker containing distilled water and exposed to light to shed cercariae which were identified to be human schistosome type. The number of infected snails for each month and season was also documented to analyze the spatiotemporal and seasonal transmission dynamics of infectivity. Out of the 2345 Bulinus snails collected, a total of 41.45% were found to be infected with S. haematobium. The monthly infectivity of Bulinus snails varied significantly (P < 0.05) throughout the study period (P = < 0.0001; F = 23.11; df = 11). Further analysis showed a strong significant association (χ2 = 23.57; df = 11; p = 0.015) between the study years. The Principal Component Analysis (PCA) results suggest that Bulinus infectivity within the Ase River catchment area was primarily associated with the months of February and January. B. truncatus consistently had the highest transmission potential, followed by B. globosus and B. senegalensis. ANOVA confirms that the monthly/study site infectivity and transmission potential in B. truncates, B. globosus and S. senegalensis were statistically, significant (P < 0.05). These results demonstrated a clear distinction in the patterns and relationships between the different months in terms of snail infectivity and seasonal transmission potential. This understanding will help in the continuous monitoring and targeted interventions to control schistosomiasis transmission in Ase River.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schistosoma haematobium is endemic in Delta State, Nigeria, particularly in the Ase River catchment as reported by Ito (2019); Emukah et al. (2012); Onyeneho et al. (2010); Ekwunife et al. (2009); Nwabueze and Opara (2007) where they are intermediately hosted by Bulinus truncatus, B. globosus and B. forskalii. The transmission and focal distribution of schistosomiasis is spatiotemporally restricted to water bodies inhabited by its obligate intermediate host snails and human water contact as documented by Merata et al. (2023); Nwoko et al. (2022a); Ito (2019); Ito and Egwunyenga 2015). Schistosoma haematobium is contracted by humans through exposure to polluted water. The parasite resides in freshwater snails, which discharge cercariae into the water. Upon contact with the contaminated water, the cercariae the intact skin and travel to the blood vessels surrounding the bladder and genital regions, where they develop into adult worms and lay eggs (Ito and Utebor 2023). These eggs are subsequently expelled in urine, contaminating the water anew and perpetuating the transmission cycle. Water sources such as rivers, lakes, and ponds inhabited by infected snails serve as the breeding grounds for this parasitic infection.
Ito (2019); Nduka et al. (2019); and Ito and Egwunyenga, (2015) have highlighted the endemicity of schistosomiasis in over 78 countries and considered its public health importance in the tropics and sub-tropical countries. In Sub-Sahara Africa (SSA), King and Dangerfield-Cha (2008) stated that approximately 436 million individuals are at risk of Schistosoma infections, of which 112 million are infected with S. haematobium. Similarly, Ito and Utebor (2023) and WHO (2013) documented that Nigeria accounts for the largest (29 million) number of infections with 101 million individuals at risk. While schistosomiasis is recognized as a debilitating disease, causing considerable morbidity, it is also estimated that 120 million individuals suffer severe complications of urinary schistosomiasis with an annual mortality estimate of about 200,000 worldwide (Ezeh et al. 2019; WHO 2016).
Schistosomiasis transmission is correlated with freshwater intermediate snail hosts and requires humans contact with cercaria-infested freshwater. The geospatial distribution and transmission pattern of schistosomiasis has been reported by Ezinna et al. (2023) and Amoah et al. (2017) to be determined by abiotic and biotic factors. The persistent transmission of schistosomiasis in Nigeria and other sub-Saharan Africa is influenced majorly by the proximity to water bodies, distribution of snail intermediate hosts, recreational/occupational activities, poverty, poor sanitation, lack/dilapidated sanitary infrastructure, none availability of potable water supply and climate change as opined by Alade et al. (2023); Ito et al. (2023); Ito (2019); Nduka (2019).
The impact of seasons on disease transmission cannot be over-emphasized (Nwoko et al. 2023; Ito and Egwunyenga 2023; Ito et al. 2023; Manyangadze et al. 2021; Amoah et al. 2017). Studies on the geospatial distribution of snails and infectivity are lacking in Delta State, Nigeria making the control and prevention of snail-borne disease difficult in affected communities. Thus, it is important to identify Schistosoma transmission sites and their dynamics in the Ase River catchment Delta State Nigeria for disease intervention and transmission interruptions. Specifically, this study is designed to: (i) Identify the geospatial transmission hotspots of S. haematobium in the Ase River and (ii) Understand snail transmission dynamics and cercarial infection rates. This could enhance an efficient resource allocation for targeted prevention and control interventions in specific transmission foci.
