Abstract
Gastrointestinal mucositis associated with the use of chemotherapeutic drugs can seriously affect the quality of life of patients. In this study, a probiotic mixture, BIO-THREE, was used to alleviate intestinal damage caused by oxaliplatin in mice and human patients. Kunming mice were injected with 15 mg/kg of oxaliplatin twice, and BIO-THREE tablets were administered to mice for 12 days. Patients with gastric cancer undergoing oxaliplatin treatment took BIO-THREE tablets for 2 weeks. The changes in the composition of fecal microbiota both in patients and mice were analyzed using 16S rRNA high-throughput sequencing. In mice, oxaliplatin caused a drop in body weight and produced lesions in the liver and small intestines. Probiotic therapy successfully mitigated the damage caused by oxaliplatin to the intestinal tract, but it was not very effective for the liver damage and weight loss caused by oxaliplatin. The sequencing of the gut microflora indicated that oxaliplatin treatment increased the abundance of Bacteroidetes and decreased the abundance of Prevotella in mice. After taking probiotics, the feces of mice and human patients both had a higher abundance of Plovitella and a lower abundance of Bacteroides. The increase in Bacteroidetes and decrease in Prevotella in the gut community might be associated with oxaliplatin-induced intestinal damage. Probiotics appeared to be beneficial, decreasing intestinal damage by restoring the abundance of Bacteroidetes and Prevotella.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancers are the second leading cause of death globally and were responsible for an estimated 9.6 million deaths in 2018 [1]. Chemotherapy is commonly used for the treatment of cancer, and many types of chemotherapy drugs have been approved, such as oxaliplatin, 5-fluorouracil, and capecitabine [2]. Numerous adverse side effects, including emergency pain, numbness, diarrhea, and mucositis, are associated with chemotherapy, which can seriously affect the continuity of treatment and compromise the quality of life of the patient [3]. The cancer survival rate is gradually improving, leading to an increased focus on understanding the experiences of patients and the side effects that can occur during cancer chemotherapy. Chemotherapy-induced diarrhea (CID) is one of the most common digestive complications in cancer patients treated with chemotherapeutic drugs [4]. CID has been found to occur in 50–80% of cancer patients, especially those with advanced cancer [5]. Severe diarrhea, colonic perforation, and gastrointestinal tumors were reported in 0.2% of cancer patients receiving platinum-based therapy [6]. These adverse effects may mean that patients are unable to receive adequate chemotherapy dosages [7]. Recently, considerable research has been conducted into the reduction of gastrointestinal reactions during chemotherapy.
The gut microbiota is a complex ecological community in the human gastrointestinal (GI) tract. The microbiota interacts with the host biochemistry to produce normal physiology, and disruption of the microbiota can lead to the development of a wide range of diseases [4]. The gut microbiota plays a crucial role in the treatment of gastrointestinal (GI) diseases. Among other functions, the gut microbiota is reported to play a role in chemotherapy-induced gastrointestinal mucositis, by modifying the intestinal barrier function, innate immunity, and intestinal repair mechanisms [4]. Patients receiving chemotherapy show obvious changes in intestinal microbiota, including decreases in the proportion of Bifidobacterium, Clostridium cluster XIVa, and Faecalibacterium prausnitzii, and an increase in Enterobacteriaceae and Bacteroides [4, 7, 8]. The changes in microbial community structure may contribute to the development of mucositis, such as diarrhea and bacteremia.
Many studies have reported that probiotics are effective against acute infectious diarrhea, antibiotic-associated diarrhea, Clostridium difficile–associated diarrhea, hepatic encephalopathy, ulcerative colitis, irritable bowel syndrome, functional gastrointestinal disorders, and necrotizing enterocolitis [9]. Probiotics have been used to maintain gastrointestinal health by regulating the balance and homeostasis of the intestinal microbiota [9, 10]. Therefore, probiotic therapy has been designed to correct the intestinal flora and reduce the intestinal diseases induced by chemotherapy, which may be valuable in cancer treatment. At present, there is no clear evidence available about the usefulness of probiotics for chemotherapy-induced gastrointestinal reactions.
Oxaliplatin is a platinum-based chemotherapeutic that is widely used in patients with gastrointestinal cancers [11]. This drug is moderately myelotoxic and causes peripheral neuropathy, in addition to nausea, vomiting, and diarrhea [12]. Its use has been associated with changes in the composition of the gastrointestinal microbiota, such as a decrease in the proportion of Parabacteroides and Prevotella, which may influence chemotherapeutic efficacy and contribute to local and systemic inflammation [13]. The probiotic drug BIO-THREE (TOA Pharmaceuticals, Japan) has been used by humans for over 50 years [14, 15]. It has been reported that fecal microflora in patients with ulcerative colitis is altered by the intake of BIO-THREE, with the abundance of bifidobacteria increased. This change appears to be beneficial for the treatment of acute infectious diarrhea and inflammatory bowel disease [14]. In this study, we investigated the effect of probiotics on the intestinal microbiota of mice and patients receiving oxaliplatin chemotherapy. A murine model was established to evaluate the physiological side effects of oxaliplatin in mice, and the effect of probiotics on various aspects of physiology. A study was then conducted on a group of eight gastric cancer patients treated with oxaliplatin, of whom four took probiotics during chemotherapy. The shift in the gut microbiome, both in patients and mice, was investigated using Illumina MiSeq sequencing. Our aim was to provide a theoretical basis for the use of BIO-THREE in the treatment of gastric cancer.
