Abstract
Background
Older adults experience age-related physiological changes that affect body weight and body composition. In general, nutrition and exercise have been identified as potent stimulators of protein synthesis in skeletal muscle. Milk proteins are excellent sources of all the essential amino acids and may represent an ideal protein source to promote muscle anabolism in older adults undergoing resistance training. However, several randomized control trials (RCTs) have yielded mixed results on the effects of milk proteins supplementation in combination with resistance training on body weight and composition.
Methods
PubMed, Web of Science and Cochrane databases were searched for literature that evaluated the effects of milk proteins supplementation on body weight and composition among older adults (age ≥ 60 years) undergoing resistance training up to September 2016. A random-effects model was used to calculate the pooled estimates and 95% confidence intervals (CIs) of effect sizes.
Results
The final analysis included 10 RCTs involving 574 participants (mean age range from 60 to 80.8 years). Overall, the combination of milk proteins supplementation and resistance training did not have significant effect on fat mass (0.30, 95% CI -0.25, 0.86 kg) or body weight (1.02, 95% CI: -0.01, 2.04 kg). However, a positive effect of milk proteins supplementation paired with resistance training on fat-free mass was observed (0.74, 95% CI 0.30, 1.17 kg). Greater fat-free mass gains were observed in studies that included more than 55 participants (0.73, 95% CI 0.30, 1.16 kg), and in studies that enrolled participants with aging-related medical conditions (1.60, 95% CI 0.92, 2.28 kg). There was no statistical evidence of publication bias among the studies.
Conclusion
Our findings provide evidence that supplementation of milk protein, in combination with resistance training, is effective to elicit fat-free mass gain in older adults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The global rapid growth of aging population has become a public health issue. The World Health Organization has estimated that the expansion of the global population over 60 years of age will increase from 605 million to greater than two billion between 2000 and 2050 (1). Older adults experience age-related physiological changes that affect body composition, including body weight gain, increased fat mass and decreased fat-free mass (2). Progressive loss of skeletal muscle mass or sarcopenia is associated with increased susceptibility of injury and disability, increased health risk, and reduced quality of life in older adults (3, 4). Although the exact mechanisms underlying sarcopenia remain to be investigated, age-related impairment in ability for skeletal muscle of older adults to respond to anabolic stimuli is believed to be one of the primary factors that contribute to muscle loss in the elderly (5). In general, nutrition and exercise have been identified as potent stimulators of protein synthesis in skeletal muscle (6-9). In that regard, milk proteins are excellent sources of all the essential amino acids, are readily available commercially, and are relatively inexpensive (10); thus, it may represent an ideal protein source to promote muscle anabolism and protein synthesis in older adults undergoing resistance training. However, several randomized control trials (RCTs) have yielded mixed results on the effects of milk protein supplementation in combination with resistance training on body weight and composition (11-14). Furthermore, recent meta-analyses by Cermak et al. (15) and Finger et al. (16) found that protein supplementation, when combined with regimented resistance training, enhanced fat-free mass in older adults. Although most intervention groups of trials in both metaanalyses were supplemented with milk proteins, the effects of milk proteins supplementation on body weight and composition (fat-free mass and fat mass) have not yet been specifically investigated. Rather, inclusion criteria encompassed studies in which at least one intervention group consumed a protein and/or amino acids supplement during resistance training. Therefore, we conducted a meta-analysis of randomized control trials (RCTs) to specifically examine the effect of milk proteins supplementation on body weight and body composition in older adults undergoing resistance training.
Methods
Search Strategy
This meta-analysis was planned, conducted, and reported according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) recommendation (17). We searched PubMed, Web of Science and Cochrane databases for literature that evaluated the effects of milk proteins supplementation on body weight and composition among older adults undergoing resistance training up to September 2016. We used the following search algorithm: (‘milk’ OR ‘whey’ OR ‘casein’ OR ‘protein supplementation’) AND (‘older adults’ OR ‘elderly’ OR ‘old age’) AND (‘body composition’ OR ‘body weight’ OR ‘body mass’ OR ‘body fat’ OR ‘fat mass’ OR ‘lean body mass’ OR ‘lean body tissue’ OR ‘fat-free mass’ OR ‘lean mass’) AND (‘resistance training’ OR ‘resistance exercise’ OR ‘training’ OR ‘exercise’). The search strategy had no language, publication date, or publication type restrictions. In addition, the reference lists of retrieved full publications and previous meta-analysis were reviewed to complement the search and to identify relevant studies that were missed during the electronic database search.
