Abstract
The prospective effects of Lactobacillus sporogenes (probiotics) and/or praziquantel (PZQ) treatment on some parasitological, histological and molecular aspects in Schistosoma mansoni infected mice were studied. The present data recorded that combination between PZQ (300 mg/Kg one dose 7 weeks post infection) and L. sporogenes (12.5 million spore/mice/week for 8 weeks from the first day of infection) reduced worm and ova count. Also, oogram patterns in liver and intestine recorded that treatment with L. sporogenes alone increase number of dead eggs especially in intestine. Histological observations showed significant (P < 0.05) reduction in the mean values of granuloma diameters in liver and intestine in infected mice groups treated with PZQ and/or L. sporogenes. Single strand breaks (comet assay) showed increase in number of damaged and strong damaged lymphocyte cells in mice infected with S. mansoni and infected treated with PZQ while L. sporogenes administration reduced DNA damage. Flow cytometry also confirmed role of L. sporogenes in reducing significantly DNA damage according to determination of cell cycle analysis apoptosis. It can be concluded that administration of L. sporogenes accompanied with PZQ treatment ameliorates the hepatic and intestinal damage caused by S. mansoni infection.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schistosomiasis is a disease of poverty that leads to chronic ill-health and affects almost 240 million people worldwide, and more than 700 million people live in endemic areas. The infection is prevalent in tropical and sub-tropical areas, in poor communities without potable water and adequate sanitation (WHO 2013).
Praziquantel (PZQ) Chemotherapy based on repeated doses is still the most effective control strategy against schistosomiasis (dell Villar et al. 2012). It is widely used for the treatment of schistosomiasis. It induces worm muscle contractions and tegumental disruption, followed by exposure of parasite surface membrane antigens to the host immunological defence mechanisms (Tallima and El-Ridi 2007). Praziquantel is currently the drug of choice for the treatment of schistosomiasis and since it is practically the only available remedy for the disease, is frequently, concern expressed that the emergence of PZQ-resistant schistosomes may eventually put it out of use (Fenwick and Webster 2006; Botros and Bennett 2007; Caffrey 2007).
Probiotics are usually live microorganisms when administered in adequate amounts confer a health benefits on host. Recently, probiotics as means for the control of parasite infections were reported covering mainly intestinal diseases but also some nongut infections that are all of human and veterinary importance (Travers et al. 2011). Probiotics can play an important role in reducing the pathogenicity of many parasites (Federica et al. 2012). A good probiotic strain should be (1) nonpathogenic, (2) resistant to very low pH, (3) confer a beneficial property like immune stimulation and protection against pathogens, (4) able to adhere to the gut epithelium. Modulation of the intestinal environment, by probiotics having the capacity to control the proliferation of surrounding microorganisms. Several studies have investigated whether probiotecs could control the proliferation of eukaryotic pathogens, either in the gut as the probiotic, or in a different compartment. They added that gut commensal microflora can play a critical role in the completion of the life cycle of the intestinal parasitic nematode Trichuris muris and in the modulation of the host immune response. It can also provide indirect protective immunostimulation against the nongut parasite, Toxoplasma gondii (Benson et al. 2009).
Bacillus coagulans or L. sporogenes is a species of lactic acid forming Bacillus bacteria, which can contaminate canned food and gives it a flat sour taste. This includes foods that are normally too acidic for most bacteria (De Vecchi and Drago 2006).
The objective of this study is to investigate the prospective effect of L. sporogenes accompanied with PZQ treatment on some parasitological, histological and molecular parameters in mice experimentally infected with S. mansoni.
Materials and methods
Animals and infection
Fifty male albino CD-1 mice (weighing 20 ± 2 g) were obtained from Schistosome Biological Supply Program (SBSP) unit at the Theodor Bilharz Research Institute (TBRI) Giza, Egypt. The animals were housed under standard caging conditions, i.e., temperature of 21 ± 1 °C and permitted ad libitum consumption of water and pellet chow. All experiments were done in compliance with the guide lines for the care and use of laboratory animals. For mice infection, the animals were injected subcutaneously by 65 ± 5 S. mansoni cercariae (Peters and Warren. 1969). The cercariae were shedd from Biomphalaria alexandrina snails infected with miracidiae of Egyptian strain of S. mansoni which purchased from SBSP Unit at TBRI.