Materials and methods
Description of study area
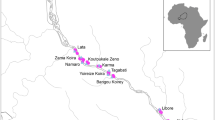
The Ase River is one of several rivers that drain the recent delta top landforms of the western Niger Delta and it is a coalesce of many tributaries originating from the southern and western slopes of the Asaba Plateau. The tributaries include the Oboshi River, which borders the Ibusa sub-urban municipal on the west, the Atakpo Creek, which borders the settlement on the east; the Nooni River, which runs through Nsukwa and Ogwashi-Uku west; while the tributaries headwaters of the Adofi River are at Ejeme Aniogor and Agbor Aladinma (Fig. 1). These tributaries meet at the southern part of Iselegwu to form the main trunk of Ase River about 292 km in length which shares a flood plain with the River Niger and flows southwards to join the Nikeroga River in the western branch while the eastern branch of the Niger River terminates at the Nun River (Ito 2017). The Ase River further flows through Ashaka, Igbuku, Ibrede, Awah, Lagos-Iyede, Iyede-Ame, Onogboko, Itobi-Ige, Ivrogbo, Ibredeni and Ase (where the river derived its name) communities which correspond to the eleven (11) geospatial sampling sites in this study. Ase is a confluence community with surface and groundwater intrusion from the Niger River. These geospatial study locations were selected based on proximity to human dwellings and human water contact sites. The population size of Ashaka, Igbuku, Ibrede, Awah, Lagos-Iyede, Iyede-Ame, Onogboko, Itobi-Ige, Ivrogbo, Ibedeni, and Ase are 2,118, 14,500, 1518, 1149, 1331, 3046, 1763, 1104, 2217, 1238, and 3217 inhabitants respectively (NPC 2006). These communities are tropical, structurally rural and typified with lowland areas subjected to seasonal flooding interspersed with streams, lakes, canals and rivulets where faecal and other anthropogenic waste are deposited (Fig. 1). Topographically, the study area falls within 10 m at Ase study site to 22 m (at Igbuku) elevation above sea level. Rainfall is about 266.50 and 190.50-mm in the coastal and extreme northern areas respectively with precipitation peaking in July. This study area is characterized by two main seasons: dry season (November—March) and Wet season (April—October). The occupation of the inhabitants of the study area are mainly fishing, farming, snail (Pila ovata) hunting and a few artisans and civil servants. More worrisome and appalling is the reliance of the inhabitants of this catchment’s communities on perennial/intermittent streams/rivulets/rivers for hydration and domestic use.

(Source: Ito et al. 2023)
Physiographic Map of Ase River, showing the study stations in Delta State, Nigeria
Research design
The study employed a longitudinal (Nov. 2020—Oct. 2022) study design in eleven (11) geospatial coordinates (Table 1) to evaluate the spatiotemporal transmission patterns of S. haematobium by Bulinus species in the Ase River catchment using standard scooping/hand-picking techniques and cercaria shedding respectively.
Snail sampling techniques and identification
Using Scoop net/dip net, snail samples were collected from eleven (11) geospatial points for identification of transmission sites for a period of twenty-four (24) months. Using forceps and safety gloves, the snails were manually hand-picked and kept in a muslin bag. This was carried out at the Ase River banks and other parts of the Ase River Catchment frequently accessed by humans. The captured snails were taken to the Animal and Environmental Biology Laboratory at Delta State University Abraka where they were further identified using keys of Brown and Kristensen (1993).
Infectivity bioassay and cercaria identification
The snail species collected were first observed in beakers for cercarial shedding. To achieve this, each Bulinus snail was placed in a 20 ml beaker containing 2 ml of distilled water and exposed to light to induce cercarial shedding (Marco and Alan 2012; and Ito and Utebor 2023). After light exposure with evidences of cercariae, 1–2 drops of iodine solution were added to each beaker and left to settle for a minute to allow cercariae staining and sedimentation. The cercariae were identified morphologically using the descriptive identification key of Frandsen and Christensen (1984). Snail found shedding bifurcate cercariae of the human schistosome type was noted and recorded. Snails that tested negative for cercariae shedding during the initial light exposure were re-examined on the twelfth day for Schistosoma infectivity.
