Abstract
Objectives
To investigate whether supplementation with low-dose dairy protein plus micronutrients augments the effects of resistance exercise (RE) on muscle mass and physical performance compared with RE alone among older adults.
Design
Randomized controlled trial.
Setting
Tokyo, Japan.
Participants
Eighty-two community-dwelling older adults (mean age, 73.5 years) were randomly allocated to an RE plus dairy protein and micronutrient supplementation group or an RE only group (n = 41 each).
Intervention
The RE plus supplementation group participants ingested supplements with dairy protein (10.5 g/day) and micronutrients (8.0 mg zinc, 12 μg vitamin B12, 200 μg folic acid, 200 IU vitamin D, and others/day). Both groups performed the same twice-weekly RE program for 12 weeks.
Measurements
Whole-body, appendicular, and leg lean soft-tissue mass (WBLM, ALM, and LLM, respectively) with dual-energy X-ray absorptiometry, physical performance, biochemical characteristics, nutritional intake, and physical activity were measured before and after the intervention. Data were analyzed by using linear mixed-effects models.
Results
The groups exhibited similar significant improvements in maximum gait speed, Timed Up-and-Go, and 5-repetition and 30-s chair stand tests. As compared with RE only, RE plus supplementation significantly increased WBLM (0.63 kg, 95% confidence interval [CI]: 0.31-0.95), ALM (0.37 kg, 95% CI: 0.16-0.58), LLM (0.27 kg, 95% CI: 0.10-0.46), and serum concentrations of 25-hydroxyvitamin D (4.7 ng/mL, 95% CI: 1.6-7.9), vitamin B12 (72.4 pg/mL, 95% CI: 12.9-131.9), and folic acid (12.9 ng/mL, 95% CI: 10.3-15.5) (all P < 0.05 for group-by-time interactions). Changes over time in physical activity and nutritional intake excluding the supplemented nutrients were similar between groups.
Conclusion
Low-dose dairy protein plus micronutrient supplementation during RE significantly increased muscle mass in older adults but did not further improve physical performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Age-related loss of skeletal muscle mass and strength, known as sarcopenia, increases the risk of functional disability in later life (1). Skeletal muscle mass is regulated by net protein balance, i.e., the dynamic equilibrium between the rates of muscle protein synthesis (MPS) and breakdown (MPB). However, the effect of feeding-induced stimulation of MPS is weaker in older adults than in younger adults (2). This anabolic resistance to MPS in older adults may cause a gradual decrease in muscle mass.
A single session of resistance exercise (RE) increases MPS and MPB (3) and anabolic sensitivity to feeding (4). However, poor nutritional status during prolonged RE results in a net negative muscle protein balance, which may result in muscle mass atrophy (5). Therefore, an intervention to combat sarcopenia should combine RE with suitable nutritional support (in particular, sufficient protein ingestion).
Although older adults may exhibit anabolic resistance, an increase in protein intake can stimulate MPS and lead to maximal MPS response, as it does in young adults (6). Evidence from systematic reviews and meta-analyses showed that a protein supplementation can augment the effect of RE on muscle mass and strength in older adults (7, 8). Moreover, short-term clinical trials (9, 10) reported that a high-protein diet increased estimated glomerular filtration rate (eGFR) in middle-aged and older adults. However, the long-term effects of a high-protein diet on renal function are uncertain (9, 11); thus, additional supplementation with relatively low amounts of protein during RE may be a more prudent option for individuals who already ingest ample protein in their normal diet and/or have mild renal insufficiency (11).
Previous studies supplied relatively low amounts of protein failed to enhance the effects of RE on muscle mass and physical performance (12, 13). Interestingly, several studies (14-16) showed greater gains in muscle mass or physical performance when both protein and vitamin D were ingested during RE, even when relatively low amounts (approximately 5-21 g/day) of supplemental protein were ingested. Some other vitamins and/or minerals may help prevent decreases in muscle mass and/or physical performance (17). Accumulating evidence suggest that anabolic resistance is regulated by the interrelationship between inflammatory markers, oxidative stress, minerals, energy and protein intakes, and insulin-like growth factor 1 (IGF-1), and micronutrient supplementation may lower the anabolic threshold (18).