Experimental materials
Lactobacillus sporogenes
It is sporolac powder manufactured in India by UNI—SANKYO LTD Plate No. B-4, MIDC, Lote parashuram MAHARASHTRA—415722. One gram sporolac powder, not less than 150 million spores of L. sporogenes was dissolved in 3.0 ml distilled water. Each mouse administrated orally with 12.5 million spores of L. sporogenes per week for 8 weeks to obtain the total dose 100 million spores/mice (Son et al. 2009).
Praziquantel
Praziquantel was produced by SEDICO pharmaceutical Co. 6 October City—Egypt. Each tablet contains 600 mg was ground into white powder and suspended in 4.8 ml distilled water. The drug was freshly prepared and orally administered to mice using a stainless steel oral cannula. One dose (300 mg/Kg of body weight) was given 7 weeks post infection (Chaiworaporn et al. 2005).
Experimental groups
Mice were divided into five groups; each group consisted of ten mice as follow: group (I) none infected control mice. Group (II) infected control mice. Group (III) infected mice treated with PZQ. Group (IV) infected mice treated with L. sporogenes. Group (V) infected mice treated with PZQ and L. sporogenes.
Parasitological aspects
Worm count
One week after treatment with PZQ, all animals were sacrificed and adult worms were recorded by portal perfusion according to Duvall and De witt (1969). The number of worms was counted for each mouse.
Ova count
Number of S. mansoni ova/g tissue (liver or intestine) was demonstrated after digestion overnight in KOH according to Cheever (1969) and kamel et al. (1977). The number was calculated in three samples.
Oogram pattern
After perfusion, three fragments from liver and intestine tissues (each about 1 cm in length) were cut off. Each fragment was placed between glass slides and cover slides and examined microscopically. One hundred of S. mansoni egg were counted in each fragment and classified according to their developmental stages (immature, mature and dead eggs) according to Pellegrino et al. (1962).
Histological procedure
Liver and intestine specimens were fixed in 10 % buffered formalin and embedded in paraffin blocks. The prepared 5 µm thick sections were stained with Ehrlich’s haematoxylin and counter stained with eosin (Harris 1990).
Measurement of mean granuloma diameter was performed at a microscopic magnification of 100× using an ocular micrometer. Only non-confluent, lobular granulomas containing eggs in their centers were measured and photographed by Olympus BX 41, Japan microscope. The mean granuloma diameter (M.G.D.) was calculated by measuring two diameters of the lesion at right angles to each other (Boros et al. 1977).
Molecular aspects
Comet assay
Single cell gel electrophoresis (Comet assay) was performed to examine DNA damage after 24 h post treatment by the detection of single stranded DNA breaks and alkali-labile site according to (Singh et al. 1988). Whole blood was collected from each mouse, incubated with 8 ml erythrocyte lysing solution (0.015 M NH4Cl, 1 mM NaHCO3, 0.1 mM EDTA) and centrifuged for 5 min at 1,000 rpm. Carefully, the blood platelets were removed; the pellets were washed two times with culture medium (RPMI 1640 medium solution with l-glutamine, supplemented with 10 % fetal calf serum and 0.1 % penicillin (5,000 IU/ml), streptomycin (5,000 mg/ml) solution). The cells were resuspended in 300 microlitre of 0.7 % of low melting agarose (BRL) and were dissolved and boiled in phosphate buffered saline (PBS) free of Ca2+ and Mg2+. It was added to clean microscope slides, immediately covered with cover slides and kept for 2 min at −12 °C, to solidify (this modification was done by Hassab El-Nabi and Sallam 2002). After solidification, the cover slides were gently removed by sliding it away. The slides were immersed in a jar containing cold lysing solution (2.5 M NaCl, 100 mM EDTA, l0 mM Tris, 1 % N-lauroyl-sarcosine, pH 10; 1 % Triton X-100 and 10 % dimethyl sulfoxide (DMSO)). The slides were kept at 4 °C for at least 20 min. After lysing, the slides were placed on a horizontal electrophoresis box. The unit was filled with a fresh alkaline electrophoresis buffer (300 mM NaOH and 1 mM EDTA, pH 13) to a