Method of data collection
The prevalence of S. haematobium infection in Bulinus snails and the cercarial output were determined and employed to establish the effect of season on transmission patterns. The Infectivity rate of Bulinus species was also calculated thus:
(Okafor 1990; Rudge et al. 2013).
The monthly and seasonal Transmission Potentials (TP) as developed by Chu and Dawood (1970) were adopted as shown below:
(b) Seasonal T.P. = Addition of the T.P.s of the months in a season.
Reliability and validity test
Prior to snail sample collection, a pilot study to identify urinary Schistosoma hot-spot was conducted among the inhabitants of the geospatially selected sites to ascertain the prevalence of S. haematobium. The few Bulinus samples collected during the pilot study also showed evidences of cercariae shedding when expose to light. This was achieved by employing a standardized protocol to assess and observe snail-related factors, such as transmission dynamics and infection rates. This iterative approach, ensures a consistent and reliable assessment of geospatial transmission hotspots and snail transmission dynamics.
Data analysis
Statistical analysis was conducted to compare the variations in infection prevalence among different Bulinus species using chi-square and ANOVA. A significance level of p = 0.05 was set and values below this threshold were deemed significant and indicative of a potential risk factor. The obtained seasonal and communal prevalence values were subjected to rigorous examination using specialized software such as Graph-Pad Prism and PAST version 4.11. The software facilitated the application of suitable statistical tests, such as chi-square (χ2) and ANOVA, to identify noteworthy differences in the data, ensuring robust and reliable analysis.
Results
Monthly and interannual prevalence of S. haematobium in Bulinus vectors
Results of the monthly and interannual prevalence variations of S. haematobium infections in the Bulinus species are presented in Table 2. Generally, a total of 972 (41.45%) of the 2345 Bulinus snails collected were infected with S. haematobium (Table 2). The monthly prevalence of S. haematobium infections in Bulinus varied throughout the study period with the highest prevalence of 69.81 and 75.00% observed in February and March of first and second year respectively. Statistically, significant differences (P = < 0.0001; F = 23.11; df = 11) in the monthly prevalence of S. haematobium in the intermediate host were noted in the first- and second-year with 95.68% variance. Table 2 also revealed that the second study year had the highest prevalence of 43.17% compared to the first year (39.51%). Chi-square showed that the association between the study years was strongly significant (χ2 = 23.57; df = 11; p = 0.015).
The Principal Component Analysis (PCA) reveals that the monthly/interannual prevalence of S. haematobium infections in Bulinus species in the Ase River catchment is primarily driven by PC1 (with an eigenvalue of 945.84), which is strongly influenced by January and February (Fig. 2). PC2 captured a smaller amount of variance and is most strongly associated with November and December with an eigenvalue of 31.01 (Fig. 2). Overall PCA reveals that the infection prevalence in the Ase River catchment is primarily characterized by strong seasonal patterns. These findings provide insights into the patterns and relationships of Bulinus infectivity between different Months.
Monthly/annual Bulinus species-specific infectivity
Table 3 displays the monthly and annual species-specific infectivity in different species of Bulinus snails. B. globosus had the highest annual infection rates of 53.86 and 44.44% in the first and second year, while B. senegalensis had the lowest rates of 26.84 and 21.78% in both years respectively. In specific months, B. globosus reached a peak infection rate of 77.05% in December of the second year, while B. truncatus had its highest rate of 76.47% in February of the first year (Table 3). The prevalence of S. haematobium infection in B. truncates (P = < 0.0001), B. globosus (P = 0.0014) and S. senegalensis (P = 0.0064) were statistically significant (P < 0.05) in the monthly prevalence with a variance of 96.14, 87.49 and 78.03% respectively but not in the annual prevalence (P > 0.05).
Geospatial prevalence of S. haematobium in Bulinus species in ase river catchment
The geospatial prevalence of S. haematobium infections varied among the three snail species (B. truncatus, B. globosus and B. senegalensis) across different sites during the study period (Table 4). The results also indicated variations in infection rates within and between years for each snail species. Overall, the infectivity increased slightly from the first year (36.59%) to the second year (40.51%) for B. truncates (Table 4). The highest infection rate for B. truncatus was observed at the Iyede-Ame study site with a value of 63.33 and 59.22% for the first and second years respectively. Similarly, B. globosus showed a significant increase in infection rates from the first year to the second year, with the highest rates observed at the Onogboko and Lagos-Iyede study sites in both years. On the other hand, B. senegalensis exhibited the lowest infection rates among the three snail species, with a decrease in infection rates from first-year to second year. However, the study sites' prevalence of S. haematobium infection in B. truncates (P = 0.0012), B. globosus (P = 0.0009) and S. senegalensis (P = 0.0172) were statistically significant (P < 0.05) with 89.26, 87.55 and 78.89% variance respectively.