Therefore, we hypothesized that low-dose dairy protein plus micronutrient supplementation augments the effects of RE on muscle mass and physical performance compared with RE alone among older adults, and aimed to examine the hypothesis in a randomized controlled trial.
Methods
Study design
This study was a 12-week, 2-arm, randomized, controlled trial that compared the short-term effects of RE and low-dose dairy protein plus micronutrient supplementation on muscle mass and physical performance with the effects of RE alone. All RE and assessment sessions were conducted at the Tokyo Metropolitan Institute of Gerontology between January and April 2015.
The required sample size was calculated by assuming a difference of 0.8 ± 1.2 kg in lean mass between the groups after 12 weeks. The estimated number of participants needed in each group is 36, for a power of 80% and an α of 0.05. With an estimated potential attrition rate of 10%, the required sample size was estimated to be greater than 40 participants in each group (80 participants in total).
The trial was registered in the UMIN Clinical Trials Registry (UMIN000015548).
Study participants and randomization
Using advertisements, we recruited study participants from residential areas surrounding Tokyo Metropolitan Institute of Gerontology. Non-disabled adults who were aged 65 to 80 years and did not exercise regularly were eligible. Among 114 individuals who participated in an explanatory meeting in December 3, 2014, 102 submitted consent forms and participated in the initial screening test in December 16, 2014. We excluded 19 individuals with at least 1 of the following exclusion criteria: 1) regular involvement in vigorous exercise and/or RE (≥30 minutes, twice a week, for a year), 2) nonstandard blood chemistry results, blood pressure, or pulse readings, 3) irregular eating or sleep schedule, 4) serious medical conditions involving major organ systems (i.e., heart, kidneys, liver, or lungs) or arthritis, 5) food allergy, lactose intolerance, or restrictions in protein intake, 6) participation in other clinical trials during the previous 3 months, and 7) ineligibility for participation, as determined by a medical doctor. The remaining 83 participants were invited to a pre-intervention assessment in January 13 and 14, 2015.
After the pre-intervention assessment, the participants were randomly allocated to either RE and low-dose dairy protein plus micronutrient supplementation (ExPM) or RE alone (Ex), using computer-generated random numbers in stratified permuted blocks with a size of four participants, stratified by age and sex. Exercise trainers and research staff who collected data were masked to the group assignments throughout the study.
Intervention
Exercise intervention
All subjects participated in the twice-weekly RE-based program for 12 weeks under the supervision of qualified exercise trainers masked to the assignment. Each exercise session consisted of a 10-minute warm-up, 45 minutes of REs (weight-bearing exercises and exercises using a resistance band and Pilates ball), and 5 minutes of cool-down routines.
The REs were designed to load the upper and lower extremities and trunk. Upper-extremity training included arm curls, shoulder lateral and front raises, pull downs, and rowing using a resistance band. Lower-extremity training included standard and split squats, as well as lunges and toe and heel raises while in a standing position. Abductions, adductions, and knee extensions were performed in a seated position. Trunk training included knee lifts while in a seated position, as well as crunches, hip lifts, and a front elbow bridge. The resistance, repetitions, sets, and speed of movements of these exercises were increased progressively using resistance bands or Pilates balls, as needed. The participants performed 2 sets of 20 repetitions by combining slow and fast movements for the respective exercises in the final month. Using the OMNI Perceived Exertion Scale for Resistance Exercise (OMNI-RES) (19), we assessed participants’ self-perceived level of exertion in the upper and lower extremities and trunk after they completed each 1-month exercise cycle and targeted an exercise intensity of approximately 5 to 7 (6 = somewhat hard).