level of 0.25 cm above the slides. The cells were exposed to alkali for 10 min to allow DNA unwinding and expression of alkali-labile sites. To analyze DNA, an electric current of 25 V (0.86 V/cm) and 300 mA was applied for 10 min. Alkali and electrophoresis treatments were performed in an ice bath. All of these steps were conducted under dim light to prevent the occurrence of additional DNA damage. After electrophoresis, the slides were placed horizontally and Tris buffer (0.4 mM Tris, pH 7.5) was added to neutralize the excess alkali. The slides were allowed to sit for 5 min. Finally, 100 microlitre ethidium bromides (20 μg/ml) was added to each slide, covered with a cover slides and stored at 4 °C for 4 days in moist chamber. Frome each slide 500 cells were examined visually and damaged DNA spots were scored according to Hassab El-Nabi (1996). All chemicals were purchased from Sigma. Examinations were done with a fluorescent microscope (Olympus BX 41, Japan) equipped with an excitation filter 510 nm and barrier filter of 590 nm (1,000× magnification).
Flow cytometry
Fresh tissue specimens (liver) were transported to the laboratory and prepared according to Tribukait et al. (1975) as follow: Liver homogenate was suspended in PBS and centrifuged at 2,000 rpm for 10 min, where upon the supernatent was aspirated. Flow-cytometry analysis was performed on single cell suspensions washed three times with PBS (pH 7.2). After washing with PBS, the cell viability was determined by flow cytometry and apoptosis was measured by using the sub G1 peak staining with propidium Iodide (Cohen and Al-Rubeai 1995). The flow cytometer used is FACS calibur flow cytometer (Becton–Dickinson, Sunnyvale, CA, USA) equipped with a compact air cooked low power 15 m watt argon ion laser beam (488 nm). The average number of evaluated nuclei per specimen 20.000 and the number of nuclei scanned was 120 per second. DNA histogram derived from flow cytometry was obtained with a computer program for Dean and Jett mathematical analysis (Dean and Jett 1974). Data analysis was conducted using DNA analysis program MODFIT (verity software house, Inc. Po Box 247, Topsham, ME 04086 USA, version: 2.0, power Mac with 131,072 KB Registration No. : 42000960827-16193213 Date made: 16-Sep., 1996).
Statistical analysis
Data are presented as mean ± standard error (M ± SE).Comparisons was made between the infected, untreated and treated groups. All numerical data were statistically analyzed using Statistical Program of Social Sciences (SPSS) software for windows, version 10.0. and calculate means, standard error and t test as in the tables in results.
Results
Worm count
The effect of L. sporogenes on total worm count in S. mansoni-infected mice was shown in Table 1. There was significant decrease (P < 0.05) in the mean numbers of total worm in mice treated with PZQ for 1 week, treated with L. sporogenes for 8 weeks from the first day of infection or with PZQ accompanied with L. sporogenes (1.4 ± 0.24, 6.0 ± 0.63 and 0.4 ± 0.24, respectively) when compared with S. mansoni-infected mice.
Ova count
The mean numbers of ova count/gram tissues (liver and intestine) in S. mansoni-infected mice were recorded (4,794.6 ± 444.2 and 1,312.7 ± 65.8, respectively). Treatment with PZQ or L. sporogenes showed significant reduction (P < 0.05) in the mean numbers of ova count in both liver and intestine when compared with infected control group. Also, administration of L. sporogenes accompanied with PZQ showed significant decrease liver and intestine ova count (629.7 ± 89.1 and 375.8 ± 44.9, respectively).
Oograme pattern
Effect of L. sporogenes and/or PZQ on oograme pattern presented in Table 1. Administration of L. sporogenes alone recorded a significant increase in mean numbers of dead eggs especially in intestine (48.11 ± 2.51). S. mansoni-infected mice treated with PZQ accompanied with L. sporogenes showed a significant decrease (P < 0.05) in the mean numbers of immature eggs and a significant increase of dead eggs in both liver and intestine (55.77 ± 2.04 and 64.66 ± 3.52, respectively) when compared with infected control group (3.11 ± 0.85 and 4.44 ± 1.17, respectively).
