Abstract
Sarcopenia is a very important issue in rehabilitation medicine and nutritional care. The prevalence of sarcopenia in older people is approximately 50% in the rehabilitation setting, and also approximately 15% of inpatients without sarcopenia upon admission developed sarcopenia during hospitalization. There is a concern that secondary sarcopenia may occur iatrogenically during hospitalization. Iatrogenic sarcopenia is defined as sarcopenia caused by the activities of medical staff including doctors, nurses, or other health care professionals in healthcare facilities. Iatrogenic sarcopenia is categorized into activity-related, nutrition-related and diseaserelated- iatrogenic sarcopenia. Especially in acute phase hospitals, concentrating on the treatment of diseases with less attention to nutrition and activity is more likely to cause iatrogenic sarcopenia. Sarcopenic dysphagia is also an important aspect in rehabilitation medicine and nutritional care. Sarcopenic dysphagia is characterized by swallowing difficulty because of a loss of mass and function in whole-body skeletal and swallowing muscles. Sarcopenic dysphagia can be diagnosed using a 5-step algorithm for the condition. Iatrogenic sarcopenia and sarcopenic dysphagia are affected by nutrition, activity and diseases in a complex manner. Therefore, treatment of iatrogenic sarcopenia and sarcopenic dysphagia requires comprehensive interventions through nutrition management and rehabilitation. Rehabilitation nutrition is effective for preventing and treating iatrogenic sarcopenia and sarcopenic dysphagia. Rehabilitation nutrition can be practiced more effectively and comprehensively by using the rehabilitation nutrition care process, which is a systematic problem-solving method. Further research is required to verify the efficacy of rehabilitation nutrition for preventing or improving iatrogenic sarcopenia and/or sarcopenic dysphagia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcopenia is very common in disabled people and frail older people. The prevalence of sarcopenia is approximately 50% in rehabilitation settings (1, 2). Of 394 inpatients without sarcopenia at hospital admission, 58 (14.7%) developed sarcopenia by hospital discharge (3). In addition, sarcopenia is associated with worse rehabilitation outcomes in older inpatients (4). Therefore, sarcopenia in disabled people and frail older people who require rehabilitation is an extremely important issue, because sarcopenia directly links to subsequent activities of daily living (ADL) and quality of life (QOL).
In hospitalized patients, sarcopenia may occur without intention, which is considered as a hospitalized-associated sarcopenia. Hospitalization-associated sarcopenia is defined as the sarcopenia which is caused by the progression of an acute and chronic inflammation and/or iatrogenic factors during hospitalization. It is difficult to completely prevent hospitalization-associated sarcopenia by the progression of an acute illness. However, some part of hospitalizationassociated sarcopenia is considered to be iatrogenic which can be prevented or treated. Iatrogenic sarcopenia is defined as sarcopenia caused by the activities of medical doctors, nurses, or other healthcare professionals in healthcare facilities (5). Iatrogenic sarcopenia is classified into activity-related sarcopenia caused by unnecessary inactivity or bed rest, nutrition-related sarcopenia caused by inappropriate nutritional care management, and disease-related sarcopenia in cases of iatrogenic diseases (5). Iatrogenic sarcopenia tends to occur in acute care hospitals due to unnecessary bed rest and not eating and inappropriate nutrition care management. Nutrition care management alone or rehabilitation alone may be inadequate for treating iatrogenic sarcopenia, because inpatients with sarcopenia tend to experience activity-, nutrition-, and diseaserelated sarcopenia simultaneously. Rehabilitation and nutrition care is useful for preventing and treating sarcopenia.
Dysphagia can occur because of sarcopenia in whole-body skeletal and swallowing muscles, which is called sarcopenic dysphagia whether the sarcopenia is iatrogenic or not. Sarcopenic dysphagia is characterized by swallowing difficulty because of a loss of mass and function in whole-body skeletal and swallowing muscles (4,6). Sarcopenic dysphagia may be common in older patients with dysphagia and sarcopenia (4,7). Sarcopenic dysphagia is not likely to occur in patients without whole body sarcopenia (8). Activity- and nutritionrelated sarcopenia during hospitalization may be attributed to iatrogenic sarcopenia in some patients. Sarcopenic dysphagia can be diagnosed with a diagnostic algorithm for sarcopenic dysphagia (9).
A concept of rehabilitation nutrition (4) is recommended for preventing and treating iatrogenic sarcopenia and sarcopenic dysphagia (Figure 1). Rehabilitation nutrition is defined as that which 1) evaluates holistically by the International Classification of Functioning, Disability and Health (ICF), and the presence and cause of nutritional disorders, sarcopenia, and excess or deficiency of nutritional intake; 2) conducts rehabilitation nutrition diagnosis and rehabilitation nutrition goal setting; and 3) elicits the highest body functions, activities, participants, and QOL by improving nutritional status, sarcopenia, and frailty using ‘nutrition care management in consideration of rehabilitation’ and ‘rehabilitation in consideration of nutrition’ in people with a disability and frail older people (5). High-quality rehabilitation nutrition is implemented by a rehabilitation nutrition care process that involves assessment and diagnostic reasoning, diagnosis, goal setting, intervention, and monitoring (5) (Figure 2). In particular, diagnosing sarcopenia and setting both a nutrition goal and a rehabilitation goal are very important to achieve the highest possible body functions, activities, participations, and QOL. In addition, aggressive nutritional management in rehabilitation can improve not only nutritional status but also function, activity, participation, and QOL (8, 10, 11). Therefore, rehabilitation nutrition for sarcopenia is very important for people with sarcopenia and it has been drawing attention.
In this review, we discuss iatrogenic sarcopenia, sarcopenic dysphagia, rehabilitation nutrition for both, and the rehabilitation nutrition care process. A literature search was conducted on the MEDLINE via PubMed and the Cochrane Database of Systematic Reviews to identify relevant articles from January 2000 to May 2018. Any language restrictions were not applied.
Iatrogenic sarcopenia
Definition of iatrogenic sarcopenia
Iatrogenic sarcopenia is a new concept (5) which is defined as sarcopenia caused by the activities of medical staff including doctors, nurses, or other health care professionals in healthcare facilities. Iatrogenic sarcopenia is categorized into the following three categories according to cause: 1) activity-related iatrogenic sarcopenia caused by unnecessary inactivity or unnecessary nil per os, 2) nutrition-related iatrogenic sarcopenia caused by inappropriate nutritional care management, and 3) disease-related iatrogenic sarcopenia in case of iatrogenic diseases. Especially in acute phase hospitals, concentrating on the treatment of diseases with less attention to nutrition and activity is more likely to cause iatrogenic sarcopenia.
The prevalence of sarcopenia is high in older inpatients: according to the GLISTEN study, 14.5% of 394 patients developed sarcopenia during hospitalization (3). Another study revealed that approximately 50% of older patients suffered from malnutrition or sarcopenia in post-acute care and rehabilitation (2, 12). In Japanese convalescent rehabilitation wards, sarcopenia was diagnosed in 53% of 637 patients and 95.1% of patients with hospital-associated deconditioning after pneumonia (13).