Materials and Methods
Probiotic Agents
The probiotic BIO-THREE (200 mg tablet) used in this study is produced by the Toa Pharmaceutical Co., Ltd., Tatebayashi Plant, Japan. Each tablet contains 10 mg of Clostridium butyricum TO-A 1 × 105 – 1 × 108, 10 mg of Bacillus mesentericus TO-A: 1 × 105 – 1 × 108, and 2 mg of Streptococcus faecalis T-110: 2 × 105 – 4 × 108.
Bacillus mesentericus TO-A and Streptococcus faecalis T-110 were selected under aerobic and anaerobic condition and grown on solid tryptone soybean media (TSB, Solarbio company, Beijing, China), respectively. Colonies of these bacteria were inoculated into liquid TSB and cultivated for 12 h at 37 °C. The cells were collected after centrifugation at 8000 × g for 10 min and repeatedly washed with ultra-pure water, followed by suspension of the cells in 0.9% sodium chloride solution (w/v). Cell concentration was determined using plate counting. Clostridium butyricum TO-A was not isolated under either aerobic or anaerobic conditions. Therefore, only Streptococcus faecalis T-110 and Bacillus mesentericus TO-A were used for the animal experiments. According to previous reports, the concentration of the probiotic consortium was in the range of 108–109 CFU/mL [16, 17]. Bacillus mesentericus TO-A and Streptococcus faecalis T-110 suspensions were mixed at concentrations of 4.52 × 108 CFU/mL and 3.70 × 108 CFU/mL, respectively, to obtain a probiotic consortium. The consortium suspension was stored at 4 °C for further use and incubated at 37 °C for 10 min just before oral administration in mice.
Experimental Animals
Forty eight-week-old Kunming female mice were obtained from the Animal Experimental Center of Lanzhou University, Gansu, China, and kept in the laboratory for seven days, to adapt to the environment before starting the experiment. The mice were kept in a standard environment of temperature 25 °C ± 2 °C, humidity 50% ± 5%, and a 12-h light/12-h dark cycle, with free access to tap water and rodent chow (Keaoxieli Company, Beijing, China).
After 1 week of acclimatization, the mice were randomly separated into four experimental groups: control (CK), oxaliplatin (OXP), BIO-THREE probiotics (BT), and oxaliplatin BIO-THREE probiotics (OXPBT) (n = 12 in each group) (Table 1). Oxaliplatin was intraperitoneally injected in groups OXP and OXPBT at 15 mg/kg twice on day 0 and day 6, and 5% glucose was used as control and injected into the mice in groups CK and BT. The oxaliplatin used in mice (AskPharma Company, Nanjing, China) was the same drug used for the treatment of cancer patients at the First Hospital of Lanzhou University. The dosage of 30 mg/kg oxaliplatin was selected on the basis of a previous study [3]. From day 1 to day 12, mice in groups BT and OXPBT received 0.5 mL of probiotic mixture daily by gavage, and those in groups CK and OXP received 0.5 mL of 0.9% NaCl daily. Consumption of water and rodent chow and the body weight of the mice were observed at intervals of 3 days.
On day 12, fresh feces of the mice were collected and stored at −80 °C. All of the mice were administered mild ether anesthesia. The liver, kidneys, and jejunum of the small intestine were excised from each mouse and washed with 0.9% NaCl solution. For histopathological studies, samples were mixed with 4% paraformaldehyde saline at room temperature for 48 h and then immersed in 2.5% glutaraldehyde (Sigma, America) for 12 h at 4 °C for transmission electron microscopy (TEM) micrograph analysis. The remaining samples were collected in clean tubes and stored at −80 °C for biochemical assays.
RT-PCR of Bacillus mesentericus TO-A and Streptococcus faecalis T-110
Genomic DNA was extracted from the mice feces. Quantitative real-time PCR (qPCR) was used to quantify the total bacteria, B. mesentericus TO-A, and S. faecalis T-110 in the DNA samples of feces from the different groups. The forward and reverse primers F-tot and R-tot were used for the amplification of 16S rDNA in order to quantify the total bacteria (tot) in the feces samples [18]. The primers, F-bm/R-bm and F-sf/R-sf, were used for the amplification of 16S rDNA of B. mesentericus TO-A (bm) and S. faecalis T-110 (sf), respectively. All the primer sequences used for qPCR are listed in Online Resource 1. The PCR products of the bm, sf, and tot 16S rDNA genes were cloned in plasmid PMD-18-T (Takara). The recombinant plasmids were used to construct standard curves for qPCR, and the number of gene copies in the samples was also calculated. Reactions were conducted in a Real-Time PCR Detection System (QuantStudio® 5, Thermo Fisher Scientific, Waltham, MA) using the SYBR Green dye method with SYBR® Premix Ex TaqTM GC (Takara Bio Inc. Kusatsu, Shiga, Japan). The relative abundances of the bm and sf genes were normalized to that of the bacterial 16S rDNA gene. All measurements were carried out in triplicate.
Biochemical Assays
Aspartate amino transferase (AST) and alanine aminotransferase (ALT) kits (Jiancheng Bioengineering Institute, Nanjing, China) were used to determine the activity of AST and ALT in tissues. Protein concentrations were measured using bicinchoninic acid (BCA) Protein Assay Kits (Solarbio Company, Beijing, China). Tumor necrosis factor alpha (TNF-α) ELISA Analysis Kits (RD, USA) were used to detect the level of TNF-α in tissues. The assays were performed according to the manufacturer’s instructions. All assays were performed in triplicate.