Histological observations
Granuloma formation occurred in all S. mansoni-infected mice. The typical formed liver granuloma was observed in S. mansoni infected groups 8 weeks post infection. Each granuloma has one or/more schistosomal ova surrounded by a dense of inflammatory cells and a fibrotic tissues. There was significant reduction in the mean values of granuloma diameter in liver and intestine of mice infected with S. mansoni treated with PZQ and/or L. sporogenes. Also, mice infected treated with L. sporogenes showed improvement in liver cells as compared with infected control mice.
Infected mice administrated L. sporogenes in weekly oral dose for 8 weeks from the first day of infection showed fibrocellular granuloma but with small size. Also, normal central vein surrounded with hepatic strands which was separated by blood sinusoids. S. mansoni-infected mice treated with PZQ 7 weeks post infection for 1 week and administrated L. sporogenes for 8 weeks from the first day of infection showed fibrocellular granuloma with reduced diameter. There was improvement in hepatic strands, central vein, Kupffer cells and blood sinusoid. On the other hand, intestinal structure of non infected control mouse consisted of villi appeared as finger like projections covered with simple columner epithelium and crypts appeared as downward invaginations lined with simple columner cells and paneth cells. Infected mice with S. mansoni showed markedly differences in the structure of intestine while multi granuloma were observed with viable eggs surrounded by chronic inflammatory cells and fibrous tissue. Treatment with PZQ and/or L. sporogenes showed mild granulomatus reaction with mild chronic inflammatory cells and little fibrous reaction around the degenerated schistosomal eggs as shown in Figs. 3 and 4.
Comet assay
Table 2 described comet assay of DNA damage leukocytic cells from different treatments. Schistosoma mansoni infected mice and treatment with PZQ showed increase in strong damage of leukocytes DNA as compared with normal control group. Treatment with L. sporogenes decreased the damage of DNA induced by infection and in combination with PZQ ameliorates its activity and decreased number of strongly damaged cells. Figure 1 showing photomicrograph of single strand breaks of DNA in mice infected with S. mansoni appeared as normal, damaged and strong damaged DNA spots.
Flow cytometry
The mean value of apoptosis in mice infected with S. mansoni; infected treated with PZQ and infected treated with PZQ and L. sporogenes showing significant increase when compared with normal control. Also, administration of L. sporogenes only showed reduction in apoptosis caused by infection to be near like non infected mice but when accompanied with PZQ showed significant decrease in apoptosis value when compared with group treated only with PZQ as shown in Fig. 2.
Computerized cell cycle analysis apoptosis of S. mansoni infected mice liver treated with PZQ and/or Lactobacillus sporogenes. a Non infected control, b infected control, c infected treated with PZQ, d infected treated with Lactobacillus sporogenes and e infected treated with PZQ and Lactobacillus sporogens
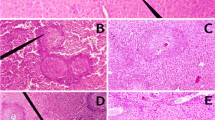
Photomicrograph of liver: a non infected mouse showing radiation of hepatic strands (HS), central vein (CV), blood sinusoids (S) and Kupffer cells (K); b mouse infected with S. mansoni showing typical schistosomal granuloma (G) with two schistosomal ova (O) surrounded by lymphocytes and fibrocytic cells, abnormal hepatocytes (H) and spaces found inside hepatic lobules; c PZQ treated mouse receiving a dose of 300 mg/kg showing fibrocellular granuloma (G) of intermediate size around non calcified schistosomal ovum (O) with hydropic degeneration (Hd) of adjacent hepatocytes; d infected mouse treated with L. sporogenes for 8 weeks from the first day of infection showing less affected liver structure, central vein surrounded with hepatic strands (HS) separated with blood sinusoids (S) and e infected mouse treated with PZQ for 1 week and L. sporogenes for 8 weeks from the first day of infection showing improvement in hepatic strands and normal central vein, normal Kupffer cells (K) and blood sinusoid (S) (H&E ×400)