Activity-related iatrogenic sarcopenia
Activity-related iatrogenic sarcopenia is mainly caused by muscle atrophy due to unnecessary bed rest and immobilization. Muscle atrophy associated with inactivity is caused by a decrease in muscle protein synthesis and an increase in muscle protein degradation. Leg muscle mass and strength decreases with only short period of inactivity (14, 15). It is reported that leg muscle mass was reduced by 1.4% and strength was decreased by 9.0% after 5 days of immobilization (15). During 10 days of bed rest, muscle protein synthesis decreased by 30% and lean body mass in the lower limbs decreased by 6%, resulting in a 16% increase in muscle weakness (16). Days in bed, low body mass index and low muscle index were risk factors for development of sarcopenia (3). In this study, patients who developed sarcopenia spent an average of 5.1 days in bed compared with 3.2 days for those with no sarcopenia at discharge (3). Tentative nil per os is also a cause of activityrelated sarcopenia, especially sarcopenic dysphagia.
Nutrition-related iatrogenic sarcopenia
Nutrition-related iatrogenic sarcopenia is caused by inappropriate nutritional management in the hospital. Malnutrition is a common problem in older people and it is significantly related to sarcopenia. The prevalence of malnutrition in older inpatients evaluated using the Mini Nutritional Assessment was 50.5% for rehabilitation and 38.7% for hospital (17). Sanchez-Rodriquez et al (12) reported that the prevalence of sarcopenia in a post-acute care geriatric unit was 37.5%, among which patients 90.9% fulfilled the European Society for Clinical Nutrition and Metabolism malnutrition criteria. Despite the high prevalence of malnutrition in older inpatients, malnutrition is not well recognized or treated. Starvation and sarcopenia were correctly recognized by only 6-58% and 43-51% of surveyed dietitians, respectively (18-20). The Nutrition Day study, a multinational audit of nutritional data collected and systematically assessed, revealed that in half of the hospital wards in European countries that participated in the audit no action was taken against malnutrition despite detecting malnutrition (21).
Nutrition-related iatrogenic sarcopenia occurs as a result of insufficient energy-related and protein intake-related starvation. None of the patients with hospital-associated deconditioning had a normal nutritional status, and 44% of those patients had starvation-related malnutrition (22). Patients undergoing rehabilitation often consume less energy and protein than they need (23). A texture-modified diet is difficult to meet the patients’ requirements for energy and protein compared to consumption of the normal hospital diet (24). The patients who consumed approximately <22 kcal/kg/day during the acute period showed significantly poorer recovery from dysphagia and poorer outcomes compared to those who consumed approximately >22 kcal/kg/day (25). Prevention of nutritionrelated iatrogenic sarcopenia is important, because starvation deteriorates rehabilitation outcomes.
Disease-related iatrogenic sarcopenia
Disease-related iatrogenic sarcopenia develops following iatrogenic diseases. Iatrogenic disease is a disease or symptoms induced in patients by the treatment or instructions of doctors, which results in harmful consequences for the patients’ health, for example adverse drug effects, falls, nosocomial infections, pressure ulcers, and complications related to surgery. One meta-analysis showed the incidence of iatrogenic disease to be between 3.4% and 33.9% (26). Causes of iatrogenic diseases include medical equipment, pharmaceuticals, medical materials, misdiagnosis by doctors, medical malpractice (e.g., inappropriate drug selection, inappropriate and immature surgery, examination), and nosocomial infection (27, 28). It should be noted that polypharmacy is one of the risk factors for sarcopenia (26, 29, 30).
Sarcopenic dysphagia
Sarcopenic dysphagia is characterized by swallowing difficulty resulting from a loss of mass and function in wholebody skeletal and swallowing muscles (4, 6). However, neuromuscular disease-related sarcopenia is not included in sarcopenic dysphagia, because dysphagia due to neuromuscular diseases appears to be a separate category (9). Whole-body skeletal muscle mass is associated with severe dysphagia in cancer patients (31, 32). Age-related decline in swallowing muscle mass has been reported in the tongue (33), geniohyoid muscle (34), and pharyngeal muscles (35), The mean crosssectional area of the geniohyoid muscle in the midsagittal plane in younger adult and older adult females is approximately 490mm2 and 340mm2, respectively (31% reduction), and approximately 520mm2 and 440mm2 in younger and older adult males, respectively (15% reduction) (34). Pharyngeal wall thickness in young and older women measured by magnetic resonance imaging showed a mean thickness at the level of the anterior inferior second cervical vertebra of 0.25cm in women in their 20’s, 0.22cm in women in their 60’s, and 0.19cm in women in their 70’s (35).
Age-related changes in swallowing muscles have been studied mainly in rats. An age-related decrease in the expression of myosin heavy chain mRNA and protein was found in the rat genioglossus but not in the masseter and geniohyoid muscles (36). In the klotho mouse, the deficiency in amino acids caused by the active movement of the masseter muscle and tongue was shown to stimulate the autophagiclysosomal pathway via down-regulation of the mTOR signaling pathway (37). Tongue muscle composition shifted to more slowly contracting muscle fibers in aged rats (38, 39). However, muscle fiber size was decreased only in the transverse and verticalis muscles (38). Although the number of primary dendrites of hypoglossal motoneurons decreased significantly with age in rats, no age-associated changes were found in the number or size of hypoglossal motoneurons (40). In older human cadavers, the number of macrophages per striated muscle fiber in the larynx and pharynx were 5–6 times greater than those in the tongue, shoulder, and anus (41). Thinning and death of striated muscle fibers may therefore occur frequently in the larynx and pharynx. In rats’ extrinsic tongue muscles, the number of muscle fibers was reduced in older rats, with significant increases in cell death due to apoptosis (42). These results indicate the presence of sarcopenia in swallowing muscles with aging.
Sarcopenia of swallowing muscles and whole body muscles are associated with dysphagia. Tongue muscle mass was less and tongue muscle echo-intensity on ultrasound was greater in patients with sarcopenic dysphagia than in patients without the disorder (43). Whole body sarcopenia and low body mass index and ADL were also independent predictors of sarcopenic dysphagia (8). Of 82 inpatients aged 65 years or older without dysphagia who had restricted oral intake for longer than 2 days, 63 (77%) had whole body sarcopenia while 21 (26%) developed dysphagia, all of whom had whole body sarcopenia (P = .002). Furthermore, sarcopenia was positively associated with dysphagia in recent systematic review and meta-analysis (44). Therefore, sarcopenic dysphagia is not likely to occur in patients without whole body sarcopenia.
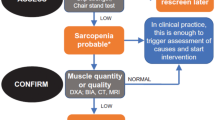
Sarcopenic dysphagia is diagnosed using a 5-step algorithm for the condition (Figure 3) (9). The diagnostic algorithm for sarcopenic dysphagia divides participants into three categories as follows: probable sarcopenic dysphagia, possible sarcopenic dysphagia, and no sarcopenic dysphagia. Patients who had a disease that was the obvious cause of the dysphagia were excluded. However, patients were included if they had stroke, brain injury, neuromuscular disease, head and neck cancer, or connective tissue disease in whom the main cause of dysphagia was considered to be age-, activity-, nutrition-, invasion-, or cachexia-related sarcopenia. Indeed, in stroke patients aged 65 years or older receiving enteral nutrition, the risk of severe malnutrition independently predicts the achievement of full oral intake (45). Swallowing muscle mass decreases separately from neurological deficits, and sarcopenic dysphagia may interrupt recovery of swallowing function in stroke patients (45). Moreover, age-related swallowing muscle atrophy affects the complex pathophysiology of dysphagia in patients with acute stroke (46).