Species-specific transmission potential (T.P) of S. haematobium in Ase River
Table 5 shows the T.P of S. haematobium by different Bulinus species across months and years. B. truncatus consistently had the highest T.P, followed by B. globosus and B. senegalensis. In first-year, B. truncatus had the highest T.P in January (23.66%) and December (16.03%), while B. globosus surpassed B. senegalensis in January (22.35%) and May (17.50%). In the second year, B. senegalensis showed higher T.P in January (38.64%) and February (20.45%), while B. globosus increased in May (10.14%). Statistical analysis confirmed significant differences (P < 0.05) in T.P among the species. However, yearly transmission potentials did not differ significantly.
Seasonal species-specific T.P of S. haematobium vectors in Ase River catchment
The results in Table 6 revealed distinct seasonal variations in the T.P of S. haematobium by different Bulinus species. During the dry season in November, B. truncatus exhibited a T.P of 7.44%, followed by B. globosus (12.73%) and B. senegalensis (5.66%). However, during the wet season, the T.P increased for all three species (Table 6). Specifically, the T.P of B. senegalensis (12.90%) higher than B. truncatus (12.20%) and B. globosus (11.66%) respectively. Monthly analysis of transmission potential for Bulinus species revealed distinct trends. In the dry season, B. truncatus, B. globosus and B. senegalensis had their highest T.P in January (Table 6). During the wet season, B. truncatus and B. globosus peaked in July, while B. senegalensis reached its peak T.P in May. Significant differences were observed between seasons (P < 0.05), but not across the years for all three species of Bulinus.
Seasonal T.P of S. haematobium infections by Bulinus species in Ase River
The results presented in Table 7 revealed the T.P of S. haematobium infections in Bulinus species from the Ase River Catchment. In the dry season, transmission potentials varied across the months, with November having the highest monthly transmission potential in first-year (8.97%) and subsequent months showing a decreasing trend. Second-year had lower T.P overall. The wet season also showed variations, with June and July having the highest monthly T.P in the first year (9.66 and 13.10% respectively), while the second year had higher T.P overall. The cumulative seasonal T.P for the two years was 55.04% in the dry season and 44.96% in the wet season. Generally, the seasonal T.P of the two years was 55.04 and 44.96% in dry and wet seasons respectively.
Vector-specific geospatial T.P of S. haematobium in Ase river catchment
Table 8 provide insights into the T.P of S. haematobium infections among different snail species across various locations and years. Ashaka consistently exhibited moderate T.P values for all three vector species. Igbuku had higher T.P values for B. truncatus in the first and second year but decreased in the second year. Conversely, Igbuku had low T.P values for B. globosus and B. senegalensis, except for a higher T.P value of B. globosus in First-year. Ibrede exhibited higher T.P values for B. truncatus and B. senegalensis in the second year. Awah had minimal T.P values for B. truncatus and B. senegalensis in the first year, indicating low T.P. The in-land communities (Lagos-Iyede, Iyede-Ame, Onogboko and Itobi-Ige) consistently maintain a very high T.P of S. haematobium infection. Specifically, Lagos-Iyede showed high T.P values for all three vector species, ranging from 16.43 to 20.45% in both years. Iyede-Ame exhibited the highest T.P values among all locations, particularly for B. truncatus and B. senegalensis in Second-year (29.46 and 34.09%, respectively). Onogboko displayed a mixed pattern, with B. truncatus having the highest T.P value of 23.66% in first-year, while B. senegalensis had its peak T.P value of 34.09% in in the second year (Table 8). Generally, Iyede-Ame had the highest geospatial T.P of 21.71% cercaria while Awah recorded 0.41%. The study sites exhibited significant differences (P < 0.05). However, no significant difference (P > 0.05) was noted in the yearly vector species T.P.
