Abstract
Salmonella is an important zoonotic pathogen and is a major cause of gastrointestinal diseases worldwide. The current serious problem of antibiotic abuse has prompted the search for new substitutes for antibiotics. JH-3 is a small antimicrobial peptide with broad-spectrum bactericidal activity. In this study, we showed that JH-3 has good bactericidal activity towards the clinical isolate Salmonella enterica serovar Typhimurium strain CVCC541. The minimum inhibitory concentration (MIC) of JH-3 against this bacterium was determined to be 100 μg/mL, which could decrease the number of CVCC541 cells by 1000-fold in vitro within 5 h. The transmission electron microscopy (TEM) results showed that JH-3 can damage the cell wall and membrane of CVCC541, leading to the leakage of cell contents and subsequent cell death. To measure the bactericidal activity of CVCC541-infected mice were treated intraperitoneally 40 or 10 mg/kg JH-3 at 2 h or 3 days postinfection. Our results showed that treatment with 40 mg/kg JH-3 at 2 h postinfection had the best therapeutic effect and could significantly protect mice from a lethal dose of CVCC541. Furthermore, the clinical symptoms, bacterial burden in blood and organs, and intestinal pathological changes were all decreased and were close to normal. This study examined the therapeutic effect of the antimicrobial peptide JH-3 against S. enterica CVCC541 infection for the first time and determined the therapeutic effect of different JH-3 doses and treatment times, laying the foundation for studies of new antimicrobial agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salmonella is an important zoonotic pathogen that is a major cause of gastrointestinal disease worldwide [1], and approximately 21 million cases of typhoid fever are reported annually worldwide [2]. The genus Salmonella is of particular clinical relevance in developed and developing countries, where this pathogen is one of the most common causes of food-borne illness and is a major cause of diarrhoeal diseases [3]. According to information published by the CDC (http://www.cdc.gov/salmonella/general/index.html), approximately 40,000 Salmonella infections are reported annually in the USA alone, although the actual number is likely 30-fold greater or more due to the absence of diagnosis or reporting of many milder cases [4]. Therefore, the pathogenesis of Salmonella infections has been extensively studied over the past few decades.
The issue of antibiotic resistance in Salmonella has become more severe with the overuse of antibiotics. A previous study showed that 39.6% of 464 Salmonella strains isolated from Moroccan (Morocco) food exhibited tolerance to at least one antibiotic. Among these samples, the greatest resistance was observed for nalidixic acid and sulfonamides, up to 27.1 and 25%, respectively [5]. Salmonella strains resistant to extended-spectrum β-lactamases (ESBLs) have been isolated from the Brazilian region in recent years, and the expression of the antibiotic resistance genes encoding CTX-M-8 and CTX-M-2 in these strains was as high as 40% [6]. Studies by Iwamoto M and others investigated Salmonella infections in humans and the presence of the bacterium in retail meat and animal food in the USA from 1996 to 2013, revealing that the incidence of ceftriaxone-resistant Salmonella was as high as 40% in adults and that ceftriaxone resistance in beef and poultry meat was widespread [7]. Ciprofloxacin (CPFX) is one of the primary drugs used for the clinical treatment of Salmonella infections, but numerous CPFX-resistant Salmonella isolates have already been reported [8]. Therefore, it is necessary to develop new anti-Salmonella drugs with high activity, reliability and to which drug resistance cannot easily be developed.
Antimicrobial peptides (AMPs) are important components of the innate immune system [9] and constitute the body’s first line of defence. AMPs have antibacterial, antiviral, antitumour and immune regulation activities, among others. Compared to antibiotics, the antibacterial mechanism of AMPs is unique, making them difficult to develop drug resistance against, and these peptides also have low toxicity [10]. Thus, AMPs may be ideal alternatives to antibiotics. A preliminary study showed that the AMP P3 from bovine red blood cells has bactericidal activity, suggesting that it has clinical development potential [11]. We subsequently increased the antibacterial activity of JH-3 by shortening its amino acid sequence to 18 amino acids, and JH-3 showed little cytotoxicity towards human red blood cells (hRBCs), HaCaT cells and mice [12]. In this study, to evaluate the bactericidal and treatment effect of the antimicrobial peptide JH-3 on Salmonella, we assayed Salmonella typhimurium strain CVCC541 and observed that JH-3 has a good killing effect on this bacterium in vitro. Furthermore, treatment of mice with JH-3 2 h after they were infected with a lethal dose Salmonella typhimurium strain CVCC541 effectively prevented mortality in these animals, laying the foundation for its use in the clinical treatment of Salmonella infections and for the development of veterinary drugs.