Eligibility Criteria
To be included in this meta-analysis, the studies had to meet the following inclusion criteria: (a) RCTs lasted at least 12 weeks; (b) one or more intervention groups receiving milk proteins supplementation (e.g. whey protein, casein) or dairy products (e.g. milk); (c) All participants were involved in resistance training; (d) The control groups could either be assigned to resistance training alone (without supplementation) or resistance training with non-protein supplementation (e.g. carbohydrate beverage); (e) participants with a mean age of ≥60 years; (f) Fat-free mass, fat mass, or body weight was evaluated. In the case of multiple publications with overlapping data from the same trial, the article with the most detailed information was selected.
Data extraction and Quality Assessment
Using a standardized data-collection form, the following study characteristics were abstracted from each study: first author’s last name, publication year and country of origin, the details of population characteristics (mean age, age range, sex distributions and health status of subjects), study duration, training details (frequency, type, and volume), supplementation details (amount, type, timing, and frequency), and outcomes assessed. The risks of selection, performance, and detection biases were evaluated from selected studies using a modified Cochrane tool for assessing risk of bias (18) (Table S1).Two investigators (K. H. and C.-G. C.) independently performed the literature search, data extraction, and quality assessment. Any discrepancies regarding inclusion were resolved by consensus. Because there was complete agreement between both reviewers regarding data searching and extraction and quality assessment, a kappa statistic was not calculated.
Statistical Analysis
Milk proteins supplementation together with resistance training was considered as the intervention arm in this metaanalysis. Resistance training with or without placebo was considered as control arm. The net changes of each outcome in the intervention and control groups were reported as differences between mean values at baseline and endpoint. For studies reporting only the mean difference between the intervention and control groups, we set the mean change of control group as zero and the mean change of intervention group as the reported mean difference. Studies with no reported Standard deviation (SD) values, had their values imputed using a standard formula (18). If only SD for the baseline and final values were provided, we computed SD for net changes using the method proposed by Folmann et al. (19) in which a correlation coefficient of 0.5 was assumed.
The degree of heterogeneity across trials was assessed using Q and I2 statistics. For the Q statistic, P<0.1 was considered statistically significant; and for the I2 statistic, the following conventional cut-off points were used: <25% (low heterogeneity), 25-50% (moderate heterogeneity), and >75% (severe heterogeneity). Both Begg’s rank correlation test and Egger’s linear regression test were performed to investigate potential publication bias (20). If evidence of publication bias was observed, the trim and fill method was applied to correct the bias (21). A random-effects model was used to calculate the pooled estimates and 95% CIs of effect sizes. To explore the possible influences of study and participant characteristics on combined effect sizes, subgroup and meta-regression analyses were performed according to sex distributions, mean age of subjects, trial duration, number of subjects, health status of subjects, protein type, amount of extra protein, and supplementation frequency. In addition, we conducted sensitivity analysis to investigate the influence of a single trial on the overall effect estimated by omitting one trial in each turn. All analyses were performed using STATA version 11.0 (StataCorp, College Station, TX, USA). A P value < 0.05 was considered to be statistically significant, unless otherwise specified.