Discussion
Understanding the schistosomiasis transmission dynamics is crucial for effective control. Ito and Egwunyenga (2015) and Kristensen et al. (2013) opined that this knowledge helps in developing integrated control strategies for managing snail populations and reducing the spread of snail-borne diseases. This study provides important insights into the monthly and annual transmission dynamics of S. haematobium infections in the Bulinus species. The overall infectivity of 41.45% in the Bulinus species indicates a significant concern of schistosomiasis in the study area. These findings corroborate those of Nwoko et al. (2023), Pam et al. (2021), N’Guessan et al. (2017) and Mohammed (2017) in South Africa, Mauritania and Sudan respectively. Interannually, the first- and second-year recorded a prevalence of 39.51 and 43.17% respectively. The observed variations in prevalence indicate the presence of climatic and seasonal fluctuations in disease transmission (Ito and Egwunyenga 2023). The differences in prevalence between first-year and second-year could also be attributed to various factors such as climatic conditions, snail population dynamics and water quality reported by Nwoko et al. (2023) and Mareta et al. (2023). Additionally, the variations in prevalence rates between the first- and second year suggest potential temporal changes in disease transmission dynamics, which should be considered when designing long-term control strategies.
The higher prevalence from January to March suggests a potential peak in transmission, while the lower prevalence in September and October indicates reduced transmission during that period. This observation is contrary to the assertions of Amoah et al., (2017) who documented higher snail infectivity in September and October. The variations in snail infectivity across different months may be attributed to several factors, including climatic/environmental conditions, water quality and snail population dynamics as opined by Abe et al. (2017), Ito et al. (2023), and Ezinna et al. (2023). The higher prevalence rates observed during certain months such as February, highlight the need for targeted interventions during specific periods of the year.
Three Bulinus species (B. truncatus, B. senegalensis and B. senegalensis) known to be intermediate hosts for S. haematobium were collected from the Ase River catchment, Delta State, Nigeria. These snail assemblages are similar to a preliminary report by Fryer and Probert (1988) and Abe et al. (2017). The Bulinus-species-specific infectivity by S. haematobium fluctuated throughout the study period. For example, B. globosus had the highest infection rate in December of Second-year, with 77.05% of the examined snails infected. Contrarily, B. truncatus had its highest infection rate in February of the first year, with 76.47% of the snails infected. These variations in monthly prevalence may be attributed to various factors, such as environmental conditions and the presence of suitable habitats for snails and parasites (Mereta et al. 2019; Nwoko et al. 2023). Overall study revealed varying levels of S. haematobium infection rates among the three snail species and across different stations. These findings suggest that different snail species may have different transmission dynamics and should be targeted for specific control interventions.
This study highlights the temporal variation in transmission potential among the Bulinus species. The highest transmission potentials generally occurred during the dry seasons, which is consistent with the cercariae infectivity period of S. haematobium and its intermediate hosts. Contrarily, in the Ase River catchment, the dry season witness a low relative Bulinus abundance compared to the wet season as documented by Ito et al. (2023). B. truncatus consistently showed the highest transmission potential, suggesting its importance in maintaining the parasite's life cycle. B. globosus and B. senegalensis also played significant roles, albeit with lower transmission potentials. The observed variations in the transmission potential of S. haematobium by different Bulinus species can be attributed to several factors such as change in temperature and seasons (dry and wet seasons). Furthermore, the seasonal variations in transmission potential suggest the existence of specific environmental conditions or behavioral patterns that contribute to increased human exposure during certain months (Ito and Egwunyenga 2023; Ito et al. 2023; Manyangadze et al. 2021).
Previous studies by Posa and Sodhi (2006), Mereta et al. (2023), Ito et al. (2023) have established the impact of environmental factors such as vegetation, water quality and human water contact pattern on snail abundance and infectivity. The differences in transmission potential between years indicate the influence of environmental factors and population dynamics of the snail intermediate hosts (Nwoko et al. 2022a). The findings from this study highlight the dynamic nature of the species-specific transmission potential of S. haematobium by different Bulinus species in the study area. The variations observed in transmission potential between different months and years suggest the influence of seasonal factors, environmental conditions, increased snail activity, potential interactions between the parasite and intermediate host species and higher parasite burdens in humans (Ito 2019; Nwoko et al. 2022b).
The results demonstrate a clear distinction in transmission potentials between the dry and wet seasons. The dry season exhibited higher transmission potentials in the initial months, gradually decreasing towards the end. This may be attributed to factors such as temperature, rainfall patterns and water availability. The dry season in the study locations of Ase River is characterized by an increase in temperature. Elevated temperatures increase snail metabolic rate, fecundity and feeding frequency, reducing the duration of the development periods and increasing the number of generations per year as reported by Ito et al. (2023); De La Rocque et al. (2008) and Kristensen et al. (2001).