Materials and Methods
Bacterial Strain and Culture Conditions
Salmonella enterica serovar Typhimurium strain CVCC541 was purchased from the China Institute of Veterinary Drug Control (Beijing, China) and was grown in lysogeny broth (LB) media at 37 °C.
Determination of the MIC
The peptide JH-3 (purity ≥ 98.0%; 18 amino acid residues [RRFKLLSHSLLVTLASHL]; molecular weight, 2091.51 Da; aliphatic index, 151.67; and net charge, 12) was synthesised by Shanghai Gil Biochemical Co., Ltd., China, and was dissolved in dH2O. The MIC of JH-3 for Salmonella typhimurium strain CVCC541 was determined in triplicate using a broth microdilution method as recommended by the Clinical and Laboratory Standards Institute [13]. The MIC was defined as the lowest concentration at which no visible growth was observed.
Killing Curve of Peptide JH-3
Salmonella typhimurium strain CVCC541 was cultured in LB broth to the logarithmic growth phase (OD600 = 1.0), and peptide JH-3 was added to two culture samples (final concentrations of 1 and 4× the MIC) and incubated in a 37 °C shaker. Fresh LB medium was used for negative controls. Subsequently, OD600 measurements and 10-fold dilution plating were performed every hour for 5 h. The killing curve of peptide JH-3 towards Salmonella typhimurium was generated as described previously [14].
Transmission Electron Microscopy
Peptide JH-3 (final concentration of 1× the MIC) was added to a Salmonella typhimurium strain CVCC541 culture in the logarithmic growth phase. After incubating for 1 h, the samples were transferred to a copper grid and stained with phosphotungstic acid (PTA; 2%, w/v). Next, the samples were observed by transmission electron microscopy (TEM) (HITACHI, HT7700, Japan) with an acceleration voltage of 80 kV [15].
Detection of Bacterial Permeability
Bacteria were cultured to the logarithmic growth phase and then centrifuged for 3 min at 8000 rpm. The cell pellets were then diluted with 0.1 mol/L phosphate buffer (pH 7.4) to 0.5 × 105–1 × 105 CFU/mL. In the positive control group, cells were killed by treatment with 2 mg/mL Triton X-100 for 1 h, whereas in the negative control group, the cells were not treated with either JH-3 or Triton X-100. For the experimental groups, JH-3 was added to two tubes (final concentration of 1 and 4× MIC), and bacteria were collected at 1, 2, 3, 4, 5 and 6 h. Next, the bacteria were filtered through 0.22-μm cellulose ester microporous filters. The absorbance of the filtrate was measured by an ultraviolet spectrophotometer at 260 nm, and the permeability was calculated (PDL = OD/ODt × 100), where OD is the absorbance of the medium treated with or without thymol, and ODt is the absorbance of the medium treated with Triton X-100 [16].
The effect of JH-3 on CVCC541 permeability was also studied by measuring propidium iodide (PI) uptake by bacterial cells. After being treated with JH-3, bacterial suspensions were mixed with PI (2.5 mg/mL in water) to a final concentration of 17 μg/mL, and bacteria were subsequently detected with a fluorescence microscope.
Agarose Diffusion Assay of the Stability of JH-3
Salmonella enterica serovar Typhimurium strain CVCC541 was used as the test strain, and the antimicrobial peptide JH-3 was selected to test the pH value stability and thermal stability at a final concentration of 1 mg/mL. The activity of JH-3 with different treatments was determined using agarose diffusion assay. To determine thermal stability, the JH-3 was incubated at 0, 20, 40, 60, 80, or 100 °C for 1 h. For pH value stability, the JH-3 was incubated in Luria–Bertani (LB) solution with different pH values of 2.0, 3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, 10.0 and 11.0 for 1 h.
The initial diameter (Do) of all samples (CVCC541) was immediately measured using Vernier callipers before adding the JH-3. After incubation with different treatments JH-3 for 24 h, the diameter (Di) of zone of growth inhibition was measured using Vernier callipers. The inhibition ration (Di-Do) of JH-3 reflects the thermal stability and pH value stability.
Measure the Leakage of Ions
In order to detect the ion release of Salmonella enterica serovar Typhimurium strain CVCC541 after treatment with the antibacterial peptide JH-3, we added antibacterial peptide JH-3 using 1 MIC and 2 MIC to bacteria in logarithmic growth period for 0.5, 1, 2, 3, 4 and 5 h, and collected the supernatant of bacterial culture solution by 0.22-μm membrane filtration, compared with the negative control (normal CVCC541 without antibacterial peptide JH-3). The cationic ion concentration of the supernatant of bacterial culture was detected by Optical Emission Spectrometer Optima 2100 DV.