Histopathological Studies
The preparation of paraffin sections, and the hematoxylin–eosin (H&E) staining of liver, kidney, and small intestine samples were conducted, and ultra-thin sections of liver and small intestine were also prepared.
Paraffin sections and staining samples were observed under a light microscope (OLYMPUS BX53, Japan), while the ultra-thin sections were examined using a TEM (Tecnai G2 Spirit Bio-TWIN, FEI, USA).
Sensitivity of Human Gut Microorganisms to Oxaliplatin
A fresh fecal sample was taken from a healthy adult with no history of intestinal problems. The feces were suspended in distilled water and inoculated into Mueller–Hinton broth (Solarbio, China) agar plates, which were cultured either aerobically or anaerobically at 37 °C for 48 h. When bacterial colonies appeared on the plates, they were picked one by one into test tubes, to avoid the formation of subjective judgments. There were more colonies on the aerobic plates, so ninety aerobic and ten anaerobic colonies of bacteria were picked and further purified by another plate streak separation.
Analysis of the sensitivity of the one hundred strains to oxaliplatin was conducted using the standard 96-well plate method [19]. Strains were inoculated in BH liquid medium (Solarbio, China) cultivated at 37 °C for 24 h, and the optical density was adjusted to 0.5 at an absorption of 600 nm. Aliquots of 100 µL of bacterial suspension were inoculated into 100 µL of BH medium containing 1 µg/mL oxaliplatin, or not containing oxaliplatin. After incubation at 37 °C for 24 h, an enzyme marker was used to detect the absorption at a wavelength of 600 nm. All experiments were performed in two duplicate, and the standard strain Escherichia coli ATCC 25,922 was used as the control.
Patients
Eight gastric cancer patients undergoing treatment at the First Hospital of Lanzhou University were selected as the subjects of the study. Four patients using oxaliplatin chemotherapy without taking probiotics were classified as the control group, while the other four patients, who were taking the probiotics, were placed in the probiotic group. The probiotic group patients received oxaliplatin chemotherapy and used probiotics from the first day of chemotherapy until the 14th day. Two 200-mg probiotics tablets were administered once a day. Fecal samples of the patients were collected at day 0, when patients were prepared to start chemotherapy, day 1, day 10, day 20, and day 30, after chemotherapy. The fecal samples were placed in an icebox immediately after they were obtained and transferred to −80 °C for storage.
DNA Extraction and 16S rRNA Gene Pyrosequencing
Genomic DNA from feces was extracted using TIANamp Stool DNA Kits, according to the manufacturer’s instructions (TIANGEN BIOTECH, Beijing, China), The DNA concentration and purity were measured using a NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA). Genomic DNA was sent to Genesky Technologies (Suzhou, China) for high-throughput sequencing of 16S rRNA. The primers F 5′-CCTACGGGNGGCWGCAG-3′ and R 5′-GACTACHVGGGTATCTAATCC 3′ were used to amplify the V3V4 region of the bacterial 16S rRNA genes. The sequencing of the 16S rRNA genes was conducted on an Illumina MiSeq platform. The sequence data were processed using QIIME Pipeline–Version 1.7.0 (http://qiime.org/), and the results were uploaded to the Sequence Read Archive (SRA) Database (https://doi.org/10.1093/nar/gkq1019) of NCBI under the SRA accession number PRJNA659425.
Statistical Analysis
Statistical analysis was performed using the GraphPad Prism version 8.0.1 software (GraphPad Software, San Diego, California USA, www.graphpad.com) and Excel 2010. One-way analysis of variance was used to calculate differences in the abundance of taxa. Values are presented as mean ± standard error. Tukey’s test was used to determine statistically significant differences.
Results and Discussion
Colonization of Probiotics in the Mouse Gut
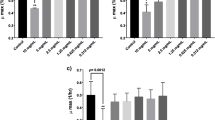
In the current study, two probiotic strains, B. mesentericus TO-A and S. faecalis T-110, were selected from the composition of the BIO-THREE tablets. A consortium of two probiotics was administered to mice for 12 days in order to determine whether the probiotics could inhabit the intestines of animals. The gene copy numbers of bm and sf, used as biomarkers of B. mesentericus TO-A and S. faecalis T-110, respectively, were determined at the end of probiotic administration after day 12 (Fig. 1 a and b). Compared with the CK group, the abundances of probiotics B. mesentericus TO-A and S. faecalis T-110 in the feces of BT and OXPBT group mice showed an evident elevation. The relative abundances of bm and sf gene in the feces of OXPBT group mice were 7.717 × 10−4% and 2.2 × 10−4%, respectively, while their relative abundance was close to zero in the feces of mice in the CK and OXP groups. The increased abundance of probiotics in the BT and OXPBT groups indicated that both B. mesentericus TO-A and S. faecalis T-110 were present in the intestines of mice after being treated with the probiotic mixture.
It has been reported that the use of BIO-THREE is safe and effective for the treatment of ulcerative colitis [14]. Yoshimatsu et al. [15] reported that probiotic BIO-THREE therapy might be effective for the patients with inactive ulcerative colitis, by improving their intestinal flora. Successful colonization of probiotic bacteria in the gut environment is an important factor for their functioning [20]. Specific primers are often used to detect the colonization of a strain in the environment [21]. The colonization of B. mesentericus TO-A and S. faecalis T-110 in the intestine was confirmed using Q-PCR, so the introduction of probiotics in the intestinal environment was considered to be successful.
