Abstract
Background
In older patients, sarcopenia is a prevalent disease associated with negative outcomes. Sarcopenia has been investigated in patients undergoing transcatheter aortic valve implantation (TAVI), but the criteria for diagnosis of the disease are heterogeneous. This systematic review of the current literature aims to evaluate the prevalence of sarcopenia in patients undergoing TAVI and to analyse the impact of sarcopenia on clinical outcomes.
Methods
A comprehensive search of the literature has been performed in electronic databases from the date of initiation until March 2020. Using a pre-defined search strategy, we identified studies assessing skeletal muscle mass, muscle quality and muscle function as measures for sarcopenia in patients undergoing TAVI. We evaluated how sarcopenia affects the outcomes mortality at ≥1 year, prolonged length of hospital stay, and functional decline.
Results
We identified 18 observational studies, enrolling a total number of 9’513 patients. For assessment of skeletal muscle mass, all included studies used data from computed tomography. Cutoff points for definition of low muscle mass were heterogeneous, and prevalence of sarcopenia varied between 21.0% and 70.2%. In uni- or multivariate regression analysis of different studies, low muscle mass was found to be a significant predictor of mortality, prolonged length of hospital stay, and functional decline. No interventional study was identified measuring the effect of nutritional or physiotherapy interventions on sarcopenia in TAVI patients.
Conclusions
Sarcopenia is highly prevalent among patients undergoing TAVI, and negatively affects important outcomes. Early diagnosis of this condition might allow a timely start of nutritional and physiotherapy interventions to prevent negative outcomes in TAVI patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Sarcopenia is defined as age-related loss of muscle mass, strength and function (1). In older people, it is a highly prevalent disease associated with functional decline and impaired ability to perform activities of daily living (2). The most recent guideline from the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) emphasizes the importance of functional status in the diagnosis of sarcopenia (3). This guideline recommends the assessment of muscle strength (e.g., grip strength and/or chair rise test) for the initial screening of sarcopenia. If muscle strength is low, further diagnostic steps for assessment of muscle mass or muscle quality (e.g., body impedance analysis, computed tomography or magnetic resonance imaging) are used to confirm the diagnosis.
Assessment of skeletal muscle mass as a marker of sarcopenia has been investigated in patients undergoing transcatheter aortic valve implantation (TAVI) for severe aortic stenosis. Two meta-analyses investigated the usefulness of skeletal muscle mass to predict mortality in TAVI patients (4, 5). Both analyses showed a significant association between reduced muscle mass and higher mortality. However, to the best of the author’s knowledge, so far no systematic review has been published on how sarcopenia affects functional outcomes in TAVI patients. Therefore, we aimed to conduct a systematic review of the literature to address the following questions: 1) What is the prevalence of sarcopenia in patients undergoing TAVI? 2) How does sarcopenia affect the outcomes mortality, length of hospital stay, and functional status after TAVI? 3) Do controlled trials measure the effect of nutritional and physiotherapy interventions on sarcopenia in TAVI patients?
Methods
Search strategy
We followed a search strategy in accordance with the PRISMA statement (6). The literature search was carried out in MEDLINE via PubMed, Embase and Cochrane Central Register of Controlled Trials from date of initiation until March 15, 2020. We designed two different search strategies: Strategy I for questions one and two, and strategy II for question three using pre-defined terms (“transcatheter aortic valve implantation” or “TAVI”) and (“sarcopenia” or “muscle mass” or “psoas muscle”) and (“protein supplementation” or “nutritional intervention” or “physiotherapy”). Detailed search strategies are shown in supplemental material. No language filters were used. References of included studies were screened for additional studies.
Inclusion and exclusion criteria
For the purpose of this literature review, we included all published observational or interventional studies reporting on sarcopenic patients undergoing transcatheter aortic valve implantation (TAVI), irrespective of which outcome was measured. Studies without original data, without an instrumental measurement of muscle mass, or reporting on mortality less than one year, were excluded from this review. We also excluded studies that were not available as full-text or were written in languages ther than English, French, German or Italian.
Data extraction
The following data were extracted from the included studies: First author, year of publication, country of origin, number of enrolled patients, mean age, mean body mass index (BMI), mean Society of Thoracic Surgeons Predicted Risk of Mortality (STS) score, median follow-up time, and methods used for assessment of sarcopenia. Variables were presented as mean (standard deviation) or median (25th to 75th interquartile range), depending on variable distribution.