In mouse aspiration pneumonia models, muscle atrophy in the diaphragm, the tibialis anterior and the tongue were observed. Furthermore, the cross-sectional area of a dorsal muscle group at the twelfth thoracic vertebra level by computed tomography decreased to 84.4% after aspiration pneumonia in human patients (47). Not only tongue strength but also lip force can be useful for diagnosing sarcopenic dysphagia (48). Assessment of swallowing muscle mass is not included in the diagnostic algorithm for sarcopenic dysphagia, because it is not easy to measure swallowing muscle mass in clinical practice. However, ultrasound examination can be used for assessing swallowing muscle mass and muscle quality (43). The prevalence of sarcopenic dysphagia assessed by a 5-step diagnostic algorithm for sarcopenic dysphagia was 32% in patients who require dysphagia rehabilitation, and sarcopenic dysphagia was independently associated with poor swallowing function at discharge (49).
Rehabilitation nutrition for iatrogenic sarcopenia and sarcopenic dysphagia
Some cases of sarcopenia are iatrogenic and are affected by nutrition, activity and diseases in a complex manner. Sarcopenic dysphagia is also likely to follow iatrogenic sarcopenia. In this respect, rehabilitation nutrition can offer better effects (11, 50-59). A recent systematic review and metaanalysis (56) showed some positive effects from a combination of exercise and nutritional interventions for older people with sarcopenia. Another systematic review showed that older people who received protein supplementation and performed resistance training showed a greater increase in lean mass and leg strength, compared to resistance training alone (51). Several randomized controlled trials have shown that exercise intervention, including resistance training, for older people with sarcopenia enables them to improve muscle mass, strength and walking ability (60-62).
Appropriate and aggressive nutritional care is an important part of rehabilitation nutrition for preventing and treating iatrogenic sarcopenia and sarcopenic dysphagia. Maintaining energy and protein intake helps to preserve muscle mass (63). Replenishment of essential amino acids is highly likely to be effective in improving muscle strength and physical functions. According to a study in community-dwelling older women with sarcopenia, ingesting 3g of a leucine-rich essential amino acid mixture for 3 months increased knee extension strength (61). The fat-free mass was high in the group with a high total protein intake calculated from the 3-day diet for older women (64).
It is important to consider the energy requirement for improving nutritional status in the case of malnutrition, together with setting the energy requirement according to the intensity of rehabilitation and activity. Nii et al. (65) reported that higher energy intake at admission (35.5kcal/kg/day [current body weight]; 95% confidence interval, 29.15-41.0) was associated with greater Functional Independence Measure efficiency in convalescent patients with cerebrovascular disorders. Hebuterne et al. (66) explained that older people require 8,800–22,600kcal to gain 1kg. Taking this into account, for nutrition care for older people with malnutrition it is suggested to add to the energy expenditure the amount of energy accumulation needed for weight gain. This suggestion is supported by several case reports in which aggressive nutrition care with an energy intake of approximately 35kcal/kg/day (ideal body weight) was implemented along with dysphagia rehabilitation (10, 11, 57). As a result, in addition to a weight gain of approximately 10 kg, both physical and swallowing function improved.
To prevent activity-related iatrogenic sarcopenia, early mobilization and early rehabilitation is important. Early rehabilitation for older patient with severe aspiration pneumonia contributes to a decrease in in the 30-day in-hospital mortality rate (67). Compared to conventional approaches, a program for enhanced recovery after surgery—a new operative care program including aggressive postoperative rehabilitation and preoperative feeding—significantly reduced overall morbidity rates, accelerated functional recovery, and shortened the primary and total hospital stay without compromising the readmission rate (68, 69).
Tentative nil per os causes wasting of swallowing-related muscles and leads to sarcopenic dysphagia and decreased swallowing function (70). In this respect, it is important to start early oral intake based on an appropriate swallowing assessment. The Kuchi-Kara Taberu Index (KT Index) is a multifaceted and comprehensive tool for preventing iatrogenic sarcopenia and encouraging early oral intake in people with eating and swallowing problems. The reliability and validity of the KT Index has been verified (71). The KT Index consists of 13 items related to comprehensive evaluation and support skills for improving oral intake: willingness to eat, overall condition, respiratory condition, oral condition, cognitive function while eating, oral preparatory and propulsive phases, severity of pharyngeal dysphagia, position and endurance while eating, eating behavior, daily living activities, food intake level, food modification, and nutrition. The KT Index is indicated by a radar chart that reveals weaknesses requiring intervention and strengths to be maintained and strengthened (Figure 4). The effects of intervention are evaluated by comparing the radar charts before and after the intervention.
Rehabilitation nutrition team management is also an important aspect of treating older people with sarcopenia. Analysis of data from an online questionnaire among members of the Japanese Association of Rehabilitation Nutrition showed that the availability of a rehabilitation nutrition team independently affected sarcopenia evaluation and practice of rehabilitation nutrition (72). For older people with severe pneumonia, multidisciplinary comprehensive care including nutritional care and encouragement of early mobilization was an independently associated with hospital stay duration and oral intake at discharge (55).
Rehabilitation Nutrition Care Process
Definition
The rehabilitation nutrition care process is defined as a systematic problem solving method for nutrition status, sarcopenia, nutrient intake, and frailty in people with a disability and frail older people. This model may help the multidisciplinary rehabilitation nutrition team to detect the overlapping problem between rehabilitation and nutrition, set the appropriate goal for older individuals, and choose an adequate rehabilitation and nutrition-related approach. Rehabilitation nutrition care process includes rehabilitation nutrition assessment and diagnostic reasoning, rehabilitation nutrition diagnosis, rehabilitation nutrition goal setting, rehabilitation nutrition intervention, and rehabilitation nutrition monitoring (5).
Along with the nutrition care process (73), the rehabilitation nutrition care process stresses the etiology of rehabilitation nutrition problems. On the other hand, unlike the nutrition care process, the rehabilitation nutrition care process focuses on a multidisciplinary approach specified for individuals with disability or older adults with frailty. Therefore, rehabilitation nutrition diagnosis includes not only nutritional problems but also broader concepts such as sarcopenia.
Rehabilitation nutrition assessment and diagnostic reasoning
Rehabilitation nutrition assessment should include a comprehensive assessment by the ICF, clinical history, a detailed nutritional assessment including nutritional status and nutritional intake, and the presence of sarcopenia and its etiology based on appropriate criteria. Diseases, trauma and injuries often cause rehabilitation nutrition problems such as malnutrition, insufficient nutritional intake, and sarcopenia. Conversely, rehabilitation nutrition problems are potential triggers of the diseases and/or injuries. For example, vitamin D deficiency may increase the risk of hip fracture (74) and cardiovascular disease (75).