Effect of Oxaliplatin on Mice
The body weight and daily water and chow intake of the mice were recorded (Fig. 2a–c). When compared to mice in group CK, no side effects on the growth or diet of the mice were apparent after probiotic administration in group BT. The body weight of the mice increased by 10% in group CK over 12 days, but the mice in groups OXP and OXPBT lost significant amounts of weight. The daily intake of water and food of mice in the OXP and OXPBT groups also decreased significantly (p < 0.02). This result indicated that oxaliplatin affected the growth and diets of the mice, while probiotics had no significant effect on the growth and diet of mice.
Influences of oxaliplatin on the physiology of mice. (a), (b) Average daily chow and water intake of mice in different groups within 12 days. (c) Changes in body weight of mice. (d), (e) ALT and AST activity in serum of mice. (f) TNF-α level in the small intestines, liver, and kidney. Comparisons were conducted using one-way analysis of variance (ANOVA) and Tukey’s multiple comparisons test. **p < 0.01 CK vs. OXP group
The increase in ALT activity in serum reflects liver tissue damage, while high levels of AST indicate severe liver tissue damage [22]. The activity of AST and ALT in mouse serum from different groups is shown in Fig. 2 d and e. No liver damage was found in mice after taking probiotics in group BT. The activity of ALT in mouse serum was increased by 50% in group OXP, while the activity of AST did not increase significantly compared to the control group. These results indicated that oxaliplatin could cause mild liver damage in mice. A small amount of β-N-acetylglucosaminidase was detected in the urine of mice, and no significant difference was found between the four groups (data not shown), indicating that oxaliplatin had no major toxic effects on the kidney. Changes in TNF-α levels are usually positively correlated with inflammation in tissues (Fig. 2f). When oxaliplatin was injected into mice in the OXP group, the TNF-α level in the small intestine increased by 42.4% over the CK group. Oxaliplatin did not cause changes in the TNF-α level in the liver and kidney (p > 0.05). Among these tissues, the small intestine was most affected by oxaliplatin. However, when probiotics were administered to the mice, the TNF-α level dropped close to that of the CK group, showing that the increase of TNF-α caused by oxaliplatin in the small intestine was ameliorated (p < 0.02). This result indicated that oxaliplatin had a negative effect on the small intestine, which was counteracted by the probiotics.
Reduced food consumption due to gastrointestinal side effects and nausea is associated with chemotherapy treatment in humans [23]. The significant weight loss in mice treated with oxaliplatin was similar to that observed in previous studies in which oxaliplatin-based chemotherapy drugs resulted in weight loss or weight gain in rodents [3, 23]. Mice treated with oxaliplatin, displayed significant changes in the daily intake of water and food, which were consistent with the loss of body weight. Significant increases in TNF-α were found in the small intestine of mice, indicating that oxaliplatin might disturb the gut and cause intestinal damage. A pervious study showed that patients with chemotherapy-induced diarrhea have a higher serum TNF-α level [24]. It has been reported that the distribution of oxaliplatin in the tissues of mice is not altered by changes in the gut microbiota [7]. The administration of probiotics reduced intestinal TNF-α but had a little adverse effect on the liver, diet, or growth of the mice. Therefore, the use of probiotics might be helpful to reduce intestinal side effects caused by oxaliplatin.
Histopathological Studies
Paraffin and ultra-thin sections of different tissues were prepared, and histopathological variations were observed. As shown in Fig. 3a–d, a loss of intact liver plates and cytoplasmic vacuolization, were observed in the liver tissue of mice in the OXP and OXPBT groups. There were no obvious differences in the kidney tissue sections among the four groups (Fig. 3e–h). Photomicrographs of the small intestines showed that oxaliplatin reduced the length of the villi of the small intestine of the OXP group and also caused erosion of the submucosa (Fig. 3i–l). When the mice were treated with probiotics in the OXPBT group, the villi of the small intestine became uniform and increased in length compared to the OXP group, and no erosion was observed in the submucosa. Further observations of the microstructure of the liver and small intestine of mice, using TEM, are shown in Fig. 3m–t. The microstructure of the liver cells of the mice changed in the cytoplasm of the OXP and OXPBT groups, implying that oxaliplatin caused injury to the liver tissue. The TEM micrographs of small intestinal tissues revealed that many chromatin fragments appeared around cells in the OXP group, and there was chromatin condensation in the nuclei, as the result of apoptosis in the small intestinal villi cells under oxaliplatin treatment. However, there were few chromatin fragments around the cells of the OXPBT group after the administration of probiotics. These results indicate that oxaliplatin caused apoptosis and shedding of villus cells in the small intestine. Probiotics reduced the intestinal side effects caused by oxaliplatin and protected the villi of the intestine.
H&E staining of liver, kidney, and small intestine tissues. a–d Micrograph of H&E staining of liver. e–h Micrograph of H&E staining of kidney. i–l Micrograph of H&E staining of small intestine. m–p Projection electron micrograph of H&E staining of liver. q–t Projection electron micrograph of H&E staining of small intestine
In the current study, the concentration of oxaliplatin in the tissues could not be detected, due to the limited detection accuracy of the approaches used. Shen et al. [7] reported that the platinum concentrations in the spinal cord, dorsal root ganglion, and serum of mice increased to 1 mmol/g, 4 mmol/g, and 2 mmol/g, respectively, after the administration of 15 mg/kg oxaliplatin. According to another study, platinum concentrations in human plasma range from 349 to 812 L, and platinum exposure values in plasma and blood cells were typically 207 ± 60.9 and 1326 ± 570 µg·h/mL, respectively [25]. Oxaliplatin could be distributed in multiple organs of the mice after intravenous injection. Therefore, oxaliplatin, as a cytotoxic substance, may have varying degrees of toxicity to tissue cells. Cancer patients experience gastrointestinal toxicity after receiving platinum-based therapy [6]. Previous researchers have reported that oxaliplatin causes the intestinal villi to shorten [26], indicating that the intestinal villus cells are very sensitive to oxaliplatin. Our study results clearly illustrate that probiotics have an effect on the repair of intestinal villi, although they have some limitations on the other adverse effects caused by oxaliplatin.