Furthermore, we summarized cut-off points for low muscle mass if a dichotomous definition for sarcopenia was used. From these studies, information on prevalence of sarcopenia was extracted. If available, we also extracted measures of sarcopenia as predictors of the outcomes mortality at ≥1 year after TAVI, length of hospital stay and functional decline from uni- and multivariate regression analysis.
Results
General characteristics of included studies
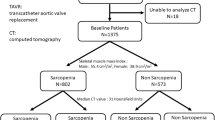
The database search identified 222 studies (Figure 1). Of these, 53 duplicate studies (23.9%) were excluded, resulting in 169 screened studies. Of these, 151 records (89.3%) were excluded according to the pre-defined exclusion criteria, which resulted in 13 single-centre and five multicentre observational longitudinal studies included in the qualitative synthesis (7–24). Ten studies were performed in Northern America, four in Europe, and two in Asia. Two further studies were performed in a collaboration between Northern America and Europe. All included studies were published between 2016 and 2020, enrolling a total number of 9’513 patients. Baseline characteristics of the studies are shown in Table 1.
Prevalence of sarcopenia
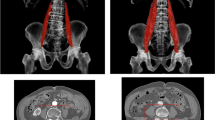
All 18 studies assessed muscle mass using computed tomography (CT) on the level of the third and/or fourth lumbar vertebra (L3 and L4) according to current guidelines (3). Of these, only seven studies (38.9%) were suited to determine the prevalence of sarcopenia, as the other studies did not report data on dichotomized muscle mass. Muscle mass was either determined using psoas muscle area (PMA) (14, 16), or total abdominal muscle area (TMA), consisting of erector spinae, quadratus lumborum, psoas major and minor, internal and external obliques, transversus and rectus abdominis muscles (7, 11, 19, 20, 22). One of these studies additionally used paravertebral muscles at level L3 (erector spinae, quadratus lumborum, psoas major and minor, internal and external obliques, transversus and rectus abdominis, latissimus dorsi) as measure for muscle mass (14). Another study additionally assessed the muscle area at 7th and 12th thoracic levels (20).
Based on the five studies, which used TMA for the assessment of muscle mass, prevalence ranged between 33.8% and 70.2% (7, 11, 19, 20, 22). For dichotomization, these studies used a validated skeletal muscle mass index with gender-specific cut-offs [25]. Despite these gender-specific cutoffs, sarcopenia was more prevalent among men than among women (7, 11, 19, 20, 22). Based on the two studies, which used PMA for the assessment of muscle mass, prevalence was 21.0% and 39.6%, respectively (14, 16). The study of Mamane et al. used low muscle strength (chair rise test ≥15 sec) as additional criterion for the definition of sarcopenia, hereby explaining the low prevalence of 21.0% (16).
Sarcopenia as predictor of outcomes
Of the seven studies, which determined prevalence of sarcopenia (7, 11, 14, 16, 19, 20, 22), five identified sarcopenia as significant predictor of mortality at ≥1 year in univariate and/or multivariate analyses (Table 2). Highest prediction of mortality was found in the multivariate analysis of Mamane et al. using low muscle mass and low muscle strength for diagnosis of sarcopenia (OR 11.30; 95% CI 2.51–50.91) [16]. Low CT density, a measure for muscle quality, as well as the combination of low CT density and sarcopenia were predictive for cumulative 3-year mortality in both uni- and multivariate models by Tokuda et al. (14). In contrast, sarcopenia alone was associated with an increased risk of mortality only in univariate, but not in multivariate analysis. One study did not reveal a significant association between sarcopenia and mortality (20), and three studies did not find an association between sarcopenia and prolonged length of hospital stay (7, 11, 20). One study investigated functional decline as an outcome, which was defined as worsening disability for basic activities of daily living (BADL) and instrumental activities of daily living (IADL). Sarcopenia was predictive for functional decline in this multivariate analysis (16).
Muscle mass as predictor of outcomes
Thirteen studies investigated muscle mass as continuous or categorized predictor of outcomes (Table 3). Eight studies used PMA or TMA as a continuous variable, and other studies calculated percentiles, tertiles or quartiles. In five out of seven studies, multivariate analysis revealed low muscle mass as a predictor of mortality at ≥1 year. Three out of five studies found a significant univariate association between low muscle mass and mortality. Michel et al. found an association in univariate analysis of males (18), two other studies in multivariate analysis of females (17, 24), and one study in multivariate analysis of both sexes (8).
Low muscle mass was predictive for prolonged length of hospital stay in the uni- and multivariate model of Dahya et al (7). Another study found a univariate association of low TMA and prolonged length of hospital stay at Level L3 and Th12, but not at Level Th7 (20), and two studies did not find any significant association (9, 23). No study investigated low muscle mass as a predictor of functional decline.