Results
Study Characteristics
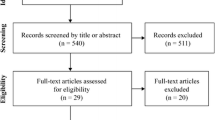
We identified 10 studies (11-14, 22-27) that fully met our inclusion criteria for this meta-analysis. A flow chart of study selection, including reasons for exclusion, is presented in Figure 1. The characteristics of the included studies are presented in Table 1. The included studies were published between 1995 and 2016. The sample size of individual trial varied from 12 to 130 participants. The mean age of the 574 participants ranged from 60 to 80.8 years. Although crossover design was not our exclusion criteria, all included studies had parallel design. Dietary compliance was measured by return of supplement packets or daily food records. Several studies (13, 24, 25, 27) reported good compliance with the supplementations, while others had no judgement. Characteristics of participants enrolled in these trials varied across studies. Notable differences in population characteristics included mobility-limited elderly in one study (25), obese elderly in one study (27), frail elderly in one study (13) and sarcopenic elderly (14) in one study. Regarding the sex of the participants, four studies were conducted exclusively in men (12, 22-24) and the remaining six were in both sexes (11, 13, 14, 25-27). Four (13, 23, 26, 27) of the included studies were conducted in Netherlands, three (11, 22, 25) in the United States, one (24) in Australia, one (14) in Italy , and one (12) in Canada.
Milk Proteins Supplementation Characteristics
The amount, frequency, and timing of milk proteins and control supplementation varied largely across studies. Regarding the amount of protein, only two studies reported protein quantity according to body weight (0.3 and 0.8 g/kg body weight/day). The remaining eight studies reported protein quantity according to daily amounts, with amounts of extra protein ranging from 14.2 g/day to 40 g/day. The sources of protein supplementation were different in intervention group of each study, consisting of milk-based beverages, a combination of whey protein, casein and egg albumin, whey protein concentrate, casein hydrolysate, fortified milk, milk protein concentrate, and whey protein. Seven studies (11, 13, 14, 24-27) provided daily protein supplementation during trial, whereas in the remaining three studies (12, 22, 23) protein supplementation was consumed on training days only. Regarding the control groups, the placebo used varied between studies and included isocaloric products, flavored beverages, low protein diet, and carbohydrate beverages.
Resistance Training Characteristics
The type, frequency, volume, and intensity of resistance exercise varied substantially across studies. The duration of interventions lasted between 12 to 72 weeks. The mean of exercise frequency was three workout sessions per week (ranged from two days per week to five days per week). The mean of resistance training intensity was 79% of 1 repetition maximum (RM) (ranged from 70-85%). Among ten studies, nine studies (11-14, 22, 24-27) prescribed whole-body training regimens, and only one study (23) prescribed lower-body exercises. The number of different exercises completed per session ranged from 2 to 10 exercises. The number of sets per exercise during each workout ranged from 2 to 4 sets. The number of repetitions per set varied from 8 to 20 repetitions.
Quality of Included Studies
Among the included studies, 40 % presented adequate random sequence generation (four of 10), 40 % reported allocation concealment (four of 10), 70 % had blinded participants and study investigators (seven of 10) and 70 % blinded assessment of outcomes (seven of 10).
Effect of Milk Proteins Supplementation on Fat-Free Mass, Fat Mass and Body Weight
Compared with control groups undergoing resistance training only, milk proteins supplementation was associated with an average net change ranging from 0.1 to 1.7 kg for fat-free mass, from -0.7 to 1.2 kg for fat mass, and -0.6 to 2.3 kg for body weight. Increase in fat-free mass was observed in the groups receiving milk proteins (0.74, 95% CI 0.30, 1.17 kg; I2 = 12.4%, P-heterogenity = 0.326) (Figure 2). However, there was no noteworthy effect of milk proteins supplementation on fat mass (0.30, 95% CI -0.25, 0.86 kg; I2 = 0%, P-heterogenity = 0.43) (Figure 3) and body weight (1.02, 95% CI -0.01, 2.04 kg; I2 = 35.3%, P-heterogenity = 0.147) (Figure 4). There was no statistical evidence of publication bias among the studies on fat-free mass (Begg, P = 0.81 and Egger, P = 0.89), fat mass (Begg, P = 1.00 and Egger, P = 0.40) and body weight (Begg, P = 0.62 and Egger, P = 0.26), respectively.