Gut Microbial Community in Mice
The microbial community of the mouse gut was studied using high-throughput sequencing (Fig. 4). Principal coordinate analysis indicated that the microbial community of the members of group OXP was different from that of the control group, indicating that oxaliplatin treatment significantly changed the intestinal microflora in mice. However, the microbial community of the OXPBT group was the same as that of the control when the mice were administered probiotics, indicating that probiotics can maintain the stability of the gut microbial structure. The composition of the microbiome did not show any statistically significant difference at the phylum level, while the genus level structure of microorganisms showed that the abundance of Prevotella and Bacteroides was significantly changed by treatment with oxaliplatin (Fig. 4b–d). Oxaliplatin decreased the abundance of Prevotella from 10.66 to 0.003%, while it increased the abundance of Bacteroides from 14.54 to 25.18%. However, when probiotics were used in the OXPBT group, the abundance of Prevotella and Bacteroides was close to that of the CK group. This result indicated that Prevotella and Bacteroides were susceptible to oxaliplatin, and probiotics played an important role in stabilizing their abundance.
Microbial community of gut of mice in different groups. (a) Principal coordinate analysis of microbial community. (b) Microbial components at the genus level. (c), (d) The relative abundance of Bacteroides and Prevotella in different group. Comparisons were performed using one-way ANOVA followed by Tukey’s post-hoc test (c, d). *p < 0.05, **p < 0.01 CK vs. OXP group
There is considerable evidence that chemotherapeutic drugs affect the gut flora [27, 28]. A study into rats treated with methotrexate showed that the animals developed mucositis accompanied by decreased microbial abundance and increased Bacteroides abundance [29]. The change in the gut microflora might be related to chemotherapy-induced mucositis. Bacteroides species are known to be the predominant anaerobes in the gut. The bacteria maintain a complex and generally mutual relationship with the host when they reside in the gut, and their role as commensals has been extensively reviewed [30]. However, particular species of Bacteroides, such as B. fragilis and B. thetaiotaomicron, have been found to be involved in anaerobic infections. According to the Wadsworth anaerobe collection database, Bacteroides species have been isolated from more than 3000 clinical specimens [30]. Bacteroides species were the most common organisms isolated from the intra-abdominal sepsis infection, accounting for 95% of these infections [30]. The polysaccharide capsule and histolytic enzymes discovered in B. fragilis have roles in abscess formation and tissue destruction [30]. Multiple studies have reported that toxic substances such as Cr(VI) and deoxynivalenol can cause intestinal damage when they are administered to mice, and the abundance of Bacteroides in the intestinal microflora also increases [16, 31]. This observation implies that the increase in Bacteroides might be associated with chemotherapy-induced mucosal damage. Prevotella strains are generally considered to be commensal bacteria, due to their extensive presence in the healthy human body and their rare involvement in infections [32]. Subjects with high levels of Prevotella usually have lower levels of Bacteroides, suggesting that taxa from these two genera compete for the same niche in the gut [33, 34]. In the current study, a decrease in the Prevotella/Bacteroides ratio was observed in the mouse gut following oxaliplatin treatment, while the Prevotella/Bacteroides ratio was restored after taking probiotics.
Effect of Oxaliplatin on the Human Gut Microbiome
To determine whether gut microbes are sensitive to oxaliplatin, 100 strains from the human gut were selected at random, and their sensitivity to 1 µg/mL oxaliplatin was analyzed. The OD600 of bacterial growth was measured, and a decrease below 20% at 1 µg/mL oxaliplatin was considered to indicate growth inhibition. We concluded that 1 µg/mL oxaliplatin had a significant inhibitory effect on 84 of the 100 strains (Fig. 5a), indicating that the intestinal microorganisms were sensitive to oxaliplatin.
(a) The effect of 1 µg/mL oxaliplatin on the growth of 100 strains of cultured intestinal bacteria, the cell concentration was detected by the microplate reader at the absorbance at 600 nm. (b) Microbial components at the genus level of patients non-taking probiotics (P1–P4) and patients taking probiotics (BP1–BP4). The number after the patient number represents the sampling time, and the chemotherapy time is the start time
Experiments in mice indicated that probiotics could repair changes in the intestinal flora caused by oxaliplatin. Fecal samples of eight patients undergoing oxaliplatin treatment were taken at different times. The patients who were not taking probiotics were denoted as P1, P2, P3, and P4, while the patients taking BIO-THREE probiotic were dubbed BP1, BP2, BP3, and BP4. Routine blood test results of patients before and after chemotherapy are shown in Table S2. The results of sequencing of the microbial community were analyzed and are shown in Fig. 5b. At the genus level, Bacteroides and Prevotella were the main genera in the gut of patients. A high abundance of Prevotella was observed, as 15.22% in BP1, 36.62% in BP2, and 42.0% in PB4, the patients taking probiotics, while a low abundance of Prevotella was observed in the patients who were not taking probiotics. The abundance of Prevotella constantly increased over time in patients BP1 and BP4, and the Bacteroides abundance decreased. The abundance of Bacteroides was recorded as 18.33% in BP1, 3.45% in BP2, and 21.60% in BP3, patients taking probiotics. However, a high abundance rate of Bacteroides was observed in patients who were not taking probiotics: 50.46% in P1, 55.39% in P2, and 53.97% in P3. Compared to the patients not taking probiotics, more Prevotella and fewer Bacteroides were found in patients taking probiotics. A similar trend was observed in the mouse experiment, in which an increase in the Prevotella/Bacteroides ratio in the gut microbiome was found after the intake of probiotics. The abundance of Streptococcus in the intestine of patients not taking probiotics was close to zero, but it reached 12.51% in patient BP3, who was taking probiotics, possibly due to the colonization of the gut by the organisms in the probiotics.