Photomicrograph of intestine: a non infected mouse showing the typical structure while villi appeared as finger like projection covered with simple columner epithelium (H&E ×200); b mouse infected with S. mansoni showing multi granuloma with viable eggs surrounded by chronic inflammatory cells and concentric fibrous tissues; c PZQ treated mouse receiving a dose of 300 mg/kg showing granuloma with degenerated schistosomal egg surrounded with chronic inflammatory cells; d S. mansoni infected mouse treated with L. sporogenes showing granuloma with schistosomal egg surrounded with little amount and mild inflammatory cells and e infected mouse treated with PZQ for 1 week and L. sporogenes for 8 weeks from the first day of infection showing granuloma with schistosomal egg surrounded with mild inflammatory cells (H&E ×400)
Discussion
Results in the present work illustrated that treatment with L. sporogenes recorded a reduction in S. mansoni total worm count in comparison with infected untreated mice group. This result is in agreement with Abdel-Salam et al. (2008) who reported that probiotic labneh containing garlic and onion oil have a protective effect against worm burden of S. mansoni infected mice compared with control infected. Also, Bautista-Garfias et al. (2001) reported that significant reduction in the number of adult Trichinella spiralis worms in the intestine and of larvae per gram of muscle tissue was observed in the mice treated with L. casei before infection with respect to the control group. They added that the immune response stimulated by L. casei may be promoted more rapid development of a specific immune response than that occurs in unperturbed naïve mice. In addition, treatment with PZQ (300 mg/kg) recorded reduction in worm count of S. mansoni compared with infected control. This report is in match with Chaiworaporn et al. (2005) who said that a few worms were found in mice infected with S. mansoni and treated with the subcurative dosage of PZQ at 1 week and 1 month after treatment (Figs. 3 and 4). Also, Morsy (2009) illustrated that S. mansoni infected mice and treated with PZQ gave reduction of total worm burden, produced marked hepatic shift and improvement of hepatic pathology, reduction in granuloma size and decrease in its number. This may be due to the mode of action of PZQ against S. mansoni which involves increasing the calcium uptake of the parasite, resulting in tegumental damage and death of the parasite as reported by Gnanasekar et al. (2009). They suggested that PZQ affected S. mansoni myosin light chain (SmMLC) function, and this may be have a role in PZQ action. Also, Tallima and EL-Ridi (2007) suggested that PZQ may bind to Schistosomal actin leading to disruption of the tegument.
In the present study, significant decrease in the mean number of ova per gram tissue in liver and intestine in infected groups of mice treated with PZQ and/or L. sporogenes was recorded. This result is in agreement with Giboda and Smith (1994) who confirmed the susceptibility of mature eggs of S. mansoni exposed to a single dose of PZQ, and they added that administration of a second dose of PZQ, 9 days after the first one was suggested as an alternative treatment strategy assuring killing of all the schistosome eggs. Also, Chaiworaporn et al. (2005) reported that the mean numbers of eggs per gram of liver and intestine in the groups treated with PZQ at curative and subcurative dosage were significantly and similarly reduced according to time after treatment. In addition, Pearce (2005) found that PZQ treatment was important to eliminate adult worms and stop eggs deposition that led to sustained diminution of eggs induced immunopathology where Schistosome eggs elevated to be highly immunogenic.
The present data reported that S. mansoni infected mice treated with L. sporogenes alone recorded significant increase in dead egg in both liver and intestine when compared with infected controls. This result is in agreement with Bautista-Garfias et al. (2001) who revealed that the stimulation of immune response by Lactobacillus spp. that caused decease in worm counts at the stage of oviposition. Also, significant decrease in immature egg and an increase in dead egg in both liver and intestine was recorded. This result is in match with report of Morsy (2009) who illustrated that complete disappearance of immature ova with increase number of dead ones after PZQ treatment.