Subgroup and Sensitivity Analyses
The results of the sub-group analyses stratified by sex distributions, mean age of subjects, trial duration, number of subjects, health status of subjects, protein type, amount of extra protein and supplementation frequency are presented in Table 2. Greater fat-free mass gains were observed in studies that included more than 55 participants (0.73, 95% CI 0.30, 1.16 kg), and in studies that enrolled participants with aging-related medical conditions (frail, mobility limited and sarcopenic) (1.60, 95% CI 0.92, 2.28 kg). There was no significant change in fat-mass and body weight across subgroups. Sensitivity analyses examining the impact of a single trial on the overall results by omitting one trial in each turn yielded a range from 0.55 kg (95% CI 0.14, 1.00) to 0.82 kg (95% CI 0.41, 1.24) for fat-free mass, from -0.11 kg (95% CI -0.78, 0.55) to 0.49 kg (95% CI -0.11, 1.09) for fat mass, and from 0.85 kg (95% CI -0.32, 1.74) to 1.71 kg (95% CI 0.92, 2.49) for bodyweight.
Discussion
The present meta-analysis is the first quantitative review of randomized control trials evaluating the effects of milk proteins supplementation on changes in body weight and body composition of older adults undergoing resistance training. Overall, the combination of milk proteins supplementation and resistance training did not have significant effect on fat mass and body weight. However, a positive effect of milk proteins supplementation paired with resistance training on fat-free mass was observed. These discrepancies are difficult and challenging to explain. Although speculative, the involvement of resistance training in these studies may have prevented changes in body weight and fat mass due to milk proteins supplementation. Most of milk proteins supplements contain around 100 calories per supplement; when milk proteins supplementation was taken three to five times per week, the participants received an additional 300 calories per week or higher. Therefore, it is expected that the additional caloric intake from milk proteins supplementation may lead to increased fat mass as well as weight gain. However, this potential body weight and fat mass gains from additional caloric intake was apparently attenuated by the increased in total energy expenditure from the resistance training, resulting in noteworthy gains in both body weight and fat mass.
Meta-analyses by Cermak et al. (15) and Finger et al. (16) suggest that protein supplementation, when combined with regimented resistance training, enhanced fat-free mass in older adults. In this present meta-analysis, we only included studies which examined the effects of milk proteins on body weight and body composition in older adults undergoing resistance training instead of studies in which at least one intervention group consumed proteins from any sources. There are several reasons why we need to specifically examine the effect of milk proteins on body weight and body composition in older adults. First, milk and dairy products are widely consumed around the world on a daily basis. Second, milk proteins are excellent sources of all the essential amino acids, are readily available commercially, and are relatively inexpensive (10). Third, although consuming whole foods is the preferable way to obtain adequate protein, older adults may also have difficulty consuming adequate protein from diet alone due to several physiological factors that may influence food intake such as appetite, disturbances in taste and smell (28, 29), poor dental health (30), changes in digestive functions (31), dementia (32), and age-related impairments in the regulation of food intake (33). For example, eggs, lean meats, poultry, fish, and soy-based foods all contain high-quality protein and they are fairly inexpensive and easy to find. However, obtaining protein from whole foods can be a serious problem for older adults since it requires the ability to cook and chew, not to mention other physiological barriers experienced by older adults. Hence, it is reasonable to consider milk proteins supplements-which usually available in powder or liquid forms-as convenience way to meet protein needs may make sense. Finally, there is emerging evidence that the combination of milk proteins supplementation and resistance training may enhance fat-free mass in older adults (13, 14, 25). Thus, we hypothesize milk proteins may represent an ideal protein source to promote muscle anabolism and protein synthesis, resulting in greater gain in fat-free mass in older adults undergoing resistance training.
