Abstract
Lysimachia ramosa (Primulaceae) is a traditionally used medicinal plant, leaves extract of which is being widely used by the Jaintia tribes of Meghalaya, India for controlling helminthiasis. Preliminary investigation carried out on helminth parasites revealed that the crude extract of the plant causes deformity in the surface topography leading to death of the parasites. Therefore, the present study was conducted to identify the specific fraction of the crude leaf extract of the plant responsible for cestocidal efficacy, through biochemical and ultrastructural studies in Raillietina echinobothrida exposed to crude extract and its different fractions namely hexane, chloroform, ethyl acetate and n-butanol. A dose dependent efficacy, with highest rate of mortality among n-butanol exposed parasites was recorded. The treated parasites exhibited complete erosion of microtriches from the tegument, disintegration of muscle bundles, cellular organelles, plasma membrane, nuclear membrane, nucleolus and vacuolization of mitochondria was also observed. Observations on histochemical distribution of some important tegumental enzymes like adenosine triphosphatase (ATPase), alkaline phosphatase (AlkPase), acid phosphatase (AcPase) and 5′Nucleotidase (5′-Nu) revealed a marked diminished stain intensity in the tegument of R. echinobothrida exposed to the crude extract and n-butanol fraction of the crude extract compared to the control. Highest reduction (77.93%) in the activity of ATPase was observed when the parasites exposed to 6 mg n-butanol fraction/ml of PBS. The results suggest that these enzymes act as target for anthelmintic stress caused by the phytochemicals present in the plant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parasitic infections are among the most common global health problem posing a serious threat to the rural and economically poor section of the society, particularly in the developing countries (Savioli and Albonico 2004). Among parasitic helminths, Raillietina echinobothrida is one of the most prevalent and pathogenic cestode parasite responsible for nodular tapeworm disease leading to large scale mortality in poultry (Kumar et al. 2007). Even though there are a number of commercial drugs available for controlling helminth infection, development of resistance by the parasites against most of these drugs has shifted attention to search for alternative strategies to control helminth infection (Kone et al. 2012; Dasgupta et al. 2013; Roy and Giri 2017).
Among helminths, cestodes lack an alimentary canal and therefore, intake of nutrient takes place through the soft body covering known as the tegument. Different enzymes viz. adenosine triphosphatase (ATPase), alkaline phosphatase (AlkPase), acid phosphatase (AcPase), and 5′nucleotidase (5′-Nu) has been reported in the cestode’s tegument which carry out various vital functions (Kwak and Kim 1996; Giri and Roy 2014). Since the tegument is the first target for any anthelmintic drug, its enzymatic study becomes an important aspect to understand the extent of anthelmintic stress exerted by the specific drug.
Meghalaya is well reputed for its floral diversity, which harbours a great variety of the total flora of the country. The tribal population of the state largely depends on plants as their primary health care regime. Lysimachia ramosa Wall (Family: Primulaceae) is one such plant, leaves extract of which is customarily used by the different tribe of Meghalaya for controlling intestinal helminth infections. Preliminary investigation carried out on different intestinal helminth parasites revealed that crude alcoholic extract of the plant causes extensive deformation of surface topography of cestode leading to death of the parasites (Challam et al. 2010). Therefore, the present in vitro investigation was carried out to identify the specific fraction of the crude alcoholic leaf extract of L. ramosa responsible for cestocidal activity and the extent of structural and functional alterations caused in the tegument of R. echinobothrida by the phytoproduct.
Materials and methods
Preparation of the plant crude extract and its various sub fractions
The plant Lysimachia ramosa Wall (Primulaceae) were collected from various parts of Jaintia Hills of Meghalaya, India. The traditionally usable part (leaves) was processed for preparation of crude ethanolic extract as described earlier (Roy et al. 2009). Different fractions of the ethanolic extract were separated out using solvents having different polarity such as hexane, chloroform, ethyl acetate and n-butanol through separating funnel and fractional distillation method (Simon et al. 2012).