Mouse Infection and Treatment In Vivo
Salmonella typhimurium strain CVCC541 was cultured in liquid medium to the logarithmic growth phase, and then, the bacteria were centrifuged at 8000 rpm for 3 min. The bacterial pellet was resuspended in PBS and washed three times. Each mouse was orally infected with 1 × 106 CFU of Salmonella typhimurium, and after 2 h or 3 days, mice were subcutaneously injected with 40 or 10 mg/kg JH-3, Salmonella typhimurium-infected mice were injected with ddH2O as a negative control or injected with 40 mg/kg ciprofloxacin (CPFX) as antibiotic control (Fig. 1). Mice were weighed on the day of infection and every day thereafter. Clinical symptoms were recorded [17] according to the following grading standards: poor spirit (1 point), loss of appetite (1 point), fur loss (1 point), increased heart rate (1 point), hunched posture (1 point), and weight loss (1 points). Scores were summed, with mice showing more severe symptoms receiving higher scores.
Blood samples were collected, and the number of Salmonella typhimurium at 1, 2, 3, 4, 5, and 6 days postinfection were determined by the colony count method [18]. The infected mice were dissected, and the lungs, livers and spleens were homogenised and used to enumerate the number of bacteria in the target organs. The spleens were also used to detect the change in T lymphocytes, macrophages and neutrophils by flow cytometry [19], and the intestines were fixed in 4% paraformaldehyde for haematoxylin–eosin (HE) staining to observe pathological changes [20].
Statistical Analysis
Data analyses (one- or two-way ANOVA) were performed with GraphPad Prism 5 to detect differences in the measured values. Differences were considered significant when *p < 0.05 and were considered extremely significant when **p < 0.01 and ***p < 0.001.
Results
JH-3 Directly Kills Salmonella typhimurium Strain CVCC541 In Vitro
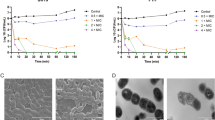
The antibacterial activity of JH-3 against Salmonella typhimurium strain CVCC541 was assayed in vitro, the results of which showed that JH-3 inhibited Salmonella typhimurium growth at 1 mg/mL. The observed size of the bacteriostatic ring of JH-3 was comparable to that of kanamycin (10 mg/mL) and thymol (10 mg/mL) but was smaller than that of CPFX (1 mg/mL) (Fig. 2a). The MIC measurements showed that the minimum inhibitory concentration of JH-3 against Salmonella typhimurium was 100 μg/mL (Fig. 2b). To evaluate the bactericidal activity of JH-3 against Salmonella typhimurium, we assessed the OD600 value of bacterial cultures in the presence and absence of the peptide, observing that the OD600 value decreased to 0.5 after a 5-h treatment with JH-3, which was significantly lower than that of the untreated control group (Fig. 2c, p < 0.001). The viable bacteria measurements showed that the bactericidal efficiency of JH-3 against Salmonella typhimurium was concentration dependent, and at 1× the MIC of JH3, the bacterial count decreased 1000-fold within 4 h (Fig. 2d, p < 0.001). In addition, the JH-3 against Salmonella typhimurium strain CVCC541 was not influenced by the range of the pH from 2 to 11, and JH-3 had the best activity when the pH value at 7 (Fig. 2e). JH-3 maintained antibacterial activities at the tested temperatures for 1 h. Although the antibacterial activity decreased with increasing temperature, the JH-3 still had better antibacterial activity after treatment at 100 °C for 1 h. The results indicated that JH-3 has good pH and thermal stability (Fig. 2f). These results indicate that JH-3 is effective at killing Salmonella typhimurium in vitro.