Our findings showed that studies using whey protein as dietary supplementation had greater fat-free mass gain, compared with studies using other kinds of milk proteins (i.e. casein, milk protein concentrated) as supplementation. We considered several possible explanations for this difference. First, whey protein is digested and absorbed rapidly, resulting in fast, high and transient elevation in plasma amino acid concentrations (hyperaminoacidemia). Hyperamidoacidemia stimulates an increase in muscle protein synthesis and anabolism (34, 35). Second, “fast-acting” protein might be more beneficial than“slow-acting” protein to limit protein loss in older people (36). Third, whey protein is rich in essential amino acid leucine, which plays a key role in muscle protein synthesis, and affects muscle hypertrophy (37, 38). Consumption of approximately 3 to 4 g of leucine is required to promote optimal protein synthesis (39, 40). Therefore, whey protein that provides at least 3 g of leucine per serving may serve as an ideal post-workout supplement in older adults. In addition, consuming repeated doses of whey protein may be beneficial to overcome the short-lived and transient nature of whey protein.
When analyzing subgroups according to health status of the participants, studies that included participants with aged-related medical conditions (frail, mobility limited and sarcopenic) achieved greater gain in fat-free mass than studies that included relatively healthy participants. Greater effect of milk proteins on fat-free mass during resistance training in participants with aged-related medical conditions is reasonable, since these individuals generally consume dietary protein below the average protein requirement, and tend to have lower fatfree mass than healthy participants (41). Moreover, genetic predisposition to poor appetite, physiological changes and medical conditions that lead to age-and disease-related anorexia, age-associated decline in physical and mental functioning that limits shopping and food preparation, and food insecurity due to financial and social constraints could be the reasons for older adults fail to meet protein requirement (42, 43).
Furthermore, participants who consumed higher doses of protein gain more fat-free mass than participants who consumed lower doses. Older adults may develop anabolic resistance, a phenomenon in which skeletal muscle fail to respond to stimulus with normal muscle protein synthesis, thus leading to slower tissue remodeling (44). The exact mechanism of anabolic resistance has not yet been determined, and several complex mechanisms have been hypothesized, including reduced protein digestion and amino acid absorption (45), reduced vasodilatory absorption of skeletal muscle to insulin (46), reduced dietary amino acid uptake in muscle (47), and impairment of mammalian target of rapamycin complex (mTORC)1 signaling (48). As the consequence of this phenomenon, older adults may require more protein/kilogram body weight than do younger adults. Thus, the observed positive effect on fat-free mass of those who consumed higher doses of protein is reasonable.
Finally, several issues warrant additional discussion. First, although older adults may indeed benefit from a higher protein intake, it should be highlighted that the optimal level of daily protein intake is not yet established and can vary greatly depending on a range of factors, including amount and type of physical activity, frequency and volume of training, etc. Second, any protein supplementation trials need to ensure an adequate energy intake to meet energy demands. In the absence of adequate energy the protein will be metabolized as an energy source in order to maintain vital organs and functions. Third, since certain types of protein happens to be digested slower or faster than any others, examining the optimal type of protein, timing of protein consumption, and distribution of protein may reveal why certain milk protein has greater effect than another on body weight and body composition outcomes. Altogether, the positive effect of milk proteins supplementation and resistance training on fat-free mass may be further extended to muscle mass through optimal dosage, type of protein, protein timing and frequency, and type and frequency of resistance exercise.
Our analysis did have limitations. First, differences in trial duration, subject characteristics (i.e. age, sex distributions, and health status), supplement protocol (i.e. dose, type of protein, timing, and frequency), and resistance training protocol (i.e. frequency, type, and volume) may all contribute to the variation in trial effects, making it harder to compare the results. Moreover, the format in which the data were presented in each studies varied widely, which made the data extraction difficult and may have influenced the extracted result. Therefore, results from our meta-analysis should always be interpreted with caution. Second, only one (11) of the ten studies included in our meta-analysis analyzed the timing of protein supplementation (before and after training) related to training session. A literature review by Stark et al. (49) put forward that the timing of protein supplementation was important, and consumption of milk protein immediately following resistance exercise was effective in promoting a positive net protein balance, which leads to increases in fatfree mass, and decreases in body fat. Third, the combination of milk protein, particularly whey protein with vitamin D (14), may also affect results. Although vitamin D supplementation is not known to augment fat-free mass gain, it is possible that interactions can take place. Fourth, although all included studies were randomized and placebo-controlled trials; blinding, allocation concealment, quality of randomization, and details of withdrawals were not always reported. Fifth, only 10 studies were eligible for this meta-analysis, four of which were conducted in participants with specific medical conditions, such as sarcopenia, frailty, mobility-limited and obesity, which may limit the generalization of the findings. Despite these limitations, this meta-analysis provides a thorough overview of the previous published studies investigating the effects of milk proteins supplementation on body weight and composition during resistance exercise training in older adults.