Moreover, we instructed the participants to wear the pedometer with a 7-day data memory capacity (Omron HJA-403C, OMRON HEALTHCARE Co., Ltd, Kyoto, Japan) in a clothing pocket on their dominant leg at least 12 h per day, except when bathing, sleeping, or performing water-based activities. Participants were instructed to increase the number of daily steps in a phased manner each month and, at the end of each day, to record the number of steps taken in an exercise diary.
Dairy protein and micronutrient supplements
We instructed participants in the ExPM group to consume a protein-fortified milk-based beverage (114 kcal energy, 10.5 g total milk protein, 3.9 g fat, 9.3 g carbohydrate, and 337 mg calcium per 200-ml pack) at lunchtime and a micronutrient beverage (45 kcal energy, 10.5 g carbohydrate, 7200 μg β-carotene, 5.0 μg [200 IU] vitamin D, 30 mg vitamin E, 3.0 mg vitamin B1, 4.0 mg vitamin B2, 32 mgNE niacin, 6.0 mg vitamin B6, 12 μg vitamin B12, 200 μg folic acid, 12 mg pantothenic acid, 320 mg vitamin C, 7.5 mg iron, 8.0 mg zinc, 0.40 mg copper, and 25 μg selenium per 125-ml pack) at breakfast every day. An independent courier not involved in this study delivered these beverages to the homes of participants in the ExPM group every month. To monitor their supplement intake accurately, we instructed the participants in the ExPM to record in a diary the time of day they took the respective supplement, and the main investigator confirmed the content of the diary at every exercise session. All participants were instructed not to alter their diet during the study period.
Measurements
Main outcome measures
Body composition
We used dual-energy X-ray absorptiometry (DXA) scanning (QDA-4500 A scanner; Hologic, Waltham, MA, USA) to assess weight, fat mass, lean soft-tissue mass, and bone mineral density. Using DXA analysis software (version 9.03 D; Hologic, Waltham, MA, USA), a skilled radiation technologist, who was masked to assignment, manually analyzed the body composition of fasting participants. We calculated appendicular lean soft-tissue mass (ALM) as the sum of the arm and leg lean soft-tissue mass (LLM) and whole-body lean soft-tissue mass (WBLM) as the sum of the ALM and trunk lean soft-tissue mass. The body weight and ALM were then normalized by the height in meters squared, to determine BMI and skeletal muscle mass index.
Physical performance
Well-trained staff who were masked to assignment measured the following physical performance measures for all participants: hand-grip strength, one-leg standing with eyes open, usual and maximum gait speeds, Timed Up-and-Go, 5-repetition and 30-s chair stand tests, and knee-extension strength. All measures were performed in 2 trials, and the average was used in the analyses. The procedures for testing physical function have been described in detail previously (20-22).
Briefly, hand-grip strength was assessed using a Smedley-type hand dynamometer in the dominant hand, while in standing position. One-leg standing with eyes open was assessed using a participant’s preferred leg. Participants were asked to place their hands at their waists while starting at a mark on the wall, raise one leg, and stand as long as possible. They were timed until they lost their balance or reached the maximum of 60 s. Usual and maximum gait speeds were measured over 5 m, with acceleration and deceleration phases of 3 m each. Upon receiving the tester’s command, they started walking at their normal and maximum paces along an 11-m course. We calculated usual and maximum gait speeds as the distance (5 m) divided by walking time (m/s). In the Timed Up-and-Go test, participants were timed as they rose from a 46-cm high chair, walked 3 m forward as quickly as possible, turned 180°, walked back to the chair, and sat down. In the 5-repetition chair stand test, the time taken to stand from a sitting position 5 times without using the arms was measured. In the 30-s chair stand test, we counted the number of times participants stood up from a chair in 30 s. In both tests, they were asked to stand up and sit down on a straight-backed 46-cm-high chair as quickly as possible. The knee-extension strength was measured using a hand-held dynamometer (μTasMF-01, ANIMA, Tokyo, Japan) during isometric contraction of the knee extensor, with the participants seated, and the hip and knees at a 90° angle. Torque was expressed as a percentage of the weight (Nm/kg).