JH-3 kills Salmonella typhimurium strain CVCC541 in vitro. a Inhibition of CVCC541 by JH-3. b Minimum inhibitory concentration of JH-3 against CVCC541. c OD600 value of CVCC541 after JH-3 treatment (p < 0.001). d Viable CVCC541 cells after JH-3 treatment (p < 0.001). e Thermal stability detection of antimicrobial peptide JH-3 (p > 0.05). f pH stability detection of antimicrobial peptide JH-3 (p > 0.05)
JH-3 Increases the Permeability of Salmonella typhimurium Strain CVCC541
TEM analyses showed that at 1× the MIC, JH-3 significantly altered the membrane integrity of Salmonella typhimurium strain CVCC541, leading to the formation of pores in the cell membrane and leakage of bacterial cell contents, indicated by the yellow and blue arrows in Fig. 3b, respectively. Salmonella typhimurium cells in the negative control group maintained an intact membrane structure (Fig. 3a), as no leakage of cellular contents was observed (Fig. 3a). JH-3 treatment led to the leakage of bacterial cell contents and decreased the bacterial internal density and colour intensity, indicated by the red arrows in Fig. 3b. However, JH-3 has no effect on the flagella of Salmonella typhimurium strain CVCC541, which are indicated by the purple arrows in Fig. 3b. Measured permeability rates indicated that at 1 and 4× the MIC, JH-3 significantly increased the total protein concentration in the CVCC541 bacterial supernatants and that CVCC541 cell permeability significantly increased within 6 h (Fig. 3c, p < 0.001). Furthermore, CVCC541 cells treated with JH-3 (at 1 and 4× the MIC) were more easily stained with PI, and this trend observed to be dose dependent as detected by fluorescence microscopy (Fig. 3d). Ca2+ and Na+ ions in bacterial supernatant after treatment with the antibacterial peptide JH-3 were significantly higher than those in the negative control group (p < 0.05, Fig. 3e, and p < 0.01, Fig. 3f). The above results demonstrate that JH-3 promoted the release of Ca2+ and Na+ ions from the cell into the culture solution after JH-3 acted on the bacterial wall, and the permeability of Ca2+ and Na+ ions were enhanced. In summary, antimicrobial peptide JH-3 could destroy the bacterial wall, resulting in the change of the ion transport channel, and then destroy the original ion exchange between bacteria and the outside world, increasing permeability, and finally thus a bactericidal effect.
Transmission electron microscopy of Salmonella typhimurium strain CVCC541 after JH-3 treatment. a Negative control. b Two hours after JH-3 treatment. c CVCC541 cell permeability significantly increased within 6 h (p < 0.001). d PI staining results by fluorescence microscopy. e Ca2+ ions in bacterial supernatant after treatment with JH-3 (p < 0.05). f Na+ ions in bacterial supernatant after treatment with JH-3 (p < 0.01)
JH-3 Effectively Treats Salmonella typhimurium Strain CVCC541 Infection in Mice
To measure the in vivo bactericidal activity of JH-3, mice were intraperitoneally treated with JH-3 (40 or 10 mg/kg) at 2 h or 3 days postinfection with Salmonella typhimurium strain CVCC541. Our results showed that the injection of 40 mg/kg JH-3 at 2 days postinfection significantly increased the body weight (yellow curve) of Salmonella typhimurium strain CVCC541-infected mice (p < 0.01, Fig. 4a). Furthermore, this effect was greater than that observed in mice treated with a lower dose of JH-3, although the body weights of the JH-3-treated mice decreased compared with those of the uninfected negative control mice (Fig. 4a). Compared with the untreated mice, the mice treated with a higher dose of JH-3 showed mild clinical symptoms, severe diarrhoea and had a significantly lower comprehensive score (p < 0.01, Fig. 4c). However, the therapeutic effect of JH-3 was relatively poor when mice were treated at 3 days after infection, as characterised by a significant decrease in body weight and extreme weight loss in the later period (Fig. 4b). The clinical symptom scores were the same for both the JH-3-treated and untreated groups (p > 0.05, Fig. 4d). When mice were treated at 2 h postinfection, the survival rate observed in the high dose-treated group was 100%, higher than that observed for the CPFX-treated group (75%) at the same dose. In addition, the observed survival rates in the 10 mg/kg JH-3 treatment group and in the untreated group were 50 and 0%, respectively (Fig. 4e). When mice were treated at 3 days postinfection, the high dose of JH-3 could protect 75% of the mice against lethal infection, higher than that observed for the CPFX-treated group (50%) at the same dose. In addition, the observed survival rates in the 10 mg/kg JH-3 treatment group and in the untreated group were 25 and 0%, respectively. Thus, the high JH-3 dose had an effective therapeutic effect when mice were treated at 2 h postinfection.