Conclusions
This meta-analysis of RCTs provides evidence that supplementation of milk proteins, in conjunction with resistance training, is effective to elicit fat-free mass gain in older adults. However, it does not have significant effect on fat mass and body weight. Our findings suggest that milk proteins supplementation in combination with resistance training may play a role in preventing sarcopenia and age-related muscle loss. Hence, adopting a balanced diet with adequate protein along with exercise may contribute to sarcopenia prevention. In addition, more RCTs are needed to reveal the optimal dosage and type of protein, protein, the timing and frequency of protein supplementation, and type and frequency of resistance training.
Conflict of Interests: All authors read and approved the final manuscript.
Financial Disclosure: None.
Ethical Standard: None.
References
Facts about ageing. http://www.who.int/ageing/about/facts/en/. Accessed April 2016
Ding J, Kritchevsky SB, Newman AB, Taaffe DR, Nicklas BJ, Visser M, Lee JS, Nevitt M, Tylavsky FA, Rubin SM, Pahor M, Harris TB; Health ABC Study. Effects of birth cohort and age on body composition in a sample of community-based elderly. Am J Clin Nutr 2007;85: 405–410. PMID: 17284736
Fujita S & Volpi E. Amino acids and muscle loss with aging. J Nutr 2006;136: 277–280. PMID: 16365098
Bunout D, de la Maza MP, Barrera G, Leiva L, Hirsch S. Association between sarcopenia and mortality in healthy older people. Australas J Ageing 2011;30: 89–92. doi: 10.1111/j.1741-6612.2010.00448.x.
Dickinson JM, Volpi E, Rasmussen BB. Exercise and nutrition to target protein synthesis impairments in aging skeletal muscle. Exerc Sport Sci Rev 2013;41: 216–223. doi: 10.1097/JES.0b013e3182a4e699.
Yang Y, Breen L, Burd NA, Hector AJ, Churchward-Venne TA, Josse AR, Tarnopolsky MA, Phillips SM. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men 2012;108: 1780–1788. doi: 10.1017/S0007114511007422.
Harber MP, Crane JD, Dickinson JM, Jemiolo B, Raue U, Trappe TA, Trappe SW. Protein synthesis and the expression of growth-related genes are altered by running in human vastus lateralis and soleus muscles. Am J Physiol Regul Integr Comp Physiol 2009;296: 708–714. doi: 10.1152/ajpregu.90906.2008.
Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab 2006;291: 381–387. doi: 10.1152/ajpendo.00488.2005
Pennings B, Koopman R, Beelen M, Senden JM, Saris WH, van Loon LJ. Exercising before protein intake allows for greater use of dietary protein-derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr 2011;93: 322–331. doi: 10.3945/ajcn.2010.29649.
JHoffman JR & Falvo MJ. Protein-Which is Best? Sports Sci Med 2004;3: 118–130. PMID: 24482589
Campbell WW, Crim MC, Young VR, Joseph LJ, Evans WJ. Effects of resistance training and dietary protein intake on protein metabolism in older adults. Am J Physiol 1995;268: 1143–1153. PMID: 7611390
Candow DG, Chilibeck PD, Facci M, Abeysekara S, Zello GA. Protein supplementation before and after resistance training in older men. Eur J Appl Physiol 2006;97: 548–556. doi: 10.1007/s00421-006-0223-8
Tieland M, Dirks ML, van der Zwaluw N, Verdijk LB, van de Rest O, de Groot LC, van Loon LJ. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 2012;13: 713–719. doi: 10.1016/j. jamda.2012.05.020.