Data obtained in the present study showed significant reduction in the mean value of granuloma diameter in liver and intestine of mice infected with S. mansoni treated with PZQ and/or L. sporogenes. In addition, there was an improvement in histology of liver and intestine in mice treated with L. sporogenes compared with infected controls. This result is in agreement with Ya et al. (2008) found that oral administration of L. casei Zhang was able to modulate immune responses and affected the composition of the intestinal microbiota. They suggested that it may be due to the significant antioxidative activity which was the basis for increases resistance of some lactobacilli strains to toxic oxidative compounds and helped some isolates of Lactobacillus spp. to serve as defective components in intestinal microbial ecosystem. Moreover, Villena et al. (2011) reported that the addition of L. casei to the repletion diet normalized the immune response against Candida albicans, allowing an efficient recruitment and activation of phagocytes as well as an effective release of pro-inflammatory cytokines. They added that, probiotic treatment induced an increase in IL-10 levels, which would help to prevent the damage caused by the inflammatory response. Also, De Moreno and Perdigón (2010) showed that lactic acid bacteria present in many foods such as yoghurt and frequently used as probiotics to improve some biological functions of the host. Results of their studies demonstrated that the consumption of fermented milk can modulate the immune system and can maintain it in a state of surveillance, which could affront different pathologies such as cancer and intestinal inflammation. Pelicano et al. (2005) evaluated the use of probiotics and prebiotics on the histological and morphological indecies of the intestinal mucosa of broilers at 21 days of age and showed beneficial effects. Khambualai et al. (2010) suggested that the intestinal villi and epithelial cells of Broiler Chickens might be hypertrophied by sugar cane extract (SCE) and commercial probiotic (SPB). The fact that a synergistic effect was observed with regard to growth performance and intestinal histology in the SCE + SPB group suggested that SCE was a good supplement to probiotics.
Data in the present work recorded significant decrease in all parasitological and histological parameters in mice infected with S. mansoni and treated with PZQ accompanied with L. sporogenes. This decrement can be considered as hall mark effect for the effectiveness of L. sporogenes to play as antischistosomal role when accompanied with PZQ.
The present study showed that infection with S. mansoni increased the percent of apoptosis and DNA fragmentationin using comet assay by single strand break and cell cycle analysis apoptosis by flow cytometry when compared with non infected control group. This result is in agreement with Ghoneim et al. (2008) who illustrated that cell death data were correlated to the degree of lymphoproliferative responses to phytohemagglutinin (PHA) as well as to the serum anti-schistosomal antibody titers. This recorded a markedly significant increase in PHA-induced apoptosis in lymphocytes isolated from S. mansoni-infected patients when compared to the corresponding healthy controls. Also, their study supported the hypothesis that activation-induced cell death (AICD) was a potentially contributing factor in T helper (Th) cell regulation during chronic stages of schistosomiasis, which represents a critically determinant factor in the host-parasite interaction. It might influence the destiny of parasitic infections either towards establishment of chronic infection or towards host death. Also, Chen et al. (2002) reported that the mechanism of schistosomes to induce apoptosis was not fully understood and there are several ways that can mediate apoptosis in schistosomiasis. They found that caspase-3 which is a key executioner of apoptosis was found in schistosomal eggs in infected mice.
The present study reported that S. mansoni-infected mice treated with L. sporogenes only or accompanied with PZQ indicated a significant decrease in DNA fragmentation in liver and spleen. This means that L. sporogenes may be considered as antiapoptotic agent and this is confirmed by Lin et al. (2008) who indicated that probiotics such as L. rhamnosus GG (LGG) may be augment intestinal host defenses in the developing intestine by stimulating antiapoptotic and cytoprotective responses. Since apoptosis may be a precursor to necrotizing enterocolitis (NEC), understanding the mechanism behind probiotic modulation of apoptotic pathways may allow for development of more specifically targeted therapies or preventive strategies in the future.
However, molecular mechanisms mediating the beneficial effects are as yet poorly understood. Studies of Travers et al. (2011) indicated that probiotics might indeed provide a strain-specific protection against parasites, probably through multiple mechanisms. But more unraveling studies are needed to justify probiotics utilization in therapeutics.