Secondary outcome measures
Biochemical measurements
We obtained morning (8:30 AM to 10:30 AM) blood samples, after a fast of longer than 12 h, from each participant’s antecubital vein and measured serum concentrations of albumin, hemoglobin, 25-hydroxyvitamin D (25[OH]D), vitamin B12, folic acid, iron, ferritin, zinc, as well as interleukin (IL)-6, tumor necrosis factor-alpha (TNF-α), high sensitivity C-reactive protein (hsCRP), and total IGF-1. Blood samples were analyzed by standardized procedures at an independent laboratory (SRL, Inc. Tokyo, Japan). We also evaluated renal function by measuring serum creatinine concentration and eGFR using equations developed for Japanese adults (23), namely, eGFR (mL/min/1.73 m2) = 194 × serum creatinine-1.094 × age-0.287 × 0.739 (if female).
Nutritional intake and physical activity
We assessed nutrient intake using a 3-day food diary. Participants were asked to record everything that they ate and drank for 3 days (2 weekdays and 1 weekend day). We provided all participants with detailed verbal and written instructions for completing their food diaries. Trained dieticians who were masked to assignment checked the submitted 3-day food diaries, and additional information on unclear items or amounts were obtained in face-to-face interviews. Total energy and nutritional intakes were calculated by using a computer program (Shokuji-shirabe 2011, National Institute of Health and Nutrition, Tokyo, Japan).
Using the Physical Activity Questionnaire for Elderly Japanese (PAQ-EJ) (24), we also assessed leisure time and habitual physical activities that included the exercise intervention, and calculated the total physical activity score (metabolic equivalents × hours/week). Moreover, we calculated the average daily step counts for 7 days before the intervention, and after 1, 2, and 3 months, using the 7-day data storage capacity of the pedometer.
Statistical analyses
All the data in the text and tables are presented as mean ± standard deviation. We used linear mixed-effects models to compare changes in all outcomes between groups, with outcomes as the dependent variables; group, time, and their interaction were defined as fixed factors; the subject was defined as a random factor; and sex, baseline age, and baseline value were defined as covariates. Similarly, changes within groups were analyzed by fitting the same model to each group, with time as the only fixed factor; and sex and baseline age as the covariates. The effect of supplementation (B) and its 95% confidence interval (CI) were calculated as an estimate of the mean differences in changes between groups, after adjusting for sex, baseline age, and baseline value. IL-6, TNF-a, and hsCRP were analyzed after log-transformation. An a of 0.05 was used to indicate statistical significance, and all statistical analyses were performed by using Stata 14.0 (StataCorp, TX, USA).
Results
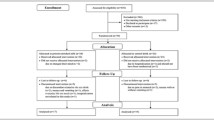
Study flow and adverse events
Figure 1 shows the study flow. Eighty-two participants were enrolled in the randomization process. Before the start of the intervention, 2 individuals (one from each group) declined to participate. The remaining 80 participants all completed the 12-week intervention and post-intervention assessments and were analyzed as the full analysis set (FAS). There were no serious injuries or severe adverse events in either group. Mild adverse effects of the intake of the protein-fortified milk-based beverage included one case each of diarrhea and an “uncomfortable feeling,” which both resolved spontaneously after consumption was suspended. These 2 individuals from the ExPM group were excluded from the FAS. Their protein-fortified milk-based beverage intake rates were 82.7% and 65.4%, respectively (the rates for the other participants were greater than 90%). Thus, 78 participants were analyzed as the per-protocol set (PPS). There were missing data (4 variables of physical performance measures, one case each in the ExPM group because of leg pain unrelated to the study) at post-intervention.