Diagnostic reasoning is considered to be the most critical skill of a physician (76). In the rehabilitation nutrition care process, the multidisciplinary team should use diagnostic reasoning to make a rehabilitation nutrition diagnosis. Two systems for decision making have been proposed: System 1, heuristic and intuitive and System 2, systematic and analytical (76). Rehabilitation nutrition diagnosis may depend on System 1 because few rehabilitation nutrition problems so far have reliable data on prevalence and specific clinical characteristics.
Rehabilitation nutrition diagnosis
Rehabilitation nutrition diagnosis simply identifies the rehabilitation- and nutrition-related problems. It comprises three major categories with 15 sub-items: nutritional status, sarcopenia, and excess and/or insufficient nutrient intake (Table 1). Unlike medical diagnosis, rehabilitation nutrition diagnosis focuses on only rehabilitation and nutrition-related problems, and should not be used to diagnose other diseases and/or injuries.
Nutritional status
The domain of nutritional status is composed of malnutrition (undernutrition), overnutrition, at risk of malnutrition, at risk of overnutrition, lack of nutrients, and excess of nutrients. Malnutrition is defined as “a state resulting from lack of intake or uptake of nutrition that leads to altered body composition (decreased fat free mass) and body cell mass leading to diminished physical and mental function and impaired clinical outcome from disease” (77) and can be used synonymously with “undernutrition” by the European Society for Clinical Nutrition and Metabolism terminology (78). Malnutrition should be identified using a validated assessment tool (79, 80) or consensus-based criteria (81, 82). Validated malnutrition screening tools would be useful to detect the individuals at risk of malnutrition who were not necessarily undernourished at the time.
Overnutrition is the excess deposition of nutrients. This term includes overweight and obesity, which are defined as abnormal or excessive fat accumulation that may impair health (79). It can be defined by body mass index, waist circumference, or waist/hip ratio (83). Body composition analysis (e.g., bio-impedance analysis, dual-energy x-ray absorptiometry, computed tomography, and magnetic resonance imaging) may help to confirm the degree and distribution of fat accumulation. Individuals at risk of overnutrition are not necessarily obese or overweight at the time. Often, a person with apparent loss or excess of dietary intake is at risk of malnutrition or overnutrition, respectively.
Lack and excess of nutrients indicates under- or overaccumulation of one or more nutrients in the body. Individuals with lack and excess of nutrients show various symptoms such as abnormal blood concentration and specific symptoms or signs of nutrient deficiency (e.g., iron-deficiency anemia) or toxicity (e.g., Parkinsonism by brain accumulation of manganese), respectively. These states can occur regardless of amount of intake of the target nutrients because it can also be affected by digestion, absorption, excretion or metabolic disorder.
Sarcopenia
In rehabilitation nutrition diagnosis, the sarcopenia category consists of three sub-items based on the component of the diagnostic criteria (84,85): sarcopenia, decreased muscle mass, and decreased muscle strength and/or physical performance. The etiology of sarcopenia should also be documented, such as aging, poor nutritional intake, inactivity and diseases/injuries (84).
Excess and/or insufficient nutrient intake
Excess or insufficient nutrient intake means that the habitual nutritional intake is too much or too little compared with the appropriate reference, such as the dietary reference intake in each country (86). Excess or insufficient nutrient intake raises the risk of various types of malnutrition and sarcopenia, although these conditions are not always accompanied by a lack or excess of nutrients. Several conditions produce a future risk of excess or insufficient nutrient intake (prediction of excess or insufficient nutrient intake). For example, if a stroke survivor with hemiplegia in a rehabilitation hospital had an excessive intake of sweetened beverages and snacks before the stroke and no compliance with the hospital diet, he or she may be predicted to have excess nutrient intake after discharge. Conversely, an individual with cancer who is scheduled for chemotherapy with a higher possibility of adverse effects such as nausea or vomiting can be predicted to have insufficient nutrient intake.
Rehabilitation nutrition goal setting
Goal setting is a fundamental step for rehabilitation nutrition intervention. It should be performed in accordance with the SMART concept: Specific, Measurable, Achievable, Relevant, and Time-bound (87). Outcome measurements need to be Specific for the rehabilitation nutrition diagnosis. They also should be Measurable quantitative variables, rather than qualitative. An Achievable goal motivates both the clients and healthcare staff. For long-term goals, Relevant indicators for the clients would be employed instead of simple biomarkers or the results of functional tests. Because all interventions will be performed within a specific time frame, the goal should be Time-bound.
Rehabilitation nutrition intervention
There are two aspects to the methods of rehabilitation nutrition intervention. Namely, Nutrition care management in consideration of rehabilitation and Rehabilitation in consideration of nutrition.
Nutrition care management in consideration of rehabilitation
This aspect means nutrition care management for maximizing functions, activities, participation and QOL through improving nutritional status and/or sarcopenia in light of the ICF and/or ongoing rehabilitation program. Several studies reported that higher energy intake or improvement of nutritional status significantly correlates with higher functional capacity in the rehabilitation patients (65, 88, 89). Rehabilitation patients with decreased muscle mass receiving an oral nutritional supplement showed greater functional recovery (53). A recent systematic review regarding rehabilitation and nutrition intervention for older adults with disability or sarcopenia is conducive to higher muscle strength (56,90), although one review reported higher mortality and hospitalization risk, probably due to selection bias of the included studies (91). Therefore, the patients with apparent malnutrition or sarcopenia and disability may benefit from Nutrition care management in consideration of rehabilitation.
Rehabilitation in consideration of nutrition
This aspect represents the rehabilitation for maximizing functions, activities, participation and QOL through improving nutritional status and/or sarcopenia in light of nutritional status, sarcopenia, the ICF, and/or ongoing nutrition care. Individuals with starvation often experience fatigue, exhaustion and apathy, which depress functional capacity (92). In the tertiary-care acute general hospital, most of the older adults (88%) referred to the department of rehabilitation medicine for hospital-associated deconditioning were malnourished (22). These findings indicated that malnutrition itself may reduce physical and mental function, which are two of the targets of rehabilitation medicine. Therefore, a rehabilitation program should be planned with consideration for nutritional status and the ongoing nutritional care plan as well as the ICF. In Japan, with the emphasis on Rehabilitation in consideration of nutrition, the Ministry of Health, Labor and Welfare revised the medical fee system in the convalescent rehabilitation hospitals. Since April 2018, the convalescent rehabilitation hospitals can claim highest medical fee, if multidisciplinary rehabilitation nutrition team implements nutritional care management. Moreover, assignment of a registered dietitian for each convalescent rehabilitation ward is encouraged (93).