In vitro treatment of parasites
Raillietina echinobothrida were collected from the intestine of fowl and were kept in 0.9% physiological buffered saline (PBS). Active and healthy worms were selected for in vitro study. The parasites were treated with varying concentrations (0.05, 1.5, 3.0, 4.5 and 6.0 mg/ml of PBS) of the crude plant extract and isolated fractions of crude extract (viz. Hexane, chloroform, ethyl acetate and n-butanol) having 0.1% DMSO (Dimethylsulphoxide) and incubated for 24 h at 37º C. During 24 h of incubation motility of the worms were assessed continuously. The same procedure was repeated with varying concentration of standard broad spectrum anthelmintic drug Praziquantel for comparison of the anthelmintic efficacy of the phytoproducts. Each incubation medium consisted of five worms and replicated for five times. The motility of the parasites at each incubation period was scored using following criteria:
Score 1 = immobile but not dead, score 2 = moving only some parts of the body, score 3 = moving whole body, (score 1–3 remained unstained with 1% vital dye, composed of 1% methylene blue diluted in 0.85% NaCl) and score 0 = immobile and dead, stained with vital dye. The paralytic state was confirmed when the scores of the parasites calculated was one and death of those worms were confirmed when the scores reached zero. Paralysed worms of the incubation medium (6 mg crude extract and active fraction/ml of PBS) were subjected for further studies. The efficacies of the tested drugs against adult R. echinobothrida were evaluated through calculating the relative motility (RM) value (Kiuchi et al. 1987), and calculated as follows:
where, n i = score N i = number of parasite with score n i.
Ultrastructural studies
For ultrastructural studies, paralysed worms were fixed and further processed for viewing under transmission microscope following standard procedure (Hayat 2000) as mentioned earlier (Roy and Giri 2015).
Histochemical localization of enzymes
Histochemical demonstration of ATPase, AlkPase and AcPase in control and phytoproduct exposed worms was carried out following Pearse (1968) as mentioned earlier (Giri and Roy 2014). To observe 5′-Nu, the lead method of Wachstein and Meisel (1957) was employed using adenosine monophosphate as substrate. Sections were incubated in a freshly prepared medium containing 10 ml of 1.25% adenosine-5-phosphate, 5 ml of 0.2 M Tris buffer at pH 7.2, 30 ml of 0.2% Pb(NO3)2 and 5 ml of 0.1 M MgSO4 at 37 °C for 30 min.
Biochemical studies
Activities for AlkPase and AcPase were estimated following Plummer (1988) as mentioned earlier (Roy and Giri 2015). ATPase activity was assayed by estimating the free phosphate released following the method of Kaplan (1957) and 5′-Nu was assayed following the method of Bunitian (1970) as mentioned earlier (Giri and Roy 2014). Protein content of all the enzyme assays was estimated following Lowry et al. (1951).
Statistical analysis
All data are reported as mean ± SEM (n = 5). The results were further analysed using Student’s t test to calculate the significance between the experimental data and respective controls. P ≤ 0.05 taken as the threshold of significance.
Results
The results of the in vitro Relative motility (RM) value showed that the time taken for the loss of mobility and mortality of parasites exposed to crude extract of the plant and its various sub-fractions worked in a dose and time dependent manner (Fig. 1). During initial 3 h of incubation in the crude and n-butanol fraction of the crude extract at a dose of 3.0 mg/ml of PBS, the relative motility (RM) of the parasites was recorded to be 91.66 and 83.33%, respectively. With increased concentration (6 mg/ml) the RM value reduced to 58.33 and 50.0% compared to other fractions (hexane, chloroform, ethyl acetate) during initial 3 h of incubation. When the helminths were incubated in crude extract at a concentration of 3.0, 4.5 and 6.0 mg/ml of PBS for 6 h, the RM value calculated was 75, 66.66 and 33.33% respectively, however, exposure to n-butanol fraction the RM value reduced to 66.66, 33.33 and 16.66% respectively. Worms exposed to 3.0, 4.5 and 6.0 mg/ml of PBS with crude, n-butanol and Praziquantel the worm motility and RM value decreased throughout the experimental period. At lower doses (0.05 mg and 1.5 mg/ml of PBS) there was no effect on RM even at 6 h of incubation, however, when the incubation period increased to 12 h the RM value showed a sharp decline to 50.0% with both crude extract and n-butanol fraction of the plant. At 24 h of incubation the RM value dropped to 0% even at the lowest dose of crude extract and n-butanol fraction of crude extract. A decline in motility was also observed among R. echinobothrida treated with Praziquantel where RM value was found to be 33.33% during 3 h, 16.66% at 6 h and 0% at 12 h of incubation.