A previous study reported that 2.1% of oxaliplatin was excreted in feces when patients received a single dose of oxaliplatin at 130 mg/m2 [25]. In our study, it was established that the gut bacteria were sensitive to 1 µg/mL oxaliplatin. Therefore, when patients are injected with oxaliplatin for treatment, their intestinal microorganisms might be affected. Patients receiving chemotherapy exhibit noticeable changes in intestinal microbiota, most frequently an increase in Bacteroides [8]. In normal gut microflora, around 25% of species are Bacteroides [30], while the abundance of Bacteroides was approximately 50% in patients who were on oxaliplatin treatment in the current study. Members of the Bacteroides group are the most prevalent anaerobic bacteria in infections [30] and are often isolated from human clinical specimens [35]. Changes in gut flora may contribute to the development of mucositis, particularly diarrhea and bacteremia [8]. In this study, the abundance of Bacteroides was close to 20% in patients BP1 and BP3 who were taking probiotics. Therefore, probiotics may be effective in repairing the imbalance of gut microflora caused by chemotherapy. In human experiments, the effect of probiotics on the intestinal tract should be the joint action of the three strains of bacteria in the tablet, and their similar effects were apparent in mouse experiments when the mice were fed two strains of probiotics.
There may be two explanations for oxaliplatin’s intestinal toxicity. First, oxaliplatin is distributed in the intestinal villi cells, causing villi cells to undergo apoptosis. Second, oxaliplatin enters the intestine, causing a change in intestinal flora and increasing the number of Bacteroides. Some bacteria, especially some types of Bacteroides, can further infect damaged mucosa and cause inflammation. A dysregulation of the intestinal flora and intestinal inflammation could lead to increased permeability of the intestinal mucosal [36]. Probiotics effectively reduce the abundance of Bacteroides and repair the changes in the intestinal flora, which may reduce the risk of bacterial infection of the intestinal mucosa, thereby reducing the damage to villi caused by oxaliplatin. Probiotics are effective in the maintenance of intestinal ecological balance, but the nature of the interactions between the bacteria is not very clear. The sensitivity of the isolated probiotics to oxaliplatin was also studied, and we found that the probiotics were similar to most intestinal microbes and were sensitive to 1 µg/mL oxaliplatin (data not shown). Probiotics might reduce the damage caused by toxic substances to the intestinal flora through their antioxidant effects [16].
Conclusions
In this study, we found that oxaliplatin affected the growth and diet of mice and damaged the liver and small intestine. The probiotics used in the current study significantly decreased oxaliplatin-induced damage in the small intestine, although it did not affect other side effects. The abundance of Bacteroides was increased while that of Prevotella was decreased in the mouse gut microbiome during oxaliplatin therapy. Probiotics were effective in reducing intestinal damage and restoring the abundance of Bacteroidetes and Prevotella. Patients taking probiotics have higher Prevotella/Bacteroides ratios in the gut microbiome.
Availability of Data and Material
The datasets generated during the current study are available in the NCBI Sequence Read Archive (SRA) Database (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA659425/).