Rondanelli M, Klersy C, Terracol G, Talluri J, Maugeri R, Guido D, Faliva MA, Solerte BS, Fioravanti M, Lukaski H, Perna S. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am J Clin Nutr 2016;103: 830–840. doi: 10.3945/ajcn.115.113357.
Cermak NM, Res PT, De Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr 2012;96: 1454–1464. doi: 10.3945/ajcn.112.037556.
Finger D, Goltz FR, Umpierre D, Meyer E, Rosa LH, Schneider CD. Effects of protein supplementation in older adults undergoing resistance training: a systematic review and meta-analysis. Sports Med 2015;45: 245–255. doi: 10.1007/s40279-014-0269-4.
Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 2009;6: e1000097. doi: 10.1371/journal.pmed.1000097.
Higgins J & Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, 2011 The Cochrane Collaboration. Available online: http://www. handbook-cochrane.org. Accessed on 20 June 2016
Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol 1992;45: 769–773. doi: http://dx.doi.org/10.1016/0895-4356(92)90054-Q
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315: 629–634. doi: http://dx.doi.org/10.1136/bmj.315.7109.629
Duval S & Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56: 455–463. doi:10.1111/j.0006-341X.2000.00455.x
Eliot KA, Knehans AW, Bemben DA, Witten MS, Carter J, Bemben MG. The effects of creatine and whey protein supplementation on body composition in men aged 48 to 72 years during resistance training. J Nutr Health Aging 2008;12: 208–212. doi:10.1007/s12603-009-0124-8
Verdijk LB, Jonkers RAM, Gleeson BG, Beelen M, Meijer K, Savelberg HH, Wodzig WK, Dendale P, van Loon LJ. Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly. Am J Clin Nutr 2009;89: 608–616. doi: 10.3945/ajcn.2008.26626.
Kukuljan S, Nowson C, Sanders K, Daly RM. Effects of resistance exercise and fortified milk on skeletal muscle mass, muscle size, and functional performance in middle-aged and older men: an 18-mo randomized controlled trial. J Appl Physiol 2009;107: 1864–1873. doi: 10.1152/japplphysiol.00392.2009.
Chale A, Cloutier GJ, Hau C, Phillips EM, Dallal GE, Fielding RA. Efficacy of whey protein supplementation on resistance exercise-induced changes in lean mass, muscle strength, and physical function in mobility-limited older adults. J Gerontol A Biol Sci Med Sci 2013;68, 682–690. doi: 10.1093/gerona/gls221.
Leenders M, Verdijk LB, van der Hoeven L, Van Kranenburg J, Nilwik R, Wodzig WK, Senden JM, Keizer HA, Van Loon LJ. Protein supplementation during resistance-type exercise training in the elderly. Med Sci Sports Exerc 2013;45: 542–552. doi: 10.1249/MSS.0b013e318272fcdb.
Verreijen AM, Verlaan S, Engberink MF, Swinkels S, de Vogel-van den Bosch J, Weijs PJ. A high whey protein-, leucine-, and vitamin D-enriched supplement preserves muscle mass during intentional weight loss in obese older adults: a double-blind randomized controlled trial. Am J Clin Nutr 2015;101: 279–286. doi: 10.3945/ajcn.114.090290.
Boyce JM, & Shone GR. Effects of ageing on smell and taste. Postgraduate Medical Journal 2006;82: 239–241. doi: 10.1136/pgmj.2005.039453
Guido D, Perna S, Carrai M, Barale R, Grassi M, Rondanelli M. Multidimensional Evaluation of Endogenous and Health Factors Affecting Food Preferences, Taste and Smell Perception. J Nutr Health Aging 2016;20: 971–981. doi: 10.1007/s12603-016-0703-4
Kazemi S, Savabi G, Khazaei S, Savabi O, Esmaillzadeh A, Keshteli AH, Adibi P. Association between food intake and oral health in elderly: SEPAHAN systematic review no. 8. Dent Res J (Isfahan) 2011;8: 15–20. PMID: 23372590
Morley JE (2001) Decreased food intake with aging. J Gerontol Med Sci 2001;56: 81–88. PMID: 11730241
Lin LC, Watson R, Wu SC. What is associated with low food intake in older people with dementia? J Clin Nurs 2010;19: 53–59. doi: 10.1111/j.1365-2702.2009.02962.x.