All sessions were completed as planned. The total adherence rate (number of training sessions attended/total number of sessions) was 94.6% (interquartile range, 91.3% to 100%) in the ExPM group and 95.3% (interquartile range, 91.3% to 100%) in the Ex group.
Participant characteristics, and physical activity, exercise intensity, and nutritional intake during the intervention
Table 1 summarizes the baseline characteristics. At baseline, there was no significant difference between the groups in any characteristic or outcome measure (Tables 1-5).
Table 2 shows PAQ-EJ total score, daily step counts, and OMNI-RES scores for the upper and lower extremities and trunk during the intervention in the ExPM and Ex groups. There were no significant group effects or group-by-time interactions in PAQ-EJ total score, daily step count, or OMNI-RES scores.
Table 3 shows nutritional intake (including supplementation) at baseline and post-intervention in both groups, according to PPS analysis. Changes were significantly different in the 2 groups for group-by-time, as follows: absolute (g/day) and relative (g/kg body weight/day) protein values, P = 0.005 and 0.015, respectively; vitamins D and B12; folic acid; calcium; iron; zinc, all P < 0.001. Changes over time in nutritional intake excluding the supplemented nutrients were similar between groups.
Main outcome measures
Table 4 shows body composition and physical performance measures at baseline and post-intervention in both groups, according to PPS analysis. Changes in WBLM, ALM, and LLM were significantly different between groups—the supplementation effects were 0.63 kg (95% CI: 0.31-0.95, P < 0.001), 0.37 kg (95% CI: 0.16-0.58, P = 0.001), and 0.27 kg (95% CI: 0.10-0.46, P = 0.001), respectively. Specifically, ALM and LLM significantly increased in the ExPM group but not in the Ex group. The results of the analyses of the FAS and PPS were similar (Figure 2).
For the physical performance measures, although the 2 groups exhibited significant improvements in maximum gait speed, Timed Up-and-Go, 5-repetition chair stand, and 30-s chair stand, no supplementation effects were observed. Other measures did not significantly change in either group.
Secondary outcome measures
Table 5 shows biochemical measurements at baseline and post-intervention in both groups, according to PPS analysis. Changes in serum 25(OH)D, vitamin B12, and folic acid concentrations significantly differed between groups—the supplementation effects were 4.7 ng/mL (95% CI: 1.6-7.9, P = 0.004), 72.4 pg/mL (95% CI: 12.9-131.9, P = 0.017), and 12.9 ng/mL (95% CI: 10.3-15.5, P < 0.001), respectively. With respect to other measures, no supplementation effects were observed. The results of the analyses of the FAS and PPS were similar.
Discussion
This study is the first randomized, controlled trial to present empirical evidence that supplementation with low-dose dairy protein plus micronutrients significantly increases muscle mass during RE. Despite the difference in change in muscle mass between groups, improvement in physical performance was similar, probably because neural adaptations have a greater role than change in muscle mass in RE-induced muscle strength and power gains (25). The difference in RE-induced muscle mass change between groups is partially supported by significant supplementation effects in the concentrations of serum 25(OH) D, vitamin B12, and folic acid.
Previous studies of MPS rates after an acute session of RE in older adults have shown that a protein intake of >20 g/day is necessary to stimulate MPS (6, 26). Tieland et al. (27) showed that WBLM and ALM increased in a group supplemented with protein twice daily (15 g each, total 30 g/day) during RE over 12 and 24 weeks but were unchanged in a placebo group. Conversely, many interventional studies of older adults supplied only approximately 10-15 g/day of protein, which failed to enhance the effects of RE. Leenders et al. (12) reported that additional supplementation with dietary protein (15 g/day) at breakfast over 24 weeks of RE did not further increase the effects of RE on muscle mass and strength. Likewise, Verdijk et al. (13) showed that protein supplementation immediately before and after RE (10 g each, total 20 g/day) did not further augment the effects of RE on muscle mass and strength over 12 weeks. Because those study subjects habitually consumed approximately 1.0 to 1.2 g/kg/day of protein during the study period, the authors (12, 13) concluded that protein supplementation does not modify improvements during RE when ample protein is already ingested in the normal diet.