Muscle quality as predictor of outcomes
Different markers for muscle quality have been investigated as predictors of outcomes in four studies (Table 4). Luetkens et al. identified a high fatty muscle fraction (FMF) as a predictor of short- and long-term mortality in uni- and multivariate analysis (15). Different markers for muscle quality were found to be predictive for mortality in multivariate analysis of males, but not in multivariate analysis of females (8).
Low psoas muscle density (PMD), but not subcutaneous (SAT) or visceral adipose tissue (VAT) were predictive for prolonged length of hospital stay (7, 23). Muscle quality was not investigated as predictor of functional decline in these studies.
Therapeutical interventions on sarcopenia in TAVI patients
Among existing literature, no study was identified measuring the effect of nutritional and physiotherapy interventions on sarcopenia in patients undergoing TAVI.
Discussion
In this systematic literature review, observational studies reported a wide range of prevalence rates for sarcopenia between 21.0% and 70.2% in patients undergoing TAVI. Further, these studies demonstrated that sarcopenia is predictive for poor outcomes, such as mortality, prolonged length of hospital stay and functional decline. The strength of this review is based on the large number of included predictive studies investigating sarcopenia in patients undergoing TAVI. Furthermore, to the best of our knowledge, this is the first systematic review to report on how sarcopenia is affecting different outcomes in TAVI patients.
Criteria for definition of sarcopenia were heterogeneous among the included studies. Whereas diagnosis of sarcopenia was based on measurement of muscle mass using data from CT scans in seventeen studies, only one study used the co-presence of low muscle mass and low muscle strength for definition of the disease as recommended in the revised guidelines of the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) (3, 16). It is not surprising that many studies have been published on CT scan-derived sarcopenia since CT scans are available as part of the pre-interventional cardiovascular routine examination in all TAVI centers. It is likely that the difference in prevalence rates among the studies using data from CT scans was due to variable cut-off points for definition of sarcopenia. However, as suggested by a recent European consensus on revised sarcopenia guidelines (3), use of CT scans alone for the diagnosis of sarcopenia has limitations, since low muscle mass without associated poor muscle function might not be sufficient for diagnosis of sarcopenia. Therefore, use of muscle mass alone most likely leads to an overestimation of the prevalence rate of this disease.
This hypothesis is corroborated by the only study using the co-presence of low muscle mass and low muscle strength for diagnosis of sarcopenia. Hereby, Mamane et al. found a much lower prevalence of sarcopenia among TAVI patients (21.0%) as compared to the studies using CT-based criteria alone (33.8% to 70.2%) (16). Moreover, this study revealed by far the highest odds ratio of sarcopenia to predict mortality (16). It is likely that the association of low muscle mass with poor muscle function actually identified the subgroup of subjects with “true” sarcopenia at risk for adverse outcomes, whereas the CT-based studies included many patients false-positively diagnosed with sarcopenia without risk for adverse outcomes. However, since this was the only study using the revised guideline-based definition of sarcopenia, further studies will be needed to determine if the revised sarcopenia definition is more specific to predict adverse outcomes in TAVI patients than a definition solely based on low muscle mass.
In addition to the limitations related to the heterogeneity of used definitions of sarcopenia in the included studies, this systematic analysis of predictive studies has further limitations. Due to methodical differences of the included studies such as different endpoints and duration of follow-up, we were not able to perform a meta-analysis. Additionally, among the 18 included studies, only one single study investigated functional status outcome (16). Previous studies have demonstrated that frail older patients undergoing TAVI are at increased risk for functional decline (26–28). In older persons, functional status is highly relevant for independency and quality of life. Therefore, additional research based on large populations will be needed to evaluate functional outcomes, such as BADL and IADL, in sarcopenic patients undergoing TAVI.
We think that our literature review has important clinical and research implications. Although we did not find any published interventional study investigating the possible effects of targeted interventions on sarcopenia and related outcomes after TAVI, various studies among older adults have demonstrated that specific, easily implementable physiotherapy and nutritional interventions can have favorable effects on sarcopenia in geriatric populations (29–31). For example, in a multicentre, randomized, controlled, double-blind, two parallel-group trial, it was demonstrated that a simple intervention of vitamin D and leucine-enriched whey protein supplementation resulted in improvements of muscle mass and lower-extremity function among sarcopenic older persons with mobility limitations (32). Other randomized controlled trials confirmed the beneficial effects of leucine supplementation on sarcopenia in patients with chronic obstructive pulmonary disease (COPD) (33) and in post-stroke patients (34). Such interventions might also be applicable in sarcopenic patients undergoing TAVI, and therefore, clinicians should pro-actively search for this highly prevalent condition and implement timely interventions. Clinical trials are needed to investigate the best approach for the treatment of sarcopenia in patients undergoing TAVI in order to make this minimally invasive technology even more meaningful for the quality of life of TAVI patients.