To control S. haematobium in the Ase River Catchment, targeted interventions are crucial. These include chemical control of snails through molluscicidal treatments and habitat modifications; health education programs can raise awareness and promote safe water practices; Mass Drug Administration (MDA) with praziquantel, especially for school-age children, is vital; improved sanitation and biological control methods can reduce transmission; seasonal interventions, community engagement, and robust monitoring systems are key for sustainable success. Adopting these measures, tailored to the area, will effectively combat schistosomiasis in the Ase River Catchment.
Conclusion
The results showed 41.45% infectivity of S. haematobium in snail vectors and are of a public health concern as water contact activities in the Ase River catchment can lead to an exponential prevalence of urogenital schistosomiasis. This study also revealed the geospatial hotspots and the dynamic nature of the species-specific transmission potential of S. haematobium by B. truncatus, B. globosus and B. senegalensis. The high transmission potential by B. truncatus and B. senegalensis in the dry season indicate their significance as potential contributors to the overall transmission of S. haematobium in the Ase River catchment. The variations observed in transmission potential between different months and years suggest the influence of seasonal factors, environmental conditions and potential interactions between the parasite and intermediate host species. These results demonstrate a clear distinction in transmission potentials between the dry and wet seasons which calls for the need for continuous monitoring and targeted interventions to control schistosomiasis transmission in the study area.
Data availability
This manuscript presents novel data from the Ase River catchment, Delta State, Nigeria.
References
Abe EM, Ombugadu A, Oluwole AS, Njila HL, Mogaji HO, Adeniran AA, Guo YH, Li SZ, Zhou XN, Ekpo UF (2017) Population abundance and bionomics of snail intermediate hosts of trematode parasites in Nasarawa State, Nigeria. Res J Parasitol 12:8–18
Alade T, Ta-Tang TH, Nassar SA, Akindele AA, Capote-Morales R, Omobami TB, Berzosa P (2023) Prevalence of S. haematobium and intestinal helminth infections among Nigerian School Children. Diagnostics 13(4):759. https://doi.org/10.3390/diagnostics13040759
Amoah LAO, Anyan WK, Aboagye-Antwi F, Abonie S, Tettey MD, Bosompem KM (2017) Environmental factors and their influence on seasonal variations of schistosomiasis intermediate snail hosts abundance in Weija Lake, Ghana. J Advocacy Res Educ 4(2):68–80
Brown DS, Kristensen TK (1993) A field guild to African Freshwater Snail: West African Species. Danish Bilharziasis Laboratory, pp 55
Chu KY, Dawood IK (1970) Cercarial transmission seasons of schistosoma mansoni in the Nile Delta Area. Bull World Health Organiz 42(4):575–580
De La Rocque S, Rioux JA, Slingenbergh J (2008) Climate change: effects on animal disease systems and implications for surveillance and control. Rev Sci Tech 27(2):339–354
Ekwunife CA, Ozumba NA, Eneanya CI (2008) Studies on the biology and population parameters of Bulinus globosus and Bulinus truncatus in the laboratory. Nigerian J Parasitol 29(1):11–14
Ekwunife CA, Agbor VO, Ozumba AN, Eneanya CI, Ukaga CN (2009) Prevalence of urinary schistosomiasis in Iyede-Ame community and environ in Ndokwa East Local Government Area, Delta State, Nigeria. Nigerian J Parasitol 30(1):2. https://doi.org/10.4314/njpar.v30i1.43986