Conclusion
It can be concluded from this study that administration of L. sporogenes accompanied with PZQ treatment ameliorates the hepatic disease and intestinal damage caused by schistosomiasis.
References
Abdel-Salam AM, Ammar N, Abdel-Hamid AZ (2008) Effectiveness of probiotic labneh supplemented with garlic or onion oil against Schistosoma mansoni in infected mice. Int J Dairy Sci 3(2):97–104
Bautista-Garfias CR, Ixta-Rodríguez O, Martínez-Gómez F, Lopez MG, Aguilar-Figueroa BR (2001) Effect of viable or dead Lactobacillus casei organisms administered orally to mice on resistance against Trichinella spiralis infection. Parasite 8(2):S226–S228
Benson A, Pifer R, Behrendt CL, Hooper LV, Yarovinsky F (2009) Gut commensal bacteria direct a protective immune response against Toxoplasma gondii. Cell Host Microbe 6(2):187–196
Boros DL, Tombord R, Warren KS (1977) Introduction of granulomatous and elicitation of cutaneous hypersensitivity by partially purified SEA of Simandin. J Immunol 118:373–376
Botros SS, Bennett JL (2007) Praziquantel resistance. Expert Opin Drug Discov 2(Suppl. 1):S35–S40
Caffrey CR (2007) Chemotherapy of schistosomiasis: present and future. Curr Opin Chem Biol 11:433–439
Chaiworaporn R, Maneerat Y, Rojekittikhun W, Ramasootal P, Janecharut T, Matsuda H, Kitikoon V (2005) Therapeutic effect of subcurative dose Praziquantel on Schistosoma mansoni infected mice and resistance to challenge infection after treatment Southeast Asian. J Trop Med Public Health 36:846–852
Cheever AW (1969) Quantitative comparison of the intensity of Schistosoma mansoni—infections in man and experimental animals. Trans R Soc Trop Med Hyg 63:781–795
Chen L, Rao KV, He YX, Ramaswamy K (2002) Skin-stage schistosomula of Schistosoma mansoni produce an apoptosis-inducing factor that can cause apoptosis of T cells. J Biol Chem 277:34329–34335
Cohen JJ, Al- Rubeai M (1995) Apoptosis-targeted therapies the next big thing in biotechnology? Trends biotechnol 13:281–283
De Moreno de Leblanc A, Perdigón G (2010) The application of probiotic fermented milks in cancer and intestinal inflammation. Proc Nutr Soc 69(3):421–428
De Vecchi E, Drago L (2006) Lactobacillus Sporogenes or Bacillus Coagulans: misidentification or mislabelling? Int J Probiot Prebiot 1:3–10
Dean PN, Jett JH (1974) Mathematical analysis of DNA distributions derived from flow microfluorometry. J Cell Biol 60:523–527
Dell Villar LP, Burguillo FJ, Lopez-Aban J, Muro A (2012) Systematic review and meta-analysis of artemisinin based therapies for the treatment and prevention of schistosomiasis. PLoS One 7(9):e45867
Duvall RH, De witt WB (1969) An improved perfusion technique for recovering adult schistosomes from laboratory animals. Am J Trop Med Hyg 16:438–486
Federica B, David DC, Serena C, Stefano DA (2012) Interactions between parasites and microbial communities in the human gut. Front Cell Infect Microbiol 2:141
Fenwick A, Webster JP (2006) Schistosomiasis: challenges for control, treatment and drug resistance. Curr Opin Infect Dis 19:577–582
Ghoneim HM, Demian SR, Heshmat MG, Ismail NS, El-Sayed LH (2008) Activation-induced apoptosis in peripheral blood mononuclear cells during hepatosplenic Schistosoma mansoni infections. Egypt J Immunol 15(2):63–72
Giboda M, Smith JM (1994) Schistosoma mansoni eggs as a target for praziquantel: efficacy of oral application in mice. J Trop Med Hyg 97(2):98–102
Gnanasekar M, Salunkhe AM, Mallia AK, He YX, Kalyanasundaram M (2009) praziquantel affects the regulatory myosin light chain of Schistosoma mansoni. Antimicrob Agents Chemother 53(3):1054–1060