Rehabilitation nutrition monitoring
In the rehabilitation nutrition care process, the following indicators are recommended for monitoring: general condition, nutritional status, nutritional intake, body weight, body composition (e.g., body cell mass, muscle mass, fat mass), physical and mental function, ADL, and social participation. When implementing rehabilitation nutrition intervention, timing and frequency of rehabilitation nutrition monitoring should be scheduled. If the current rehabilitation nutrition intervention is not effective, the rehabilitation nutrition plan would be changed, if applicable. Monitoring frequency can be determined by patients’ condition, type and severity of rehabilitation nutrition diagnosis and specific indicators must be followed for the patients. In underweight stroke patients who undergo tubefeeding, monitoring the patients’ nutritional status once per week may be superior to once per month (94).
Conclusion
Iatrogenic sarcopenia is caused by the activities of medical staff including doctors, nurses, or other health care professionals in healthcare facilities. Sarcopenic dysphagia is swallowing difficulty due to loss of mass and function in whole-body skeletal and swallowing muscles, which is often found in older patients after aspiration pneumonia and/or acute illness. These condition is promoted by unnecessary inactivity or unnecessary nil per os (activity-related), inappropriate nutritional care management (nutrition-related), and iatrogenic diseases (disease-related). Rehabilitation nutrition is effective to prevent or treat both iatrogenic sarcopenia and sarcopenic dysphagia. Rehabilitation nutrition care process can promote more theoretical and comprehensive practice of rehabilitation nutrition. Further research is required to verify the efficacy of rehabilitation nutrition for preventing or improving iatrogenic sarcopenia and/or sarcopenic dysphagia.
Acknowledgments: This work was supported by a research Grant-in-Aid for Scientific Research C (no. 16K01460) from the Ministry of Education, Science, Culture, Sports, Science, and Technology of Japan.
Disclosure statement: Hidetaka Wakabayashi reports a grant from the Ministry of Education, Science, Culture, Sports, Science, and Technology of Japan (grant number: 16K01460), during the conduct of the study. Ayano Nagano and Shinta Nishioka have nothing to disclose
Ethical Standards: This study has been performed in accordance with the ethical standards established in the 1964 Declaration of Helsinki and later amendments.
References
Sánchez-Rodríguez D, Calle A, Contra A, Ronquillo N, Rodríguez-Marcos A, Vázquez-Ibar O, Colominas M, Inzitari M. Sarcopenia in post-acute care and rehabilitation of older adults: A review. Eur Geriatr Med 2016;7:224–231. doi:10.1016/j.eurger.2015.11.001.
Churilov I, Churilov L, MacIsaac RJ, Ekinci EI. Systematic review and meta-analysis of prevalence of sarcopenia in post acute inpatient rehabilitation. Osteoporos Int 2018;29:805–812. doi:10.1007/s00198-018-4381-4.
Martone AM, Bianchi L, Abete P, Bellelli G, Bo M, Cherubini A, Corica F, Di Bari M, Maggio M, Manca GM, Marzetti E, Rizzo MR, Rossi A, Volpato S, Landi F. The incidence of sarcopenia among hospitalized older patients: results from the Glisten study. J Cachexia Sarcopenia Muscle 2017;8:907–914. doi:10.1002/jcsm.12224.
Wakabayashi H, Sakuma K. Rehabilitation nutrition for sarcopenia with disability: a combination of both rehabilitation and nutrition care management. J Cachexia Sarcopenia Muscle 2014;5:269–277. doi:10.1007/s13539-014-0162-x.
Wakabayashi H. Rehabilitation nutrition in general and family medicine. J Gen Fam Med 2017;18:153–154. doi:10.1002/jgf2.116.
Wakabayashi H. Presbyphagia and Sarcopenic Dysphagia: Association between Aging, Sarcopenia, and Deglutition Disorders. J Frailty Aging 2014;3:97–103. doi:10.14283/jfa.2014.8.
Maeda K, Akagi J. Sarcopenia is an independent risk factor of dysphagia in hospitalized older people. Geriatr Gerontol Int 2016;16:515–521. doi:10.1111/ggi.12486.
Maeda K, Takaki M, Akagi J. Decreased Skeletal Muscle Mass and Risk Factors of Sarcopenic Dysphagia: A Prospective Observational Cohort Study. J Gerontol A Biol Sci Med Sci 2017;72:1290–1294. doi:10.1093/gerona/glw190.
Mori T, Fujishima I, Wakabayashi H, Oshima F, Itoda M, Kunieda K, Kayashita J, Nishioka S, Sonoda A, Kuroda Y, Yamada M. Development and reliability of a diagnostic algorithm for sarcopenic dysphagia. JCSM Clinical Reports 2017;2:e00017. doi:10.17987/jcsm-cr.v2i2.17.
Wakabayashi H, Uwano R. Rehabilitation Nutrition for Possible Sarcopenic Dysphagia After Lung Cancer Surgery: A Case Report. Am J Phys Med Rehabil 2016;95:e84–89. doi:10.1097/phm.0000000000000458.
Hashida N, Shamoto H, Maeda K, Wakabayashi H, Suzuki M, Fujii T. Rehabilitation and nutritional support for sarcopenic dysphagia and tongue atrophy after glossectomy: A case report. Nutrition 2017;35:128–131. doi:10.1016/j. nut.2016.11.003.
Sanchez-Rodriguez D, Marco E, Ronquillo-Moreno N, Miralles R, Vazquez-Ibar O, Escalada F, Muniesa JM. Prevalence of malnutrition and sarcopenia in a post-acute care geriatric unit: Applying the new ESPEN definition and EWGSOP criteria. Clin Nutr 2017;36:1339–1344. doi:10.1016/j.clnu.2016.08.024.
Yoshimura Y, Wakabayashi H, Bise T, Tanoue M. Prevalence of sarcopenia and its association with activities of daily living and dysphagia in convalescent rehabilitation ward inpatients. Clin Nutr 2018;37:2022–2028;doi:10.1016/j.clnu.2017.09.009.
Wall BT, Dirks ML, van Loon LJ. Skeletal muscle atrophy during short-term disuse: implications for age-related sarcopenia. Ageing Res Re 2013;12:898–906. doi:10.1016/j.arr.2013.07.003.
Wall BT, Dirks ML, Snijders T, Senden JM, Dolmans J, van Loon LJ. Substantial skeletal muscle loss occurs during only 5 days of disuse. Acta Physiol (Oxf) 2014;210:600–611. doi:10.1111/apha.12190.
Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA 2007;297:1772–1774. doi:10.1001/jama.297.16.1772-b.
Kaiser MJ, Bauer JM, Ramsch C, Uter W, Guigoz Y, Cederholm T, Thomas DR, Anthony PS, Charlton KE, Maggio M, Tsai AC, Vellas B, Sieber CC. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc 2010;58:1734–1738. doi:10.1111/j.1532-5415.2010.03016.x.
Yaxley A, Miller MD. The challenge of appropriate identification and treatment of starvation, sarcopenia, and cachexia: a survey of Australian dietitians. J Nutr Metab 2011:603161. doi:10.1155/2011/603161.
Ter Beek L, Vanhauwaert E, Slinde F, Orrevall Y, Henriksen C, Johansson M, Vereecken C, Rothenberg E, Jager-Wittenaar H. Unsatisfactory knowledge and use of terminology regarding malnutrition, starvation, cachexia and sarcopenia among dietitians. Clin Nutr 2016;35:1450–1456. doi:10.1016/j.clnu.2016.03.023.