Interestingly, although baseline protein consumption was greater among our study participants than among the subjects of their studies (12, 13), significant supplementation effects on muscle tissues were observed in the present participants receiving 10.5 g/day of dairy protein and micronutrient supplementation, as compared with RE only. The decrease in muscle tissue observed in the Ex group might have resulted from an increase in the need for dietary protein caused by increased physical activity (28). Another obvious difference between our study and the studies described above (12, 13) was the presence or absence of micronutrient supplementation.
Evidence from several studies suggests that 25(OH)D has a positive effect on MPS (17) and that a serum 25(OH)D concentration above 75 to 80 nmol/L (30 to 32 ng/mL) is needed for optimal muscle function (29). Although 47.4% of our participants in each group had a 25(OH)D deficiency (25[OH]D <20 ng/mL) [30], only the participants in the ExPM group attained sufficient levels (25[OH]D >30 ng/mL) during the study period. Moreover, B vitamins (B6, B12, and folic acid) are involved in protein and homocysteine metabolism (31), and deficiencies in these vitamins predict subsequent disability. The potential mechanisms include altered protein metabolism and increased levels of homocysteine, oxidative stress, and inflammatory markers, resulting in protein damage, and reduced muscle mass and strength (32). In a previous study of Kukuljan et al. (33), the baseline (approximately 1.3 g/kg/day) and supplemental (13.2 g/day) protein intakes of the participants were very similar to those of our present subjects. Although their study (33) included supplementation with 800 IU of vitamin D together, the interventions did not enhance the effects of RE on muscle mass and strength. With these in mind, co-ingestion of not only vitamin D, but also other micronutrients, such as B vitamins and minerals, with protein might further support MPS.
However, the definitive mechanism was not evident in the present study. Our results suggest that serum concentrations of 25(OH)D and B vitamins contributed to the discrepancy in RE-induced muscle tissue changes between groups, whereas no supplementation effects were observed in serum concentrations of IGF-1, inflammatory markers, or zinc, which are believed to influence anabolic resistance (18). Moreover, concentrations of antioxidants that were not measured in the current study might potentially play a role in preservation of muscle mass (34). Secondary analyses and future studies should continue to explore the mechanisms responsible for differences in muscle tissue change between groups and identify micronutrients that significantly contribute to RE-induced changes in muscle tissue.
Our study has several other limitations. First, we did not include a group given RE plus low-dose dairy protein alone. Second, the proportion of male subjects was low. However, our results are strengthened by the randomized controlled design, high adherence rates, participant compliance with supplement intake, and carefully controlled training. Other strengths include the large amount of biochemical data as well as the data on physical activity and nutrient intakes, which were ascertained using a 3-day food diary. Our findings may shed light on the possibility of micronutrient co-ingestion as nutritional support—most previous studies focused on the protein dose ingested.
In conclusion, supplementation with low-dose dairy protein plus micronutrients during a 12-week RE regimen significantly increased muscle mass in community-dwelling older adults, although no further improvements in physical performance were observed.
Conflict of interest statement: This study was conducted under the contract of the Tokyo Metropolitan Institute of Gerontology and Meiji Co., Ltd. and supported by research grants from Meiji Co., Ltd. KS and K. Ashida are employed by Meiji Co., Ltd. Study supplements were provided by Meiji Co., Ltd. The terms of this arrangement have been reviewed and approved by the Tokyo Metropolitan Institute of Gerontology in accordance with its policy on objectivity in research. Meiji Co., Ltd. provided support in the form of salaries for KS and K. Ashida but did not have any role in the study design, methods, subject recruitment, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgements: We are very grateful to our participants, staff members, and exercise trainers, Masahiro Koyama and Akiko Iwasaki.