Harris HF (1990) On the rapid conversion of haematoxylin into haematin staining reaction. J Appl Microsc lab Methods 3:777
Hassab El-Nabi SE (1996) The anti-genotoxic effect of propolies and cloves on human human lymphocytes culture treated with lead nitrate as a heavy metal. J Union Arab Biol Zool 5(A):479–498
Hassab El-Nabi SE, Sallam F (2002) The protective effect of ellagic acid against the mutagenic portal of berelex as one of plant growth regulator hormones using different mutagenic tests. J Egypt Ger Soc Zool 37(C):77–98
Kamel IA, Cheever AW, Elwi AM, Mosimann JE, Danner R (1977) Schistosoma mansoni and S. haematobium infections in Egypt: technique for recovery of worms at necropsy. Am J Trop Med Hyg 26:696–701
Khambualai O, Yamauchi K, Ruttanavut J, Incharoen T, Jun Kashimura J (2010) Effect of sugar cane extract, commercial probiotic and their mixture on growth performance and intestinal histology in broiler chickens. Am J Anim Vet Sci 5(2):132–138
Lin PW, Nasr TR, Berardinelli AJ, Kumar A, Neish AS (2008) The probiotic Lactobacillus GG may augment intestinal host defense by regulating apoptosis and promoting cytoprotective responses in the developing murine gut. Pediatr Res 64(5):511–516
Morsy GH (2009) Parasitological and histo-pathological studies on schistosomiasis mansoni infected mice and treated with praziquatel and/or oltipraz. J Egypt Soc Parasitol 39(2):687–701
Pearce EJ (2005) Priming the immune response by schistosome eggs. Parasite Immunol 27:265–270
Pelicano ERL, Souza PA, Souza HBA, Figueiredo DF, Boiago MM, Carvalho SR, Bordon VF (2005) Intestinal mucosa development in broiler chickens fed natural growth promoters. Braz J Poult Sci 1516-635X:221–229
Pellegrino J, Oliveria CA, Faria J, Cunhan A (1962) New approach to the screening of drugs in experimental S. mansoni in mice. Am J Trop Med Hyg 11:201–215
Peters PA, Warren KS (1969) A rapid method of infecting mice and other laboratory animals with Schistosoma mansoni: subcutaneous injection. J Parasitol 55:558
Singh NP, Mccoy MT, Tice RR, Schneider EL (1988) A simple technique for quantification of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Son VM, Chang CC, Wu MC, Guu YK, Chiu CH, Cheng W (2009) Dietary administration of the Probiotic, Lactobacillus plantarum, enhanced the growth, innate immune responses, and disease resistance of the grouper Epinephelus coioides. Fish Shellfish Immunol 26(5):691–698
Tallima H, El-Ridi R (2007) Praziquantel binds Schistosoma mansoni adult worm actin. Int J Antimicrob Agents 29:570–575
Travers MA, Florent I, Kohl L, Grellier P (2011) Probiotics for the control of parasites: an overview. J Parasitol Res 610769:11
Tribukait B, Moberger G, Zetterberg A (1975) Methodological aspects of rapid-flow cytoflurometry for DNA analysis of human urinary bladder cells. In: Pulse-cytophotometry Part 1, p 50–60
Villena J, Salva S, Agüero G, Alvarez S (2011) Immunomodulatory and protective effect of probiotic Lactobacillus casei against Candida albicans infection in malnourished mice. Microbiol Immunol 55(6):434–445
W H O (2013) Word Health Organization, Geneva. Epidemiological Record No. 8, 88:81–88
Ya T, Zhang Q, Chu F, Merritt J, Bilige M (2008) Immunological evaluation of Lactobacillus casei Zhang: a newly isolated strain from koumiss in Inner Mongolia, China. BMC Immunol 9:68–76
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohamed, A.H., Osman, G.Y., Zowail, M.E.M. et al. Effect of Lactobacillus sporogenes (probiotic) on certain parasitological and molecular aspects in Schistosoma mansoni infected mice. J Parasit Dis 40, 823–832 (2016). https://doi.org/10.1007/s12639-014-0586-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-014-0586-4