Nakahara S, Wakabayashi H, Maeda K, Nishioka S, Kokura Y. Sarcopenia and cachexia evaluation in different healthcare settings: a questionnaire survey of health professionals. Asia Pac J Clin Nutr 2018;27:167–175. doi:10.6133/apjcn.032017.15.
Ostrowska J, Jeznach-Steinhagen A. Fight against malnutrition (FAM): Selected results of 2006–2012 nutrition day survey in Poland. Rocz Panstw Zakl Hig 2016;67:291–300.
Wakabayashi H, Sashika H. Malnutrition is associated with poor rehabilitation outcome in elderly inpatients with hospital-associated deconditioning a prospective cohort study. J Rehabil Med 2014;46:277–282. doi:10.2340/16501977-1258.
Walton K, Williams P, Tapsell L, Batterham M. Rehabilitation inpatients are not meeting their energy and protein needs. Clin Nutr ESPEN 2007;2:e120–e126. doi:10.1016/j.eclnm.2007.09.001.
Wright L, Cotter D, Hickson M, Frost G. Comparison of energy and protein intakes of older people consuming a texture modified diet with a normal hospital diet. J Hum Nutr Diet 2005;18:213–219. doi:10.1111/j.1365-277X.2005.00605.x.
Iwamoto M, Higashibeppu N, Arioka Y, Nakaya Y. Swallowing rehabilitation with nutrition therapy improves clinical outcome in patients with dysphagia at an acute care hospital. J Med Invest 2014;61:353–360. doi:10.2152/jmi.61.353.
Atiqi R, van Bommel E, Cleophas TJ, Zwinderman AH. Prevalence of iatrogenic admissions to the Departments of Medicine/Cardiology/Pulmonology in a 1,250 bed general hospital. Int J Clin Pharmacol Ther 2010;48:517–524. doi:10.5414/CPP48517.
Permpongkosol S. Iatrogenic disease in the elderly: risk factors, consequences, and prevention. Clin Interv Aging 2011;6:77–82. doi:10.2147/CIA.S10252.
Krishnan NR, Kasthuri AS. Iatrogenic Disorders. Med J Armed Forces India 2005;61:2–6. doi:10.1016/S0377-1237(05)80107-8.
Konig M, Spira D, Demuth I, Steinhagen-Thiessen E, Norman K. Polypharmacy as a Risk Factor for Clinically Relevant Sarcopenia: Results From the Berlin Aging Study II. J Gerontol A Biol Sci Med Sci 2017;73:117–122. doi:10.1093/gerona/glx074.
Hao Q, Hu X, Xie L, Chen J, Jiang J, Dong B, Yang M. Prevalence of sarcopenia and associated factors in hospitalised older patients: A cross-sectional study. Australas J Ageing 2018;37:62–67. doi:10.1111/ajag.12492.
Wakabayashi H, Matsushima M, Uwano R, Watanabe N, Oritsu H, Shimizu Y. Skeletal muscle mass is associated with severe dysphagia in cancer patients. J Cachexia Sarcopenia Muscle 2015;6:351–357. doi:10.1002/jcsm.12052.
Saitoh M, Ishida J, Konishi M, Springer J. The concept that focuses on oral motor and feeding function in cancer patients with muscle wasting: Skeletal muscle mass is associated with severe dysphagia in cancer patients. J Cachexia Sarcopenia Muscle 2016;7:233–234. doi:10.1002/jcsm.12119.
Tamura F, Kikutani T, Tohara T, Yoshida M, Yaegaki K. Tongue thickness relates to nutritional status in the elderly. Dysphagia 2012;27:556–561. doi:10.1007/s00455-012-9407-z.
Feng X, Todd T, Lintzenich CR, Ding J, Carr JJ, Ge Y, Browne JD, Kritchevsky SB, Butler SG. Aging-related geniohyoid muscle atrophy is related to aspiration status in healthy older adults. J Gerontol A Biol Sci Med Sci 2013;68:853–860. doi:10.1093/gerona/gls225.
Molfenter SM, Amin MR, Branski RC, Brumm JD, Hagiwara M, Roof SA, Lazarus CL. Age-Related Changes in Pharyngeal Lumen Size: A Retrospective MRI Analysis. Dysphagia 2015;.30:321–327. doi:10.1007/s00455-015-9602-9.
Kaneko S, Iida RH, Suga T, Morito M, Yamane A. Age-related changes in rat genioglossus, geniohyoid and masseter muscles. Gerodontology 2014;31:56–62. doi:10.1111/ger.12004.
Iida RH, Kanko S, Suga T, Morito M, Yamane A. Autophagic-lysosomal pathway functions in the masseter and tongue muscles in the klotho mouse, a mouse model for aging. Mol Cell Biochem 2011;348:89–98. doi:10.1007/s11010-010-0642-z.
Cullins MJ, Connor NP. Alterations of intrinsic tongue muscle properties with aging. Muscle Nerve 2017;56:E119–E125. doi:10.1002/mus.25605.
Schaser AJ, Wang H, Volz LM, Connor NP. Biochemistry of the anterior, medial, and posterior genioglossus in the aged rat. Dysphagia 2011;26:256–263. doi:10.1007/s00455-010-9297-x.
Schwarz EC, Thompson JM, Connor NP, Behan M. The effects of aging on hypoglossal motoneurons in rats. Dysphagia 2009;24:40–48. doi:10.1007/s00455-008-9169-9.
Rhee S, Yamamoto M, Kitamura K, Masaaki K, Katori Y, Murakami G, Abe SI. Macrophage density in pharyngeal and laryngeal muscles greatly exceeds that in other striated muscles: an immunohistochemical study using elderly human cadavers. Ana Cell Biol 2016;49:177–183. doi:10.5115/acb.2016.49.3.177.
Kletzien H, Hare AJ, Leverson G, Connor NP. Age-related effect of cell death on fiber morphology and number in tongue muscle. Muscle Nerve 2018;57:E29–E37. doi:10.1002/mus.25671.
Ogawa N, Mori T, Fujishima I, Wakabayashi H, Itoda M, Kunieda K, Shigematsu T, Nishioka S, Tohara H, Yamada M, Ogawa S. Ultrasonography to Measure Swallowing Muscle Mass and Quality in Older Patients With Sarcopenic Dysphagia. J Am Med Dir Assoc 2018;19:516–522. doi:10.1016/j.jamda.2017.11.007.
Zhao W-T, Yang M, Wu H-M, Yang L, Zhang X-m, Huang Y. Systematic Review and Meta-Analysis of the Association Between Sarcopenia and Dysphagia. J Nutr Health Aging. 2018; 22:1003–1009 doi:10.1007/s12603-018-1055-z.
Nishioka S, Okamoto T, Takayama M, Urushihara M, Watanabe M, Kiriya Y, Shintani K, Nakagomi H, Kageyama N. Malnutrition risk predicts recovery of full oral intake among older adult stroke patients undergoing enteral nutrition: Secondary analysis of a multicentre survey (the APPLE study). Clin Nutr 2017;36:1089–1096. doi:10.1016/j. clnu.2016.06.028.