References
Newsroom (2018) Cancer. World Health Organization. https://www.who.int/health-topics/cancer#tab=tab_1
Ruiz-González R, Milán P, Bresolí-Obach R, Stockert JC, Villanueva A, Cañete M, Nonell S (2017) Photodynamic synergistic effect of pheophorbide a and doxorubicin in combined treatment against tumoral cells. Cancers (Basel) 9:18. https://doi.org/10.3390/cancers9020018
Feather CE, Lees JG, Makker PGS, Goldstein D, Kwok JB, Moalem-Taylor G, Polly P (2018) Oxaliplatin induces muscle loss and muscle-specific molecular changes in mice. Muscle Nerve 57:650–658. https://doi.org/10.1002/mus.25966
Wang J, Feng W, Zhang S, Chen L, Tang F, Sheng Y, Ao H, Peng C (2019) Gut microbial modulation in the treatment of chemotherapy-induced diarrhea with Shenzhu Capsule. BMC Complement Altern Med 19:126. https://doi.org/10.1186/s12906-019-2548-y
Stein A, Voigt W, Jordan K (2010) Chemotherapy-induced diarrhea: pathophysiology, frequency and guideline-based management. Ther Adv Med Oncol 2:51–63. https://doi.org/10.1177/1758834009355164
Abu-Sbeih H, Mallepally N, Goldstein R, Chen E, Tang T, Dike UK, Al-Asadi M, Westin S, Halperin D, Wang Y (2020) Gastrointestinal toxic effects in patients with cancer receiving platinum-based therapy. J Cancer 11:3144–3150. https://doi.org/10.7150/jca.37777
Shen S, Lim G, You Z, Ding W, Huang P, Ran C, Doheny J, Caravan P, Tate S, Hu K, Kim H, McCabe M, Huang B, Xie Z, Kwon D, Chen L, Mao J (2017) Gut microbiota is critical for the induction of chemotherapy-induced pain. Nat Neurosci 20:1213–1216. https://doi.org/10.1038/nn.4606
Touchefeu Y, Montassier E, Nieman K, Gastinne T, Potel G, Bruley des Varannes S, Le Vacon F, de La Cochetiere MF, (2014) Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis - current evidence and potential clinical applications. Aliment Pharmacol Ther 40:409–421. https://doi.org/10.1111/apt.12878
Wilkins T, Sequoia J (2017) Probiotics for gastrointestinal conditions: a summary of the evidence. Am Fam Physician 96:170–178
Seminario-Amez M, Lopez-Lopez J, Estrugo-Devesa A, Ayuso-Montero R, Jane-Salas E (2017) Probiotics and oral health: a systematic review. Med Oral Patol Oral Cir Bucal 22(3):e282–e288. https://doi.org/10.4317/medoral.21494
Wan C-F, Zheng L-L, Liu Y, Yu X (2016) Houttuynia cordata Thunb reverses oxaliplatin-induced neuropathic pain in rat by regulating Th17/Treg balance. Am J Transl Res 8:1609–1614
Marmol I, Quero J, Rodriguez-Yoldi MJ, Cerrada E (2019) Gold as a possible alternative to platinum-based chemotherapy for colon cancer treatment. Cancers (Basel) 11(6):780. https://doi.org/10.3390/cancers11060780
Chamaillard M, Stojanovska V, McQuade RM, Fraser S, Prakash M, Gondalia S, Stavely R, Palombo E, Apostolopoulos V, Sakkal S, Nurgali K (2018) Oxaliplatin-induced changes in microbiota, TLR4+ cells and enhanced HMGB1 expression in the murine colon. PLoS ONE 13(6):e0198359. https://doi.org/10.1371/journal.pone.0198359
Tsuda Y, Yoshimatsu Y, Aoki H, Nakamura K, Irie M, Fukuda K, Hosoe N, Takada N, Shirai K, Suzuki Y (2007) Clinical effectiveness of probiotics therapy (BIO-THREE) in patients with ulcerative colitis refractory to conventional therapy. Scand J Gastroenterol 42:1306–1311. https://doi.org/10.1080/00365520701396091
Yoshimatsu Y, Yamada A, Furukawa R, Sono K, Osamura A, Nakamura K, Aoki H, Tsuda Y, Hosoe N, Takada N, Suzuki Y (2015) Effectiveness of probiotic therapy for the prevention of relapse in patients with inactive ulcerative colitis. World J Gastroenterol 21:5985–5994. https://doi.org/10.3748/wjg.v21.i19.5985
Feng P, Ye Z, Han H, Ling Z, Zhou T, Zhao S, Virk AK, Kakade A, Abomohra AE, El-Dalatony MM, Salama ES, Liu P, Li X (2020) Tibet plateau probiotic mitigates chromate toxicity in mice by alleviating oxidative stress in gut microbiota. Commun Biol 3:242. https://doi.org/10.1038/s42003-020-0968-3
Huber VC, Abdo Z, LeCureux J, LaVoy A, Eklund B, Ryan EP, Dean GA (2019) Impact of oral probiotic Lactobacillus acidophilus vaccine strains on the immune response and gut microbiome of mice. PLoS ONE 14(12):e0225842. https://doi.org/10.1371/journal.pone.0225842
Liu M, Lu X, Khan A, Ling Z, Wang P, Tang Y, Liu P, Li X (2019) Reducing methylmercury accumulation in fish using Escherichia coli with surface-displayed methylmercury-binding peptides. J Hazard Mater 367:35–42. https://doi.org/10.1016/j.jhazmat.2018.12.058
Schultz KK, Strait EL, Erickson BZ, Levy N (2012) Optimization of an antibiotic sensitivity assay for Mycoplasma hyosynoviae and susceptibility profiles of field isolates from 1997 to 2011. Vet Microbiol 158:104–108. https://doi.org/10.1016/j.vetmic.2012.02.002
Yu X, Shi J, Khan A, Yun H, Zhang P, Zhang P, Kakade A, Tian Y, Pei Y, Jiang Y, Huang H, Wu K, Li X (2020) Immobilized-microbial bioaugmentation protects aerobic denitrification from heavy metal shock in an activated-sludge reactor. Bioresour Technol 307:123185. https://doi.org/10.1016/j.biortech.2020.123185
Yao Y, Lu Z, Zhu F, Min H, Bian C (2013) Successful bioaugmentation of an activated sludge reactor with Rhodococcus sp. YYL for efficient tetrahydrofuran degradation. J Hazard Mater 261:550–558. https://doi.org/10.1016/j.jhazmat.2013.08.007
Sookoian S, Pirola CJ (2015) Liver enzymes, metabolomics and genome-wide association studies: from systems biology to the personalized medicine. World J Gastroenterol 21:711–725. https://doi.org/10.3748/wjg.v21.i3.711