Conclusions
Prevalence of sarcopenia is likely very high among older patients undergoing TAVI, but reliable prevalence estimates are not available because most previous studies did not use the revised guideline-based criteria for diagnosis of the disease. Sarcopenia negatively affects important outcomes in TAVI patients, and therefore, clinicians should pro-actively search for this condition. This might allow a timely start of nutritional and physiotherapy interventions aimed at building up muscle strength and function before and after TAVI procedure.
References
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–23.
Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle. 2016;7(1):28–36.
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31.
Soud M, Alahdab F, Ho G, Kuku KO, Cejudo-Tejeda M, Hideo-Kajita A, et al. Usefulness of skeletal muscle area detected by computed tomography to predict mortality in patients undergoing transcatheter aortic valve replacement: a meta-analysis study. Int J Cardiovasc Imaging. 2019;35(6):1141–7.
Takagi H, Hari Y, Kawai N, Ando T. Meta-analysis of the prognostic value of psoas-muscle area on mortality in patients undergoing transcatheter aortic valve implantation. Am J Cardiol. 2018;122(8):1394–400.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12.
Dahya V, Xiao J, Prado CM, Burroughs P, McGee D, Silva AC, et al. Computed tomography-derived skeletal muscle index: a novel predictor of frailty and hospital length of stay after transcatheter aortic valve replacement. Am Heart J. 2016;182:21–7.
Foldyna B, Troschel FM, Addison D, Fintelmann FJ, Elmariah S, Furman D, et al. Computed tomography-based fat and muscle characteristics are associated with mortality after transcatheter aortic valve replacement. J Cardiovasc Comput Tomogr. 2018;12(3):223–8.
Garg L, Agrawal S, Pew T, Hanzel GS, Abbas AE, Gallagher MJ, et al. Psoas muscle area as a predictor of outcomes in transcatheter aortic valve implantation. Am J Cardiol. 2017;119(3):457–60.
Hebeler KR, Baumgarten H, Squiers JJ, Wooley J, Pollock BD, Mahoney C, et al. Albumin is predictive of 1-year mortality after transcatheter aortic valve replacement. Ann Thorac Surg. 2018;106(5):1302–7.
Heidari B, Al-Hijji MA, Moynagh MR, Takahashi N, Welle G, Eleid M, et al. Transcatheter aortic valve replacement outcomes in patients with sarcopaenia. EuroIntervention. 2019;15(8):671–7.
Kleczynski P, Tokarek T, Dziewierz A, Sorysz D, Bagienski M, Rzeszutko L, et al. Usefulness of psoas muscle area and volume and frailty scoring to predict outcomes after transcatheter aortic valve implantation. Am J Cardiol. 2018;122(1):135–40.
Kofler M, Reinstadler SJ, Mayr A, Stastny L, Reindl M, Dumfarth J, et al. Prognostic implications of psoas muscle area in patients undergoing transcatheter aortic valve implantation. Eur J Cardiothorac Surg. 2019;55(2):210–6.
Krishnan A, Suarez-Pierre A, Zhou X, Lin CT, Fraser CD, 3rd, Crawford TC, et al. Comparing frailty markers in predicting poor outcomes after transcatheter aortic valve replacement. Innovations (Phila). 2019;14(1):43–54.
Luetkens JA, Faron A, Geissler HL, Al-Kassou B, Shamekhi J, Stundl A, et al. Opportunistic computed tomography imaging for the assessment of fatty muscle fraction predicts outcome in patients undergoing transcatheter aortic valve replacement. Circulation. 2020;141(3):234–6.
Mamane S, Mullie L, Lok Ok Choo W, Piazza N, Martucci G, Morais JA, et al. Sarcopenia in older adults undergoing transcatheter aortic valve replacement. J Am Coll Cardiol. 2019;74(25):3178–80.
Mamane S, Mullie L, Piazza N, Martucci G, Morais J, Vigano A, et al. Psoas muscle area and all-cause mortality after transcatheter aortic valve replacement: the Montreal-Munich study. Can J Cardiol. 2016;32(2):177–82.
Michel J, Pellegrini C, Rheude T, von Scheidt M, Trenkwalder T, Elhmidi Y, et al. The clinical impact of psoas muscle cross-sectional area on medium-term mortality after transcatheter aortic valve implantation. Heart Lung Circ. 2019.