Sporns PB, Muhle P, Hanning U, Suntrup-Krueger S, Schwindt W, Eversmann J, Warnecke T, Wirth R, Zimmer S, Dziewas R. Atrophy of Swallowing Muscles Is Associated With Severity of Dysphagia and Age in Patients With Acute Stroke. J Am Med Dir Assoc 2017;18:635.e631–635.e637. doi:10.1016/j.jamda.2017.02.002.
Komatsu R, Okazaki T, Ebihara S, Kobayashi M, Tsukita Y, Nihei M, Sugiura H, Niu K, Ebihara T, Ichinose M. Aspiration pneumonia induces muscle atrophy in the respiratory, skeletal, and swallowing systems. J Cachexia Sarcopenia Muscle 2018; 9:643–653 doi:10.1002/jcsm.12297.
Sakai K, Nakayama E, Tohara H, Takahashi O, Ohnishi S, Tsuzuki H, Hayata M, Takehisa T, Takehisa Y, Ueda K. Diagnostic accuracy of lip force and tongue strength for sarcopenic dysphagia in older inpatients: A cross-sectional observational study. Clin Nutr. 2018;doi:10.1016/j.clnu.2018.01.016.
Wakabayashi H, Takahashi R, Murakami T. The prevalence and prognosis of sarcopenic dysphagia in patients who require dysphagia rehabilitation. J Nutr Health Aging, 2018;doi: 10.1007/s12603-018-1117-2.
Parmar MP, Vanderbyl BL, Kanbalian M, Windholz TY, Tran AT, Jagoe RT. A multidisciplinary rehabilitation programme for cancer cachexia improves quality of life. BMJ Support Palliat Care 2017;7:441–449. doi:10.1136/bmjspcare-2017-001382.
Liao CD, Tsauo JY, Wu YT, Cheng CP, Chen HC, Huang YC, Chen HC, Liou TH. Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: a systematic review and metaanalysis. Am J Clin Nutr 2017;106:1078–1091. doi:10.3945/ajcn.116.143594.
Kouw IW, Holwerda AM, Trommelen J, Kramer IF, Bastiaanse J, Halson SL, Wodzig WK, Verdijk LB, van Loon LJ. Protein Ingestion before Sleep Increases Overnight Muscle Protein Synthesis Rates in Healthy Older Men: A Randomized Controlled Trial. J Nutr 2017;147:2252–2261. doi:10.3945/jn.117.254532.
Yoshimura Y, Uchida K, Jeong S, Yamaga M. Effects of Nutritional Supplements on Muscle Mass and Activities of Daily Living in Elderly Rehabilitation Patients with Decreased Muscle Mass: A Randomized Controlled Trial. J Nutr Health Aging 2016;20:185–191. doi:10.1007/s12603-015-0570-4.
Ueshima J, Maeda K, Wakabayashi H, Nishioka S, Nara S, Nakatani H. Availability of Early, Intensive, and Continuous Nutrition Management for Fournier’s Gangrene with Rectal Cancer: A Case Report. J Acad Nutr Diet 2016;116:909–916. doi:10.1016/j. jand.2015.09.021.
Koyama T, Shamoto H, Anzai H, Koganei Y, Maeda K, Wakabayashi H. Multidisciplinary Comprehensive Care for Early Recommencement of Oral Intake in Older Adults With Severe Pneumonia. J Gerontol Nurs 2016;42:21–29. doi:10.3928/00989134-20160913-05.
Yoshimura Y, Wakabayashi H, Yamada M, Kim H, Harada A, Arai H. Interventions for Treating Sarcopenia: A Systematic Review and Meta-Analysis of Randomized Controlled Studies. J Am Med Dir Assoc 2017;18:553.e551–553.e516. doi:10.1016/j. jamda.2017.03.019.
Maeda K, Akagi J. Treatment of Sarcopenic Dysphagia with Rehabilitation and Nutritional Support: A Comprehensive Approach. J Acad Nutr Diet 2016;116:573–577. doi:10.1016/j.jand.2015.09.019.
Nishida Y, Wakabayashi H, Maeda K, Nishioka S. Nutritional status is associated with the return home in a long-term care health facility. J Gen Fam Med 2018;19:9–14. doi:10.1002/jgf2.142.
Kokura Y, Wakabayashi H, Maeda K, Nishioka S, Nakahara S (2017) Impact of a multidisciplinary rehabilitation nutrition team on evaluating sarcopenia, cachexia and practice of rehabilitation nutrition. J Med Invest 2017;64:140–145. doi:10.2152/jmi.64.140.
Kim H, Suzuki T, Saito K, Kojima N, Hosoi E, Yoshida H. Long-term effects of exercise and amino acid supplementation on muscle mass, physical function and falls in community-dwelling elderly Japanese sarcopenic women: A 4-year follow-up study. Geriatr Gerontol Int 2016;16:175–181. doi:10.1111/ggi.12448.
Kim H, Kim M, Kojima N, Fujino K, Hosoi E, Kobayashi H, Somekawa S, Niki Y, Yamashiro Y, Yoshida H. Exercise and Nutritional Supplementation on Community-Dwelling Elderly Japanese Women With Sarcopenic Obesity: A Randomized Controlled Trial. J Am Med Dir Assoc 2016;17:1011–1019. doi:10.1016/j. jamda.2016.06.016.
Kim H, Suzuki T, Saito K, Yoshida H, Kojima N, Kim M, Sudo M, Yamashiro Y, Tokimitsu I. Effects of exercise and tea catechins on muscle mass, strength and walking ability in community-dwelling elderly Japanese sarcopenic women: a randomized controlled trial. Geriatr Gerontol Int 2013;13:458–465. doi:10.1111/j.1447-0594.2012.00923.x.
Wall BT, van Loon LJ. Nutritional strategies to attenuate muscle disuse atrophy. Nutr Rev 2013;71:195–208. doi:10.1111/nure.12019.
Isanejad M, Mursu J, Sirola J, Kroger H, Rikkonen T, Tuppurainen M, Erkkila AT. Association of protein intake with the change of lean mass among elderly women: The Osteoporosis Risk Factor and Prevention -Fracture Prevention Study (OSTPRE-FPS). J Nutr Sci 2015;4:e41. doi:10.1017/jns.2015.31.
Nii M, Maeda K, Wakabayashi H, Nishioka S, Tanaka A. Nutritional Improvement and Energy Intake Are Associated with Functional Recovery in Patients after Cerebrovascular Disorders. J Stroke Cerebrovasc Dis 2016;25:57–62. doi:10.1016/j. jstrokecerebrovasdis.2015.08.033.
Hebuterne X, Bermon S, Schneider SM. Ageing and muscle: the effects of malnutrition, re-nutrition, and physical exercise. Curr Opin Clin Nutr Metab Care 2001;4:295–300.
Momosaki R, Yasunaga H, Matsui H, Horiguchi H, Fushimi K, Abo M. Effect of early rehabilitation by physical therapists on in-hospital mortality after aspiration pneumonia in the elderly. Arch Phys Med Rehabil 2015;96:205–209. doi:10.1016/j. apmr.2014.09.014.