Ethics: The study protocol was developed in accordance with the guidelines proposed in the Declaration of Helsinki and the current laws of the country, and was approved by the Ethical Committee of the Tokyo Metropolitan Institute of Gerontology (September 5, 2014). Written informed consent was obtained from all subjects.
References
Chen LK, Liu LK, Woo J et al. Sarcopenia in Asia: consensus report of the asian working group for sarcopenia. J Am Med Dir Assoc 2014;15:95–101. doi: 10.1016/j. jamda.2013.11.025.
Breen L, Phillips SM. Skeletal muscle protein metabolism in the elderly: Interventions to counteract the ‘anabolic resistance’ of ageing. Nutr Metab (Lond) 2011;8:68. doi: 10.1186/1743-7075-8-68.
Biolo G, Maggi SP, Williams BD et al. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol 1995;268:E514–E520.
Breen L, Phillips SM. Interactions between exercise and nutrition to prevent muscle waste during ageing. Br J Clin Pharmacol 2013;75:708–715. doi: 10.1111/j.1365-2125.2012.04456.x.
Campbell WW, Barton ML Jr, Cyr-Campbell D et al. Effects of an omnivorous diet compared with a lactoovovegetarian diet on resistance-training-induced changes in body composition and skeletal muscle in older men. Am J Clin Nutr 1999;70:1032–1039.
Yang Y, Breen L, Burd NA et al. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br J Nutr 2012;108:1780–1788. doi: 10.1017/S0007114511007422.
Cermak NM, Res PT, de Groot LC et al. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a metaanalysis. Am J Clin Nutr 2012;96:1454–1464. doi: 10.3945/ajcn.112.037556.
Malafarina V, Uriz-Otano F, Iniesta R et al. Effectiveness of nutritional supplementation on muscle mass in treatment of sarcopenia in old age: a systematic review. J Am Med Dir Assoc 2013;14:10–17. doi: 10.1016/j.jamda.2012.08.001.
Juraschek SP, Appel LJ, Anderson CA et al. Effect of a high-protein diet on kidney function in healthy adults: results from the OmniHeart trial. Am J Kidney Dis 2013;61:547–554. doi: 10.1053/j.ajkd.2012.10.017.
Ramel A, Arnarson A, Geirsdottir OG et al. Glomerular filtration rate after a 12-wk resistance exercise program with post-exercise protein ingestion in community dwelling elderly. Nutrition 2013;29:719–723. doi: 10.1016/j.nut.2012.10.002.
Knight EL, Stampfer MJ, Hankinson SE et al. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med 2003;138:460–467.
Leenders M, Verdijk LB, Van der Hoeven L et al. Protein supplementation during resistance-type exercise training in the elderly. Med Sci Sports Exerc 2013;45(3):542–52. doi: 10.1249/MSS.0b013e318272fcdb.
Verdijk LB, Jonkers RA, Gleeson BG et al. Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am J Clin Nutr 2009;89:608–616. doi: 10.3945/ ajcn.2008.26626.
Yamada M, Arai H, Yoshimura K et al. Nutritional supplementation during resistance training improved skeletal muscle mass in community-dwelling frail older adults. J Frailty Aging 2012;1:64–70.
Holm L, Olesen JL, Matsumoto K et al. Protein-containing nutrient supplementation following strength training enhances the effect on muscle mass, strength, and bone formation in postmenopausal women. J Appl Physiol, 2008;105:274–281. doi: 10.1152/japplphysiol.00935.2007.
Verreijen AM, Verlaan S, Engberink MF et al. A high whey protein-, leucine-, and vitamin D-enriched supplement preserves muscle mass during intentional weight loss in obese older adults: a double-blind randomized controlled trial. Am J Clin Nutr 2015;101:279–286. doi: 10.3945/ajcn.114.090290.
Mithal A, Bonjour JP, Boonen S et al. Impact of nutrition on muscle mass, strength, and performance in older adults. Osteoporos Int 2013;24:1555–1566. doi: 10.1007/ s00198-012-2236-y.