Emukah E, Gutman J, Miri ES, Yinkore P (2012) Urine hem dipstick are useful in monitoring the impact of praziquantel treatment on S. haematobium in sentinel communities of Delta State, Nigeria. Acta Trop 122:126–131
Ezeh CO, Onyekwelu KC, Akinwale OP, Shan L, Wei H (2019) Urinary schistosomiasis in Nigeria: a 50-year review of prevalence, distribution and disease burden. La schistosomiase urinaire au Nigeria: un bilan de 50 ans sur la prévalence, la distribution et le fardeau de la maladie. Parasite 26:19. https://doi.org/10.1051/parasite/2019020
Ezinna EE, Obisike VU, Dike MC (2023) Fresh water snails (Bulinus and Lymnaea) in Canals in Imo State, Nigeria: their public health importance and implications for control. J Infect Dis Case Reports 4(1):1–6. https://doi.org/10.47363/jidscr/2023(4)170
Frandsen F, Christensen N (1984) An introductory guide to the identification of cercariae from African freshwater snails with special reference to cercariae of trematode species of medical and veterinary importance. Acta Trop 41:181–202
Fryer S, Probert A (1988) The cercarial output from three Nigerian bulinids infected with two strains of S. haematobium. J Helminthol 62(2):133–140. https://doi.org/10.1017/S0022149X00011378
Ito EE (2017) Survey of parasites of two fish species (Tilapia zillii and Clarias gariepinus) in Ase River Catchment, Delta State, Nigeria. J Coastal Life Med 5(10):417–421
Ito EE (2019) Hyper-endemicity of urinary schistosomiasis among school children in two communities in the Lower Niger-Delta, Nigeria. Nigerian J Parasitol 40(1):76–81
Ito EE, Egwunyenga AO (2015) Schistosomiasis: the aftermath of 2012 Floods in Delta State, Southern Nigeria. Int Med J 22(4):218–223
Ito EE, Egwunyenga AO (2023) Seasonal prevalence, intensity and risk factors of geohelminthiasis among children in Peri-Urban settings, Abraka, Southern, Nigeria. Nigerian J Parasitol 44(1):219–232
Ito EE, Utebor EK (2023) Anti-schistosoma efficacy of Occimum gratissimum (Scent Leaf) and Andrographis paniculata (King of Bitters) against S. haematobium Cercaria. In Press, Scientific African
Ito EE, Eze CN, Nduka FO (2023) Geospatial seasonal environmental impacts on the distribution and abundance of S. haematobium vectors in two districts of the Ase River Catchment, Delta State, Nigeria. Tropical Freshwater Biol 32:19–36
King CH, Dangerfield-Cha M (2008) The unacknowledged impact of chronic schistosomiasis. Chronic Illn 4(1):65–79
Kristensen TK, Malone JB, McCarroll JC (2001) Use of satellite remote sensing and geographic information systems to model the distribution and abundance of snail intermediate hosts in Africa: a preliminary model for Biomphalaria pfeifferi in Ethiopia. Acta Trop 79:73–78
Kristensen KT, Madsen H, Stensgaard AS (2013) The role of snails in schistosomiasis research—from taxonomy to forecasting disease outbreaks. In: Olsen A, Ørnbjerg N, Winkel K (eds) A succes story in Danish development aid DBL (1964–2012). Institute for Health Research and Development. University of Copenhagen, Denmark, pp 61–65
Manyangadze T, Chimbari MJ, Rubaba O, Soko W, Mukaratirwa S (2021) Spatial and seasonal distribution of Bulinus globosus and Biomphalaria pfeifferi in Ingwavuma, uMkhanyakude district, KwaZulu-Natal, South Africa: implications for schistosomiasis transmission at micro-geographical scale. Parasit Vectors 14(1):222. https://doi.org/10.1186/s13071-021-04720-7
Marco AAS, Alan LM (2012) Ecological aspect of Biomphalaria in endemic areas for schistosomiasis in Brazil. In: Mohammed BR (Eds.) Schistosomiasis. Available at http://www.interchopen.com/booksschistosomiasis.