Lassen K, Soop M, Nygren J, Cox PB, Hendry PO, Spies C, von Meyenfeldt MF, Fearon KC, Revhaug A, Norderval S, Ljungqvist O, Lobo DN, Dejong CH. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg 2009;144:961–969. doi:10.1001/archsurg.2009.170.
Song W, Wang K, Zhang RJ, Dai QX, Zou SB. The enhanced recovery after surgery (ERAS) program in liver surgery: a meta-analysis of randomized controlled trials. Springerplus 2016;5:207. doi:10.1186/s40064-016-1793-5.
Maeda K, Koga T, Akagi J. Tentative nil per os leads to poor outcomes in older adults with aspiration pneumonia. Clin Nutr 2016;35:1147–1152. doi:10.1016/j. clnu.2015.09.011.
Maeda K, Shamoto H, Wakabayashi H, Enomoto J, Takeichi M, Koyama T. Reliability and Validity of a Simplified Comprehensive Assessment Tool for Feeding Support: Kuchi-Kara Taberu Index. J Am Geriatr Soc 2016;64:e248–e252. doi:10.1111/jgs.14508.
Kokura Y, Wakabayashi H, Maeda K, Nishioka S, Nakahara S. Impact of a multidisciplinary rehabilitation nutrition team on evaluating sarcopenia, cachexia and practice of rehabilitation nutrition. J Med Invest 2017;64:140–145. doi:10.2152/jmi.64.140.
Writing Group of the Nutrition Care Process/Standardized Language Committee. Nutrition care process and model part ?: The 2008 update. J Am Diet Assoc 2008;108:1113-1117. doi:10.1016/j.jada.2008.04.027.
Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 2008;87:1080s–1086s. doi:10.1093/ajcn/87.4.1080S.
Zhang R, Li B, Gao X, Tian R, Pan Y, Jiang Y, Gu H, Wang Y, Wang Y, Liu G. Serum 25-hydroxyvitamin D and the risk of cardiovascular disease: dose-response meta-analysis of prospective studies. Am J Clin Nutr 2017;105:810–819. doi:10.3945/ajcn.116.140392.
Croskerry P. A universal model of diagnostic reasoning. Acad Med 2009;84:1022–1028. doi:10.1097/ACM.0b013e3181ace703.
Sobotka L, Allison SP, 2011. Basics in clinical nutrition. 4th edn. Galen, Prague
Vellas B, Villars H, Abellan G, Soto ME, Rolland Y, Guigoz Y, Morley JE, Chumlea W, Salva A, Rubenstein LZ, Garry P. Overview of the MNA—Its history and challenges. J Nutr Health Aging 2006;10:456–463; discussion 463–455.
Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, Compher C, Correia I, Higashiguchi T, Holst M, Jensen GL, Malone A, Muscaritoli M, Nyulasi I, Pirlich M, Rothenberg E, Schindler K, Schneider SM, de van der Schueren MA, Sieber C, Valentini L, Yu JC, Van Gossum A, Singer P. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr 2017;36:49–64. doi:10.1016/j. clnu.2016.09.004.
Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, Jeejeebhoy KN. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr 1987;11:8–13. doi:10.1177/014860718701100108.
Cederholm T, Bosaeus I, Barazzoni R, Bauer J, Van Gossum A, Klek S, Muscaritoli M, Nyulasi I, Ockenga J, Schneider SM, de van der Schueren MA, Singer P. Diagnostic criteria for malnutrition -An ESPEN Consensus Statement. Clin Nutr 2015;34:335–340. doi:10.1016/j.clnu.2015.03.001.
White JV, Guenter P, Jensen G, Malone A, Schofield M. Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). JPEN J Parenter Enteral Nutr 2012;36:275–283. doi:10.1177/0148607112440285.
The obesity society, 2016. What is obesity. https://doi.org/www.obesity.org/obesity/resources/facts-about-obesity/what-is-obesity. Accessed 12 August 2018
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. doi:10.1093/ageing/afq034.
Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, Lee JS, Lee WJ, Lee Y, Liang CK, Limpawattana P, Lin CS, Peng LN, Satake S, Suzuki T, Won CW, Wu CH, Wu SN, Zhang T, Zeng P, Akishita M, Arai H. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95–101. doi:10.1016/j.jamda.2013.11.025.
Ministry of Health, Labour and Welfare. Overview of Dietary Reference Intakes for Japanese, 2015. https://doi.org/www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/Overview.pdf. Accessed 12 August 2018.
Bovend’Eerdt TJ, Botell RE, Wade DT. Writing SMART rehabilitation goals and achieving goal attainment scaling: a practical guide. Clin Rehabil 2009;23:352–361. doi:10.1177/0269215508101741.
Nishioka S, Wakabayashi H, Nishioka E, Yoshida T, Mori N, Watanabe R. Nutritional Improvement Correlates with Recovery of Activities of Daily Living among Malnourished Elderly Stroke Patients in the Convalescent Stage: A Cross-Sectional Study. J Acad Nutr Diet 2016;116:837–843. doi:10.1016/j.jand.2015.09.014.
Nishioka S, Wakabayashi H, Momosaki R. Nutritional status changes and activities of daily living after hip fracture in convalescent rehabilitation wards: a retrospective observational cohort study from the Japan Rehabilitation Nutrition Database. J Acad Nutr Diet 2018;118:1270–1276. doi: 10.1016/j.jand.2018.02.012.
Tsuboi M, Momosaki R, Vakili M, Abo M. Nutritional supplementation for activities of daily living and functional ability of older people in residential facilities: A systematic review. Geriatr Gerontol Int 2018;18:197–210. doi:10.1111/ggi.13160.
Beck AM, Dent E, Baldwin C. Nutritional intervention as part of functional rehabilitation in older people with reduced functional ability: a systematic review and meta-analysis of randomised controlled studies. J Hum Nutr Diet 2016;29:733–745. doi:10.1111/jhn.12382.
Kalm LM, Semba RD. They starved so that others be better fed: remembering Ancel Keys and the Minnesota experiment. J Nutr 2005;135:1347–1352. doi:10.1093/jn/135.6.1347.
Ministry of Health, Labour and Welfare. Revision of Medical fee in 2018. https://doi.org/www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000188411.html. Accessed 12 August 2018. In Japanese.
Nishioka S, Sugawara H, Takayama M, Urushihara M, Watanabe M, Kiriya Y, Shintani K, Nakagomi H, Kageyama N, Okamoto T, Sumita S, Fujita M, Hashimoto S, Ishikawa M, Tsushima E, Ogawa A. Relationship between weight gain, functional recovery and nutrition monitoring in underweight tube-fed stroke patients. Jpn J Compr Rehabil Sci 2018;9:3–10. doi:10.11336/jjcrs.9.3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagano, A., Nishioka, S. & Wakabayashi, H. Rehabilitation Nutrition for Iatrogenic Sarcopenia and Sarcopenic Dysphagia. J Nutr Health Aging 23, 256–265 (2019). https://doi.org/10.1007/s12603-018-1150-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-018-1150-1