Sorensen JC, Petersen AC, Timpani CA, Campelj DG, Cook J, Trewin AJ, Stojanovska V, Stewart M, Hayes A, Rybalka E (2017) BGP-15 protects against oxaliplatin-induced skeletal myopathy and mitochondrial reactive oxygen species production in mice. Front Pharmacol 8:137. https://doi.org/10.3389/fphar.2017.00137
Stringer AM, Al-Dasooqi N, Bowen JM, Tan TH, Radzuan M, Logan RM, Mayo B, Keefe DM, Gibson RJ (2013) Biomarkers of chemotherapy-induced diarrhoea: a clinical study of intestinal microbiome alterations, inflammation and circulating matrix metalloproteinases. Support Care Cancer 21:1843–1852. https://doi.org/10.1007/s00520-013-1741-7
Graham MA, Lockwood GF, Greenslade D, Brienza S, Bayssas M, Gamelin E (2000) Clinical pharmacokinetics of oxaliplatin: a critical review. Clin Cancer Res 6:1205–1218
Mi H, Dong Y, Zhang B, Wang H, Peter CCK, Gao P, Fu H, Gao Y (2017) Bifidobacterium infantis ameliorates chemotherapy-induced intestinal mucositis via regulating T cell immunity in colorectal cancer rats. Cell Physiol Biochem 42:2330–2341. https://doi.org/10.1159/000480005
Zwielehner J, Lassl C, Hippe B, Pointner A, Switzeny OJ, Remely M, Kitzweger E, Ruckser R, Haslberger AG (2011) Changes in human fecal microbiota due to chemotherapy analyzed by TaqMan-PCR, 454 sequencing and PCR-DGGE fingerprinting. PLoS ONE 6(12):e28654. https://doi.org/10.1371/journal.pone.0028654
Alexander JL, Wilson ID, Teare J, Marchesi JR, Nicholson JK, Kinross JM (2017) Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol 14:356–365. https://doi.org/10.1038/nrgastro.2017.20
Fijlstra M, Ferdous M, Koning AM, Rings EH, Harmsen HJ, Tissing WJ (2015) Substantial decreases in the number and diversity of microbiota during chemotherapy-induced gastrointestinal mucositis in a rat model. Support Care Cancer 23:1513–1522. https://doi.org/10.1007/s00520-014-2487-6
Wexler HM (2007) Bacteroides: the good, the bad, and the nitty-gritty. Clin Microbiol Rev 20:593–621. https://doi.org/10.1128/CMR.00008-07
Saint-Cyr MJ, Perrin-Guyomard A, Houee P, Rolland JG, Laurentie M (2013) Evaluation of an oral subchronic exposure of deoxynivalenol on the composition of human gut microbiota in a model of human microbiota-associated rats. PLoS ONE 8(11):e80578. https://doi.org/10.1371/journal.pone.0080578
Precup G, Vodnar DC (2019) Gut Prevotella as a possible biomarker of diet and its eubiotic versus dysbiotic roles: a comprehensive literature review. Br J Nutr 122:131–140. https://doi.org/10.1017/S0007114519000680
Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, Hallen A, Martens E, Bjorck I, Backhed F (2015) Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab 22:971–982. https://doi.org/10.1016/j.cmet.2015.10.001
Koren O, Knights D, Gonzalez A, Waldron L, Segata N, Knight R, Huttenhower C, Ley RE (2013) A guide to enterotypes across the human body: meta-analysis of microbial community structures in human microbiome datasets. PLOS Comput Biol 9:e1002863. https://doi.org/10.1371/journal.pcbi.1002863
Veloo ACM, Baas WH, Haan FJ, Coco J, Rossen JW (2019) Prevalence of antimicrobial resistance genes in Bacteroides spp. and Prevotella spp. Dutch clinical isolates. Clin Microbiol Infect 25:1156 e1159–1156 e1113. https://doi.org/10.1016/j.cmi.2019.02.017
Forsgard RA, Marrachelli VG, Korpela K, Frias R, Collado MC, Korpela R, Monleon D, Spillmann T, Osterlund P (2017) Chemotherapy-induced gastrointestinal toxicity is associated with changes in serum and urine metabolome and fecal microbiota in male Sprague-Dawley rats. Cancer Chemother Pharmacol 80:317–332. https://doi.org/10.1007/s00280-017-3364-z
Acknowledgements
We would like to thank the central laboratory of School of Life Science, Lanzhou University, for providing various instruments and equipment.
Funding
This work was supported by Fundamental Research Funds for the Central Universities grant (grant numbers: lzujbky-2017-br01), National Natural Science Foundation grant (grant numbers: 31870082), and Lanzhou science and technology plan project (grant numbers: 2019-4-40).
Author information
Authors and Affiliations
Contributions
Conceptualization, Wenzhen Yuan and Xingpeng Xiao; Methodology, Xingpeng Xiao; Software, Fuquan Xie, Jingyuan Wu; Investigation, Xingpeng Xiao, Xuan Yu, Pengya Feng, and Ze Ye; Resources, Peng Zhang, Wenzhen Yuan, and Xiangkai Li; Writing—original draft preparation, Xuan Yu; Writing—review and editing, Kamran Malik; Project administration, Xiangkai Li. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
All the animal experiments were performed in accordance with the approval of the Committee on the Ethics of Animal Experiments of School of Life Sciences of Lanzhou University, China (201912021).
Consent to Participate
In the research that involves human subjects, all subjects gave their informed consent for inclusion before they participated in the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yuan, W., Xiao, X., Yu, X. et al. Probiotic Therapy (BIO-THREE) Mitigates Intestinal Microbial Imbalance and Intestinal Damage Caused by Oxaliplatin. Probiotics & Antimicro. Prot. 14, 60–71 (2022). https://doi.org/10.1007/s12602-021-09795-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-021-09795-3