Mok M, Allende R, Leipsic J, Altisent OA, Del Trigo M, Campelo-Parada F, et al. Prognostic value of fat mass and skeletal muscle mass determined by computed tomography in patients who underwent transcatheter aortic valve implantation. Am J Cardiol. 2016;117(5):828–33.
Nemec U, Heidinger B, Sokas C, Chu L, Eisenberg RL. Diagnosing sarcopenia on thoracic computed tomography: quantitative assessment of skeletal muscle mass in patients undergoing transcatheter aortic valve replacement. Acad Radiol. 2017;24(9):1154–61.
Paknikar R, Friedman J, Cron D, Deeb GM, Chetcuti S, Grossman PM, et al. Psoas muscle size as a frailty measure for open and transcatheter aortic valve replacement. J Thorac Cardiovasc Surg. 2016;151(3):745–51.
Tokuda T, Yamamoto M, Kagase A, Koyama Y, Otsuka T, Tada N, et al. Importance of combined assessment of skeletal muscle mass and density by computed tomography in predicting clinical outcomes after transcatheter aortic valve replacement. Int J Cardiovasc Imaging. 2020;36(5):929–38.
Tzeng YH, Wei J, Tsao TP, Lee YT, Lee KC, Liou HR, et al. Computed tomography-determined muscle quality rather than muscle quantity is a better determinant of prolonged hospital length of stay in patients undergoing transcatheter aortic valve implantation. Acad Radiol. 2020;27(3):381–8.
van Mourik MS, Janmaat YC, van Kesteren F, Vendrik J, Planken RN, Henstra MJ, et al. CT determined psoas muscle area predicts mortality in women undergoing transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2019;93(4):E248–E54.
Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–63.
Schoenenberger AW, Stortecky S, Neumann S, Moser A, Juni P, Carrel T, et al. Predictors of functional decline in elderly patients undergoing transcatheter aortic valve implantation (TAVI). Eur Heart J. 2013 Mar;34(9):684–92.
Kim DH, Afilalo J, Shi SM, Popma JJ, Khabbaz KR, Laham RJ, et al. Evaluation of Changes in Functional Status in the Year After Aortic Valve Replacement. JAMA Intern Med. 2019 Feb 4.
Afilalo J, Lauck S, Kim DH, Lefevre T, Piazza N, Lachapelle K, et al. Frailty in Older Adults Undergoing Aortic Valve Replacement: the FRAILTY-AVR Study. J Am Coll Cardiol. 2017 Aug 8;70(6):689–700.
Martinez-Arnau FM, Fonfria-Vivas R, Cauli O. Beneficial effects of leucine supplementation on criteria for sarcopenia: a systematic review. Nutrients. 2019;11(10).
Kim HK, Suzuki T, Saito K, Yoshida H, Kobayashi H, Kato H, et al. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: a randomized controlled trial. J Am Geriatr Soc. 2012;60(1):16–23.
Fiatarone MA, O’Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330(25):1769–75.
Bauer JM, Verlaan S, Bautmans I, Brandt K, Donini LM, Maggio M, et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2015;16(9):740–7.
Dal Negro RW, Testa A, Aquilani R, Tognella S, Pasini E, Barbieri A, et al. Essential amino acid supplementation in patients with severe COPD: a step towards home rehabilitation. Monaldi Arch Chest Dis. 2012;77(2):67–75.
Yoshimura Y, Bise T, Shimazu S, Tanoue M, Tomioka Y, Araki M, et al. Effects of a leucine-enriched amino acid supplement on muscle mass, muscle strength, and physical function in post-stroke patients with sarcopenia: a randomized controlled trial. Nutrition. 2019;58:1–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest: Dominic Bertschi (DB): No conflicts of interest. Funding: This work was in part supported by the „Forschungsfonds der Geriatrischen Universitätsklinik“, Bern/Switzerland. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Caroline M. Kiss (CMK): No conflicts of interest. Andreas W. Schoenenberger (AWS): No conflicts of interest. Andreas E. Stuck (AES): No conflicts of interest. Reto W. Kressig (RWK): No conflicts of interest.
Ethical standards: This study did not include any animal or human experiments.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Bertschi, D., Kiss, C.M., Schoenenberger, A.W. et al. Sarcopenia in Patients Undergoing Transcatheter Aortic Valve Implantation (TAVI): A Systematic Review of the Literature. J Nutr Health Aging 25, 64–70 (2021). https://doi.org/10.1007/s12603-020-1448-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-020-1448-7