Mereta ST, Bedewi J, Yewhalaw D, Mandefro B, Abdie Y, Tegegne D, Birke W, Mulat WL, Kloos H (2019) Environmental determinants of distribution of freshwater snails and trematode infection in the Omo Gibe River Basin, southwest Ethiopia. Infect Dis Poverty 8(1):93. https://doi.org/10.1186/s40249-019-0604-y
Mereta ST, Abaya SW, Tulu FD, Takele K, Ahmednur M, Melka GA, Nanyingi M, Vineer HR, Graham-Brown J, Caminade C, Siobhan MM (2023) Effects of land-use and environmental factors on snail distribution and trematode infection in Ethiopia. Tropical Med Infect Dis 8:154. https://doi.org/10.3390/tropicalmed8030154
Mohammed MHMA (2017) Distribution, ecological factors and trematode infectivity of snails in Mosana Lake, Kassala State. University of Gezira, Sudan, p 2017
N’Guessan GC, Silué KD, Ba O, Ba H, Tian-Bi NT, Yapi GY, Kaba A, Koné B, Utzinger J, Koudou BG (2017) Prevalence and seasonal transmission of S. haematobium infection among school-aged children in Kaedi town, southern Mauritania. Parasit Vectors 10(1):353. https://doi.org/10.1186/s13071-017-2284-4
Nduka F, Nebe OJ, Njepuome N, Dakul DA, Anagbogu IA, Ngege E, Jacob SM, Nwoye IA, Nwankwo U, Urude R, Aliyu SM, Garba A, Adamani W, Nwosu C, Clark A, Mayberry A, Mansiu K, Nwobi B, Isiyaku S, Dixon R, Adeoye GO, Amuga GA (2019) Epidemiological mapping of schistosomiasis and soiltransmitted helminthiasis for intervention strategies in Nigeria. Nigerian J Parasitol 40(2):218–225
Nwabueze AA, Opara KN (2007) Outbreak of urinary schistosomiasis among school children in riverine communities of Delta State, Nigeria: impact of road and bridge construction. J Med Sci 7(4):572–578
Nwoko OE, Kalinda C, Manyangadze T, Chimbari MJ (2022a) Species diversity, distribution, and abundance of freshwater snails in KwaZulu-Natal, South Africa. Water 14:2267
Nwoko OE, Manyangadze T, Chimbari MJ (2022b) Spatial distribution, abundance, and infection rates of human schistosome transmitting snails and related physicochemical parameters in KwaZulu-Natal (KZN) province, South Africa. Heliyon 9:e12463
Nwoko OE, Manyangadze T, Chimbari MJ (2023) Spatial and seasonal distribution of human schistosomiasis intermediate host snails and their interactions with other freshwater snails in 7 districts of KwaZulu-Natal province, South Africa. Sci Rep 13:7845. https://doi.org/10.1038/s41598-023-34122-x
Okafor FC (1990) Schistosoma haematobium Cercariae transmission patterns in freshwater systems of anambra State, Nigeria. Angew Parasitol 31:159–166
Onyeneho NG, Yinkore P, Egwuage J, Emukah E (2010) Perceptions, attitudes and practices on schistosomiasis in Delta State, Nigeria. Tanzania J Health Res 12(4):287–298
Pam VA, Daramola OS, Uzoigwe NR, Ombugadu A, Maikenti JI, Ahmed HO, Aimankhu PO, Aliyu AA, Ayuba SO, Odey SA, Anyebe GE, Adejoh VA, Attah AS, Dogo KS (2021) Freshwater snails infection status and predisposing risk factors to schistosomiasis in Doma local government area, Nasarawa State, Nigeria. Biomed J Sci Technol Res 37(2):005963
Posa MRC, Sodhi NS (2006) Effects of anthropogenic land use on forest birds and butterflies in Subic Bay, Philippines. Biol Cons 129:256–270
Rudge JW, Webster JP, Lu DB (2013) Identifying host species driving transmission of schistosomiasis Japonica; a multi host parasite system, in China. Proc Nat Acad Sci United States Amer 110:114–117
WHO (2013) Sustaining the drive to overcome the global impact of neglected tropical diseases. WHO, Geneva
WHO. (2016). Schistosomiasis: WHO reports substantial treatment progress for school age children. Available from: http://www.who.int/neglected_diseases/news/WHO schistosomiasis reports substantial treatment_progresssac/en/ Accessed June 21, 2023
Acknowledgements
We want to appreciate the undeniable role of Shalom Research Laboratories in lending her staff as field research assistants and providing research-enabling laboratory environments for this investigation. The role of Delta State University, Abraka for also providing research fellows and laboratory equipment is highly appreciated. Special thanks to Dr. Babalola of the Nigerian Institute of Medical Research (NIMR) for assisting in geospatial mappings of S. haematopium transmission hotspots in the Ase, River Catchment.
Funding
No funds, grants, or other support were received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Ethical clearance was obtained from the ethical review board, University of Port-Harcourt, with reference number UPH/CEREMAD/REC/MM90/039 for this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ito, E.E., Eze, C.N. & Nduka, F.O. Spatiotemporal and seasonal transmission dynamics of Schistosoma haematobium and snail infectivity in Ase River catchment, Delta State, Nigeria. J Parasit Dis 48, 235–246 (2024). https://doi.org/10.1007/s12639-024-01656-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-024-01656-4