Therapeutic effect of JH-3 in CVCC541-infected mice. a Body weight of mice treated with JH-3 2 h postinfection (p < 0.01). b Body weight of mice treated with JH-3 3 days postinfection. c Clinical scores of mice treated with JH-3 2 h postinfection (p < 0.01). d Clinical scores of mice treated with JH-3 3 days postinfection (p > 0.05). e Survival rate of mice treated with JH-3 2 h postinfection. f Survival rate of mice treated with JH-3 3 days postinfection
JH-3 Significantly Decreases Bacterial Burden in Blood and Distal Organs
The blood bacterial burdens at different time points after Salmonella typhimurium strain CVCC541 infection were measured to estimate the therapeutic effect of JH-3. The results showed that treatment with 40 mg/kg JH-3 at 2 h postinfection significantly reduced the amount of Salmonella typhimurium strain CVCC541 in the blood (Fig. 5a–f; p < 0.05 for day 2 after infection; p < 0.01 for days 3 and 4 after infection; and p < 0.001 for days 5 and 6 after infection). In addition, treatment of mice with 10 mg/kg JH-3 at 2 h postinfection also significantly reduced the abundance of Salmonella typhimurium strain CVCC541 in the blood (Fig. 5a–f; p < 0.05 for day 2 after infection; p < 0.01 for days 3 and 4 after infection; and p < 0.001 for days 5 and 6 after infection), with this treatment effect not significantly different from that observed for CPFX. However, treatment with peptide JH-3 and CPFX 3 days after infection significantly reduced the blood bacterial burden on days 4 and 6, although the clearance of Salmonella typhimurium strain CVCC541 in the blood was poor (p < 0.05 for days 4 and 6, Fig. 5a–f). In addition, the highest bacterial burden was observed in the spleen, and the 40 mg/kg JH-3 treatment at 2 h postinfection significantly reduced the abundance of Salmonella typhimurium strain CVCC541 in the spleens, lungs and livers of mice (p < 0.01, Fig. 5g–i), with results not significantly different from those observed for the CPFX-treated group. Furthermore, treatment with 10 mg/kg JH-3 at 2 h postinfection also reduced the colonisation of Salmonella typhimurium strain CVCC541 (p < 0.05 for spleen and lung, p < 0.05 for liver, Fig. 5g–i). However, the therapeutic effect in the group treated 3 days postinfection was poor, and large amounts of Salmonella typhimurium strain CVCC541 were observed in those organs (Fig. 5g–i).
Salmonella typhimurium strain CVCC541 burden in the blood and distal organs after treatment with JH-3 or ampicillin. a–f The bacterial count in blood at 1 to 6 days in the JH-3- or ampicillin-treated groups. g–i The bacterial counts in the spleens, lungs, and livers of mice at 1 to 6 days in the JH-3- or ampicillin-treated groups
Antibacterial Peptide JH-3 Treatment Can Alter the Proportion of Immune Cells in Infected Spleens
The percentages of T cells, neutrophils and macrophages in the spleens of mice were measured by flow cytometry (FCM). Our results showed that the percentage of spleen neutrophils was significantly increased in the Salmonella typhimurium strain CVCC541-infected mice compared to that observed in the control group (51.3 vs. 7.5%, respectively), and the JH-3 treatment significantly reduced the proportion of neutrophils (reduced to 8.9 and 31.4% by the 40 and 10 mg/kg treatments, respectively; Fig. 6a), indicating that the antimicrobial peptide JH-3 can reduce Salmonella typhimurium strain CVCC541 infection-mediated spleen inflammation. Salmonella typhimurium infections can induce macrophage apoptosis [21]. Our results showed that Salmonella typhimurium infection reduced the proportion of macrophages in the spleen compared to that observed in the control group (22.5 vs. 34.8%, respectively), and the JH-3 treatment restored the proportion of macrophages (increased to 35.0 and 29.5% by the 40 and 10 mg/kg treatments, respectively; Fig. 6b), suggesting that the antimicrobial peptide JH-3 improved the phagocytosis of spleen macrophages. Salmonella typhimurium strain CVCC541 infection significantly reduced the proportion of spleen T lymphocytes compared to that observed in the control group (18.0 vs. 42.3%, respectively), and the 40 mg/kg JH-3 treatment significantly increased the proportion of T lymphocytes (increased to 36.2%; Fig. 6c). Mice treated with 10 mg/kg JH-3 showed less dramatic changes in the proportion of immune cells in the spleen of Salmonella typhimurium strain CVCC541-infected mice compared to mice treated with 40 mg/kg JH-3. Taken together, these results suggest that the antimicrobial peptide JH-3 can change the proportion of immune cells in the spleen of Salmonella typhimurium strain CVCC541-infected mice.