Relative motility (%) of R. echinobothrida caused by different concentration of ethanolic crude (Crd) extract of Lysimachia ramose (leaves) and its different sub-fractions viz. Hexane (Hex), Chloroform (Chl), Ethyl acetate (Eth), n-butanol (n-bu) and reference drug Praziquantel (Prz). Values are significant at *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 versus control value
Ultrastructural studies
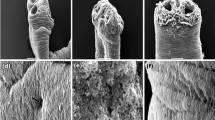
Transmission electron microscopic studies on the control worm showed normal characteristics of surface as well as distal and proximal cytoplasm having microtriches (Fig. 2a), disc-shaped bodies, basal lamina (BL), circular muscles (CM), longitudinal (LM) muscle blocks, nucleus with double nuclear membrane (NM), nucleolus (NL), chromatin granules (Fig. 2b) and mitochondria (Fig: 2c). Plant extract (crude) exposed worm showed complete erosion of distal cytoplasm along with microtriches leading to exposure of basal lamina to outer surface of the parasite. Basal lamina and muscle bundles appeared to be deformed (Fig. 2d). Swollen nucleus with irregular nuclear membrane and disintegrated nucleolus (Fig. 2e). Most of the mitochondria became deformed and vacuolized in the plant extract treated parasites (Fig. 2f). n-Butanol fraction exposed R. echinobothrida also showed erosion of surface tegument leading to exposure of basal lamina into the medium, vacuolization and disintegration of syncytial organelle (Fig. 3a), disruption of nuclear membrane, condensation of chromatin granules (Fig. 3b), disintegration and vacuolization of mitochondrial membrane was also evident in the n-butanol fraction exposed worms (Fig. 3c). Praziquantel treated parasites showed immense damaging effect in the microtrix layer and distal cytoplasm (Fig. 3d). Swollen nucleus with irregular singlet nuclear membrane, (Fig. 3e) and deformed mitochondria without having cristae was also recorded (Fig. 3f).