Maggio M, De Vita F, Lauretani F et al. IGF-1, the cross road of the nutritional, inflammatory and hormonal pathways to frailty. Nutrients 2013;5:4184–4205. doi: 10.3390/nu5104184.
Robertson RJ, Goss FL, Rutkowski J et al. Concurrent validation of the OMNI perceived exertion scale for resistance exercise. Med Sci Sports Exerc 2003;35:333–341. doi: 10.1249/01.MSS.0000048831.15016.2A.
Kim HK, Suzuki T, Saito K et al. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: a randomized controlled trial. J Am Geriatr Soc 2012;60:16–23. doi: 10.1111/j.1532-5415.2011.03776.x.
Seino S, Shinkai S, Fujiwara Y et al. Reference values and age and sex differences in physical performance measures for community-dwelling older Japanese: a pooled analysis of six cohort studies. PLoS One 2014;9:e99487. doi: 10.1371/journal. pone.0099487.
Nakazono T, Kamide N, Ando M. The reference values for the chair stand test in healthy japanese older people: determination by meta-analysis. J Phys Ther Sci 2014;26:1729–1731. doi: 10.1589/jpts.26.1729.
Matsuo S, Imai E, Horio M et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009;53:982–992. doi: 10.1053/j. ajkd.2008.12.034.
Yasunaga A, Park H, Watanabe E et al. Development and evaluation of the physical activity questionnaire for elderly Japanese: The Nakanojo Study. J Aging Phys Act 2007;15:398–411.
Hakkinen K, Kallinen M, Izquierdo M et al. Changes in agonist-antagonist EMG, muscle CSA, and force during strength training in middle-aged and older people. J Appl Physiol 1998;84:1341–1349.
Pennings B, Groen B, de Lange A et al. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am J Physiol Endocrinol Metab 2012;302:e992-E999. doi: 10.1152/ ajpendo.00517.2011.
Tieland M, Dirks ML, van der Zwaluw N et al. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 2012;13:713–719. doi: 10.1016/j.jamda.2012.05.020.
Lemon PW, Dolny DG, Yarasheski KE. Moderate physical activity can increase dietary protein needs. Can J Appl Physiol 1997;22:494–503.
Bischoff-Ferrari HA, Giovannucci E, Willett WC et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 2006;84:18–28.
Lee JH, O’Keefe JH, Bell D et al. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol 2008;52:1949–1956. doi: 10.1016/j.jacc.2008.08.050.
Selhub J. The many facets of hyperhomocysteinemia: studies from the Framingham cohorts. J Nutr 2006;136:1726S–1730S.
Bartali B, Semba RD, Frongillo EA et al. Low micronutrient levels as a predictor of incident disability in older women. Arch Intern Med 2006;166:2335–2340. doi: 10.1001/archinte.166.21.2335.
Kukuljan S, Nowson CA, Sanders K et al. Effects of resistance exercise and fortified milk on skeletal muscle mass, muscle size, and functional performance in middle-aged and older men: an 18-mo randomized controlled trial. J Appl Physiol 2009;107:1864–1873. doi: 10.1152/japplphysiol.00392.2009.
Labonte M, Dionne IJ, Bouchard DR et al. Effects of antioxidant supplements combined with resistance exercise on gains in fat-free mass in healthy elderly subjects: a pilot study. J Am Geriatr Soc 2008;56:1766–1768. doi: 10.1111/j.1532- 5415.2008.01810.x.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seino, S., Sumi, K., Narita, M. et al. Effects of Low-Dose Dairy Protein Plus Micronutrient Supplementation during Resistance Exercise on Muscle Mass and Physical Performance in Older Adults: A Randomized, Controlled Trial. J Nutr Health Aging 22, 59–67 (2018). https://doi.org/10.1007/s12603-017-0904-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-017-0904-5