Rolls BJ, Dimeo KA, Shide DJ. Age-related impairments in the regulation of food intake. Am J Clin Nutr 1995;62: 923–931. PMID: 7572737
Dangin M, Boirie Y, Guillet C, Beaufrère B. Influence of the protein digestion rate on protein turnover in young and elderly subjects. J. Nutr 2002;132: 3228–3233. PMID: 12368423
Tang JE & Phillips SM. Maximizing muscle protein anabolism: the role of protein quality. Curr Opin Clin Nutr Metab Care 2009;12, 66–71. doi: 10.1097/MCO.0b013e32831cef75.
Dangin M, Guillet C, Garcia-Rodenas C, Gachon P, Bouteloup-Demange C, Reiffers-Magnani K, Fauquant J, Ballèvre O, Beaufrère B. The rate of protein digestion affects protein gain differently during aging in humans. J. Physiol 2003;549: 635–644. doi: 10.1113/jphysiol.2002.036897
Kimball SR & Jefferson LS. New functions for amino acids: effects on gene transcription and translation. Am J Clin Nutr 2006;83: 500–507. PMID: 16470021
Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, Dhanani S, Volpi E, Rasmussen BB. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab 2008;294: 392–400. doi: 10.1152/ajpendo.00582.2007
Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab 2004;286: 321–328. doi: 10.1152/ajpendo.00368.2003
Tipton KD, Ferrando AA, Phillips SM, Doyle D, Wolfe RR. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol 1999;276: 628–634. PMID: 10198297
Tieland M, Borgonjen-Van den Berg KJ, van Loon LJ, de Groot LC. Dietary protein intake in community-dwelling, frail, and institutionalized elderly people: scope for improvement. Eur J Nutr 2012;51: 173–179. doi: 10.1007/s00394-011-0203-6.
Volpi E, Campbell WW, Dwyer JT, Johnson MA, Jensen GL, Morley JE, Wolfe RR. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J Gerontol A Biol Sci Med Sci 2013;68: 677–681. doi: 10.1093/gerona/gls229.
Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, Cederholm T, Cruz-Jentoft A, Krznariç Z, Nair KS, Singer P, Teta D, Tipton K, Calder PC. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr 2014;33: 929–936. doi: 10.1016/j. clnu.2014.04.007.
Burd NA, Gorissen SH, van Loon LJ. Anabolic resistance of muscle protein synthesis with aging. Exerc sport Sci Rev 2013;41: 69–73. doi: 10.1097/JES.0b013e318292f3d5.
Boirie Y, Gachon P, Beaufrere B. Splanchnic and whole-body leucine kinetics in young and elderly men. Am J Clin Nutr 1997;65: 489–495. PMID: 9022534
Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, Volpi E. Insulin resistance of muscle protein metabolism in aging. FASEB J 2006;20: 768–769. doi: 10.1096/fj.05-4607fje
Dickinson JM, Drummond MJ, Coben JR, Volpi E, Rasmussen BB. Aging differentially affects human skeletal muscle amino acid transporter expression when essential amino acids are ingested after exercise. Clin Nutr 2012;32: 273–280. doi: 10.1016/j.clnu.2012.07.009.
Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Dhanani S, Volpi E, Rasmussen BB. Aging impairs contraction induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle 2011;1: 11. doi: 10.1186/2044-5040-1-11.
Stark M, Lukaszuk J, Prawitz A, Salacinski A. Protein timing and its effects on muscular hypertrophy and strength in individuals engaged in weight-training. J Int Soc Sports Nutr 2012;9: 54. doi: 10.1186/1550-2783-9-54.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hidayat, K., Chen, GC., Wang, Y. et al. Effects of milk proteins supplementation in older adults undergoing resistance training: A meta-analysis of randomized control trials. J Nutr Health Aging 22, 237–245 (2018). https://doi.org/10.1007/s12603-017-0899-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-017-0899-y