Transmission electron micrographs of control (a–c) and crude extract of L. ramosa treated (d–f) Raillietina echinobothrida: a Control tegument with intact microtrix layer (MT), distal cytoplasm (DC), non-disrupted basal lamina (BL) and longitudinal muscle layer (LM); b normal nucleus showing intact double layered nuclear membrane (NM) and distinct nucleolus (NL); c normal electron dense mitochondra with distinct cristae; d L. ramosa crude extract exposed parasite showing complete erosion of microtriches (MT) and distorted microtrix layer (arrow); e abnormal nucleus with disappearance of nucleolus, damage in the nuclear membrane (arrow) and chromatin condensation; f deformed mitochondra (*) showing dissapearance of mitochondrial membrane (arrow) and reduced cristae
Transmission electron micrographs of n-butanol fraction of L.ramosa (a–c) and Praziquantel treated (d–f) Raillietina echinobothrida: a tegumental cyton showing extensive damage in the microtrix layer (arrows) with remnants of microtriches (MT), distorted basal lamina (BL) and longitudinal muscle (LM); b deformed nucleus, disappearance of nucleolus and clumping of chromatin (*); c distorted and vacuolated mitochondria (*) with reduced number of cristae; d Praziquantel exposed parasite showing complete erosion of microtriches (MT); e abnormal swollen nucleus with wavy nuclear membrane (arrow) and vacuolization of cytoplasm (V); f deformed mitochondria (*) with broken mitochondrial membrane (arrow) and absence of cristae
Histochemical studies
The tegument of control R. echinobothrida showed intense activity of ATPase, AlkPase, AcPase and 5′Nu compared to sub-tegument and somatic musculature (Figs. 3a, e, 5a, e). ATPase activity was almost negligible in the tegument and sub-tegument of the parasite treated with crude and n-butanol fraction of the plant extract compared to control (Fig. 4b, c). A reduced activity of AlkPase was observed throughout the treated sections of the parasite (Fig. 4f, g). Fig: 5a–d depicts the histochemical localization of AcPase, where in the control section, pronounced stain intensity was observed in the tegument and sub- tegument region however, there was minimal activity throughout the section of parasite exposed to crude, n-butanol fraction and Praziquantel. The 5′-Nu activity was also found to be reduced throughout the tegument and sub-tegumental region in the phytoproducts exposed worms compared to the control (Fig. 5e–h).
Histochemical demonstration of ATPase (a–d) and AlkPase (e–h) activity in the tegument (T), sub-tegument (ST) and somatic musculature (SM) of Raillietina echinobothrida (a) control, b L. ramosa crude extract treated, c n-butanol fraction treated and d Praziquantel treated, e control, f L. ramosa crude extract treated, g n- butanol fraction treated and h Praziquantel treated (all scale bar = 100 µm)
Histochemical demonstration of AcPase (a–d) and 5′-Nu (e–h) activity in the tegument (T), sub-tegument (ST) and somatic musculature (SM) of Raillietina echinobothrida (a) control, b L. ramosa crude extract treated, c n-butanol fraction treated and d Praziquantel treated, e control, f L. ramosa crude extract treated, g n- butanol fraction treated and h Praziquantel treated (all scale bar = 100 µm)
Biochemical studies
Table 1 shows the effect of the plant phytoproducts on the quantitative analysis of the tegumental enzymes in R. echinobothrida. Following exposure to the crude extract, n-butanol fraction of the crude extract and that of Praziquantel, the activities of AlkPase decreased by 38.89, 73.24 and 52.41%, AcPase activities reduced by 44.09, 65.70 and 54.35%, ATPase activities reduced by 57.08, 77.93, 63.40% and 5′-Nu activity decreased by 42.33, 30.21 and 39.35%, respectively, compared to the control. Highest inhibition was observed in case of ATPase followed by AlkPase activity in the worms treated with n-butanol fraction of the plant.
Discussion