Antimicrobial Peptide JH-3 Alleviates the Intestinal Pathological Changes in Infected Mice
Salmonella typhimurium strain CVCC541 infection caused severe intestinal damage in mice. The observed lesions were primarily concentrated in the jejunum, ileum and caecum, and the characteristic symptoms of mice included intestinal swelling, congestion, bleeding, oedema, thinning of the intestinal wall that became easily ruptured, and severely affected regions of the intestine were filled with blood. However, treatment with 40 mg/kg JH-3 at 2 h postinfection was able to significantly relieve these symptoms, with less bleeding and no visible pathological changes observed, equivalent to the effect observed in mice treated with 40 mg/kg of CPFX at 2 h postinfection. However, when treated 3 days after infection, both JH-3 and CPFX showed poor therapeutic effects (Fig. 7). HE staining of the intestine showed that Salmonella typhimurium strain CVCC541 infection led to numerous pathological changes, including severe damage to the villi in the intestinal cavity, intestinal epithelial cell degeneration, necrosis, shedding, high levels of inflammatory cell infiltration in the lamina propria, the production of large amounts of cellulose exudate, and the generation of intestinal tissue necrosis, cellulose and necrosis that often condensed together to form films that were difficult to remove. These pathological changes indicated that Salmonella typhimurium strain CVCC541 infection led to necrotizing enterocolitis and cellulose necrotizing enterocolitis (Fig. 7, blue arrow). However, treatment with 40 mg/kg of peptide JH-3 at 2 h postinfection significantly alleviated the pathological changes to the intestine, maintaining the normal intestinal villi and intestinal epithelial cells, which appeared the same as those observed in the uninfected control mice (Fig. 7, green and red arrows). Treatment with 40 mg/kg of CPFX also showed a good therapeutic effect, as only local damage was observed in the intestinal villi (Fig. 7, yellow arrows). Treatment with the antibacterial peptide JH-3 and CPFX at 3 days postinfection showed a poor therapeutic effect and did not alleviate the severe intestinal injury (Fig. 7). These results indicated that treatment with 40 mg/kg JH-3 at 2 h postinfection significantly reduced intestinal damage induced by Salmonella typhimurium strain CVCC541 infection.
Discussion
Salmonella typhimurium is an important zoonotic pathogen that is a major cause of gastrointestinal diseases worldwide [22]. In recent years, the unreasonable use of traditional antibiotics has resulted in bacterial resistance and environmental drug residues, and these problems are becoming increasingly prominent, causing a great threat to human health [23]. In the search for traditional antibiotic substitutes, antimicrobial peptides are being closely considered because of their wide distribution, low rate of drug resistance development and the production of fewer drug residues [9, 10]. In this study, we showed that JH-3 has a good bactericidal effect against Salmonella typhimurium strain CVCC541 and identified the minimum inhibitory concentration of JH-3 against this bacterium. JH-3 treatment led to the leakage of bacterial contents, resulting in bacterial cell death. In addition, our results showed that treatment with 40 mg/kg JH-3 at 2 h postinfection had the best therapeutic effect in vivo. At this concentration, mice were significantly protected from a lethal dose of Salmonella typhimurium strain CVCC541, with an observed survival rate of 100% and reduced clinical symptoms and pathological changes. In this study, we evaluated the therapeutic effect of different doses and treatment times of the antimicrobial peptide JH-3 in mice infected with Salmonella typhimurium strain CVCC541 for the first time, laying the foundation for future research on new antimicrobial agents.
The study of antimicrobial peptides against Salmonella typhimurium has become a hot research topic. Antimicrobial peptide Bac7 was shown to exhibit anti-Salmonella typhimurium activity both in vivo and in vitro, and Monica et al. observed that Bac7 has very low toxicity in mice in vivo (30 mg/kg) and can effectively combat lethal doses of Salmonella typhimurium infection [24]. Human antibacterial peptide LL-37 can inhibit the synthesis of collagen and reduce the fibrosis of dermal fibroblasts. Jun et al. confirmed that LL-37 is able to inhibit Salmonella infection of fibroblasts by activating the MAPK signalling pathway and through the expression and synthesis of collagens, reducing Salmonella infection-mediated colitis [25]. Na et al. treated Salmonella typhimurium-infected mice with the antimicrobial peptide VRW3 (a β-hairpin-like peptide) and observed that it could alleviate the occurrence of mouse peritonitis and reduce the Salmonella typhimurium colonisation of mice, improving the survival rate of mice [26]. Wu et al. observed that modification of the antimicrobial peptide DP7 increased its antimicrobial activity against Salmonella typhimurium by 4–8-fold, suggesting that modification of the amino acid residues of antimicrobial peptides can be an effective means of improving their activity [27]. The antimicrobial peptide JH-3 used in this study was obtained by amino acid modification of the original antimicrobial peptide P3, with the resulting peptide exhibiting an improved antimicrobial activity and antibacterial spectrum (Hu et al., 2011). In addition, the molecular weight of JH-3 is 6.3 times greater that of CPFX, such that if the same amount of JH-3 and CPFX are used for mouse treatment studies, the effect of JH-3 may be better than that of CPFX. Thus, Jh-3 is an ideal candidate for the treatment of Salmonella infection.