Raillietina echinobothrida exposed to different sub-fractions of crude extract of L. ramosa exhibited reduced motility in a concentration and time dependent manner. A similar type of concentration dependent mortality was also observed by Challam et al. (2010) when parasites were exposed to ethanolic crude extract of the plant. Though the chloroform and hexane fractions of the extract did not affect motility of the parasites, both crude and n-butanol fraction of the crude extract showed a comparable paralytic effect, which suggest that the n-butanol fraction contains the most active anthelmintic component of the plant.The soft tegument of cestodes is the host-parasite interface and principal site for protection, absorption and secretion (Lumsdem 1975). However, disruption of host-parasite interface in the phytoproducts treated cestode leads to complete blocking of the vital functions required for survival of the parasite (Shivers 1986; Roy et al. 2007). In cestodes, microtriches increase the effective surface area for enhanced absorption of nutrients however, an altered microtrichs as observed in the experimental worm caused by n-butanol fraction indicate that the parasites were completely devoid of nutrients leading to starvation (Bricker 1983; Dasgupta et al. 2010). Further, damage in the nucleus and mitochondria of the parasite caused by the plant extract indicates the extent of anthelmintic efficacy of the plant in terms of energy metabolism and nucleic acid synthesis in the parasite. In the present study, high intensity of tegumental enzymes viz. ATPase, AlkPase, AcPase and 5′Nu were observed in the tegumental and sub-tegumental region of control parasites indicating the active involvement of these enzymes in various metabolic processes. The role of ATPase has been reported in the transportation of various ions, phospholipids along the brush border plasma membrane subtending the tegument using ATP hydrolysis for generating energy within the parasite (Barrett 1981; McCranken and Taylor 1983; Burenina 2007). Hovever, a significant reduction in activity in the somatic musculature and complete absence in the tegument and sub –tegumental layers in most active fraction exposed parasite further support the claim that the active fraction disrupt the energy metabolism pathway of the parasite. The present investigation also shows decrease in lysosomal enzyme AcPase activity by the crude and n-butanol fraction of the crude extract which indicates disruption of lysosomes as a result of absorption of phytoproducts (Smyth and McManus 1989; Giri and Roy 2014). Inhibition of AlkPase poses hindrance on glucose uptake, thereby resulting in starvation (Burenina 2007; Roy and Giri 2015). 5′-Nu was also noted to be reduced following treatment with the plant extract compared to control. In helminths 5′-Nu has been suggested to be involved with other enzymes in the uptake of nucleosides or their hydrolysis to purine and pyrimidine bases (Walter 1985; Pal and Tandon 1998).
The present investigation suggests that n-butanol fraction of the plant L. ramosa has effective anthelmintic property against R. echinobothrida and tegumental enzymes could be a potential target for chemotherapy. However, further investigation is required to identify the active compound(s) from n-butanol fraction of the plant to unveil the precise mode of action in the parasite.
References
Barrett J (1981) Biochemistry of parasitic helminthes. Mac Pub Ltd., London
Bricker CS, Depenbusch JW, Bennett JL, Thompson DP (1983) The relationship between tegumental disruption and muscle contraction in Schistosoma mansoni exposed to various compounds. Z Parasitenkd 69:61–71
Bunitian HC (1970) Deamination of nucleotides and the role of their deaminoforms in ammonia formation from amino acids. In: Lajtha A (ed) Handbook of neurochemistry. Plenum, New York, pp 399–413
Burenina EA (2007) Adenosine triphosphatases of cestodes Bothriocephalus scorpii. Zh Evol Biokhim Fiziol 43:240–245
Challam M, Roy B, Tandon V (2010) Effect of Lysimachia ramosa (Primulaceae) on helminth parasites: motility, mortality and scanning electron microscopic observations on surface topography. Vet Parasitol 169(1–2):214–218
Dasgupta S, Roy B, Tandon V (2010) Ultrastructural alterations of the tegument of Raillietina echinobothrida treated with the stem bark of Acacia oxyphylla (Leguminosae). J Ethnopharmacol 127:568–571
Dasgupta S, Roy B, Giri BR (2013) Ultrastructural observations on Raillietina echinobothrida exposed to crude extract and active compound of Securingra virosa. Micron 50:62–67