The bactericidal mechanism of most antimicrobial peptides is the perforation of the bacterial membrane to form pores, resulting in the destruction of the bacterial cell membrane structure and increasing its permeability, causing the leakage of intracellular water-soluble substances that eventually leads to bacterial death [28,29,30,31]. The cathelicidin PMAP-36 derivative G124 can destroy the E. coli cell membrane, increasing the cell membrane permeability and resulting in leakage of the intracellular contents, resulting in cell death [30]. HBV core protein (HBc) can bind to the surface of the gram-negative bacteria P. aeruginosa and E. coli by binding to LPS, increasing bacterial membrane permeability and leading to cell death [28]. The antimicrobial peptide R-FV-I16 affects the depolarization of the outer and the inner bacterial membranes of the gram-negative bacteria E. coli ATCC25922 and P. aeruginosa ATCC27853, interfering with the formation of the cell membrane and increasing the permeability of the bacterial membrane and resulting in cell death [31]. The C-terminal hydrophobic aromatic amino acid residues of the antimicrobial peptide papiliocin can damage the permeability of the outer and the inner bacterial membranes of E. coli, providing a reference for the study of selective antibacterial mechanisms of antimicrobial peptides [29]. In this study, the antimicrobial peptide JH-3 was observed to be able to rapidly destroy the inner and outer membranes of Salmonella typhimurium CVCC541 (within 1 h), resulting in leakage of the intracellular contents of the bacteria, leading to bacterial cell death.
The effect of antimicrobial peptides is closely related to the time of administration. Clostridium difficile-infected mice develop enteritis due to the production of toxin A, and colon administration of antimicrobial peptide cathelicidin 1 day prior to infection and continuously 3 days after infection significantly reduced C. difficile-induced damage to the colons of C57BL/6 mice, reducing toxin A-mediated intestinal inflammation [32]. Adding the antibacterial peptide coprisin to the drinking water at 1 day prior to infection and continuously for 6 days after infection relieved C. difficile-induced mice inflammatory diarrhoea and pseudomembranous colitis [33]. The administration of the insect peptide Copa3 in drinking water continuously for 1 week before infection was observed to effectively prevent C. difficile infection, promote colon epithelial cell proliferation, enhance intestinal mucosal barrier function and improve intestinal inflammation in mice [34]. The administration of the antimicrobial peptide Peptoid 1 at 4 h postinfection significantly reduced the colonisation of S. aureus (ATCC 25923) in CD1 mice by more than 100-fold [35]. The administration of horse antimicrobial peptide eCATH1 1 day after the infection of mice with Rhodococcus equi and then continuously for 7 days thereafter was shown to have a good therapeutic effect [36]. P. aeruginosa 15159-infected mice treated with the antimicrobial peptide FFF21 at 30 min or 2 h postinfection significantly reduced the colonisation of P. aeruginosa in the spleens, kidneys and livers of mice, improving their survival [37]. In this study, the treatment of Salmonella typhimurium-infected BALB/c mice with 40 mg/kg JH-3 at 2 h postinfection significantly reduced Salmonella typhimurium CVCC541-mediated intestinal damage and improved the survival rate to 100%. However, treatment with JH-3 at 3 days postinfection showed a poor therapeutic effect, further confirming that early administration or preventive administration of antimicrobial peptides has a better therapeutic effect.
References
Owen KA, Meyer CB, Bouton AH, Casanova JE (2014) Activation of focal adhesion kinase by Salmonella suppresses autophagy via an Akt/mTOR signaling pathway and promotes bacterial survival in macrophages. PLoS Pathog 10(6):e1004159. https://doi.org/10.1371/journal.ppat.1004159
Crump JA, Luby SP, Mintz ED (2004) The global burden of typhoid fever. Bull World Health Organ 82(5):346–353. https://doi.org/10.1590/S0042-96862004000500008
Kozak GK, MacDonald D, Landry L, Farber JM (2013) Foodborne outbreaks in Canada linked to produce: 2001 through 2009. J Food Prot 76(1):173–183. https://doi.org/10.4315/0362-028X.JFP-12-126