Giri BR, Roy B (2014) Resveratrol induced structural and biochemical alterations in the tegument of Raillietina echinobothrida. Parasitol Int 63:432–437
Hayat MA (2000) Principles and techniques of electron microscopy. Biological application, 4th edn. Cambridge University Press, Cambridge, p 543
Kaplan C (1957) Methods in enzymology, vol III. Acad Press, New York
Kiuchi F, Miyashita N, Tsuda Y, Konda K, Yoshimusa H (1987) Studies on crude drug effective on visceral larval migrans. I. Identification of larvicidal principles in betelnuts. Chem Pharma Bul 35:2880–2886
Kone WM, Vargas M, Keiser J (2012) Anthelmintic activity of medicinal plants used in Cote d’Ivoire for treating parasitic diseases. Parasitol Res 110:2351–2362
Kumar PR, Ravindran R, Lakshmanan B, Senthamil Selvan P, Subramanan H, Sreekumaran T (2007) Pathology of nodular tapeworm in backyard poultry. J Parasit Dis 31:54–55
Kwak KH, Kim CH (1996) Characteristics of alkaline and acid phosphatase in Spirometra erinacei. Kor J Parasitol 34(1):69–77
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with folin-phenol reagent. J Biol Chem 193:265–275
Lumsdem RD (1975) The tapeworm tegument: a model system for studies on membrane structure and function in host-parasite relationships. Tran Am Microsc Soc 94:501–507
McCranken RO, Taylor DD (1983) Biochemical effects of thiabendazole and camendazole on Hymenolepis dimunata (Cestoda) in vivo. J Parasitol 69:295–301
Pal P, Tandon V (1998) Anthelmintic efficacy of Flemingia vestita (Fabaceae): genistein induced alterations in the activity of tegumental enzymes in the cestode, Raillietina echinobothrida. Parasitol Int J 47:233–243
Pearse AGE (1968) Histochemistry: Theoretical and Applied: 3rd edition, Vol1. Churchill Livingstone, Edinburg, pp 720–721
Plummer DT (1988) An introduction to practical biochemistry, 3rd edn. Tata McGraw- Hill Pub Comp. Ltd., New Delhi
Roy B, Giri BR (2015) α-viniferin induced structural and functional alterations in Raillietina echinobothrida, a poultry tapeworm. Microsc Microanyal 21(2):377–384
Roy B, Giri BR (2017) Carex baccans Nees, an anthelmintic medicinal plant in northeast India. In: Birla Singh K (ed) Medicinal plants and its therapeutic uses. OMICS Grp Int, New York, pp 60–81
Roy B, Lalchhandama K, Dutta BK (2007) Anticestodal efficacy of Acacia oxyphylla on Raillietina echinobothrida: a light and electron microscopic studies. Pharmaonline 1:279–287
Roy B, Dasgupta S, Tandon V (2009) Ultrastructuaral observations on Fasciolopsis buski and its alterations caused by shoot extract of Alpinia nigra. Microsc Res Tech 72:61–66
Savioli L, Albonico M (2004) Soil-transmitted helminthiasis. Nat Rev Micro 2:618–619
Shivers RR, Siddiquui AA, Podesta RB (1986) Integument of the tapeworm scolex. 1 freeze-fraction of the syncytial layer, microvilli and discoid bodies. Tis And Cel 18:869–885
Simon MK, Nafanda WD, Obeta SS (2012) In vivo evaluation for anthelmintic effect of alkaloids extracted from the stem bark of Afzelia africana in rats. J Adv Sc Res 3(1):100–104
Smyth JD, McManus DP (eds) (1989) The physiology and biochemistry of cestodes. Cambridge University, Cambridge
Wachstein M, Meisel E (1957) Histochemistry of hepatic phosphatase at a physiologic pH. Am J Clin Pathol 27:13–23
Walter RD, Albeiz EJ (1985) Amoscannate—derivative CGP inhibits 5′ Nucleotidase from Onchocera volvulus and Dinofilaria immitis. Trop Med Parasitol 36:29
Acknowledgments
This study was supported by University Grants Commission (New Delhi) to BR. Infrastructural facilities provided by Sophisticated Analytical Instrument Facilities and UGC-SAP programme to the Department of Zoology, North Eastern Hill University, Shillong are also acknowledged.
Author information
Authors and Affiliations
Contributions
B. Roy framed the work, guided the experiments and interpreted the results. P. Dey performed the experiments and wrote the paper.
Corresponding author
Rights and permissions
About this article
Cite this article
Dey, P., Roy, B. Biochemical and ultrastructural changes in Raillietina echinobothrida in vitro exposed to extract of Lysimachia ramosa. J Parasit Dis 42, 212–219 (2018). https://doi.org/10.1007/s12639-018-0985-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-018-0985-z