Fabrega A, Vila J (2013) Salmonella enterica serovar Typhimurium skills to succeed in the host: virulence and regulation. Clin Microbiol Rev 26(2):308–341. https://doi.org/10.1128/CMR.00066-12
Amajoud N, Bouchrif B, Maadoudi ME, Senhaji NS, Karraouan B, Harsal AE, Abrini JE (2017) Prevalence, serotype distribution, and antimicrobial resistance of Salmonella isolated from food products in Morocco. J Infect Dev Ctries 11(2):136–142. https://doi.org/10.3855/jidc.8026
Fernandes SA, Camargo CH, Francisco GR, Bueno MFC, Garcia DO, Doi Y, Tiba Casas MR (2017) Prevalence of extended-spectrum beta-lactamases CTX-M-8 and CTX-M-2-producing Salmonella serotypes from clinical and nonhuman isolates in Brazil. Microb Drug Resist 23(5):580–589. https://doi.org/10.1089/mdr.2016.0085
Iwamoto M, Reynolds J, Karp BE, Tate H, Fedorka-Cray PJ, Plumblee JR, Hoekstra RM, Whichard JM, Mahon BE (2017) Ceftriaxone-resistant nontyphoidal Salmonella from humans, retail meats, and food animals in the United States, 1996-2013. Foodborne Pathog Dis 14(2):74–83. https://doi.org/10.1089/fpd.2016.2180
Correia S, Nunes-Miranda JD, Pinto L, Santos HM, de Toro M, Saenz Y, Torres C, Capelo JL, Poeta P, Igrejas G (2014) Complete proteome of a quinolone-resistant Salmonella Typhimurium phage type DT104B clinical strain. Int J Mol Sci 15(8):14191–14219. https://doi.org/10.3390/ijms150814191
Brown KL, Poon GFT, Birkenhead D, Pena OM, Falsafi R, Dahlgren C, Karlsson A, Bylund J, Hancock REW, Johnson P (2011) Host defense peptide LL-37 selectively reduces proinflammatory macrophage responses. J Immunol 186(9):5497–5505. https://doi.org/10.4049/jimmunol.1002508
Zhang M, Volpert O, Shi YH, Bouck N (2000) Maspin is an angiogenesis inhibitor. Nat Med 6(2):196–199. https://doi.org/10.1038/72303
Hu J, Xu M, Hang B, Wang L, Wang Q, Chen J, Song T, Fu D, Wang Z, Wang S, Liu X (2011) Isolation and characterization of an antimicrobial peptide from bovine hemoglobin α-subunit. World J Microbiol Biotechnol 27(4):767–771. https://doi.org/10.1007/s11274-010-0514-4
Zhang Q, Xu Y, Wang Q, Hang B, Sun Y, Wei X, Hu J (2015) Potential of novel antimicrobial peptide P3 from bovine erythrocytes and its analogs to disrupt bacterial membranes in vitro and display activity against drug-resistant bacteria in a mouse model. Antimicrob Agents Chemother 59(5):2835–2841. https://doi.org/10.1128/AAC.04932-14
Pereira V, Lopes C, Castro A, Silva J, Gibbs R, Teixeira P (2009) Characterization for enterotoxin production, virulence factors, and antibiotic susceptibility of Staphylococcus aureus isolates from various foods in Portugal. Food Microbiol 26(3):278–282. https://doi.org/10.1016/j.fm.2008.12.008
Jones A, Georg M, Maudsdotter L, Jonsson A (2009) Endotoxin, capsule, and bacterial attachment contribute to Neisseria meningitidis resistance to the human antimicrobial peptide LL-37. J Bacteriol 191(12):3861–3868. https://doi.org/10.1128/JB.01313-08
Wang L, Qin W, Zhai R, Liu S, Zhang H, Sun C, Feng X, Gu J, Du C, Han W, Langford PR, Lei L (2015) Differential gene expression profiling of Actinobacillus pleuropneumoniae during induction of primary alveolar macrophage apoptosis in piglets. Microb Pathog 78:74–86. https://doi.org/10.1016/j.micpath.2014.11.017
Wang L, Zhao X, Zhu C, Xia X, Qin W, Li M, Wang T, Chen S, Xu Y, Hang B, Sun Y, Jiang J, Richard LP, Lei L, Zhang G, Hu J (2017) Thymol kills bacteria, reduces biofilm formation, and protects mice against a fatal infection of Actinobacillus pleuropneumoniae strain L20. Vet Microbiol 203:202–210. https://doi.org/10.1016/j.vetmic.2017.02.021
Martz SE, McDonald JAK, Sun J, Zhang Y, Gloor GB, Noordhof C, He S, Gerbaba TK, Blennerhassett M, Hurlbut DJ, Allen-Vercoe E, Claud EC, Petrof EO (2015) Administration of defined microbiota is protective in a murine Salmonella infection model. Sci Rep 5(16094). https://doi.org/10.1038/srep16094
Wang L, Qin W, Zhang J, Bao C, Zhang H, Che Y, Sun C, Gu J, Feng X, Du C, Han W, Richard PL, Lei L (2016) Adh enhances Actinobacillus pleuropneumoniae pathogenicity by binding to OR5M11 and activating p38 which induces apoptosis of PAMs and IL-8 release. Sci Rep 6(24058). https://doi.org/10.1038/srep24058
Yang F, Ma Q, Lei L, Huang J, Ji Q, Zhai R, Wang L, Wang Y, Li L, Sun C, Feng X, Han W (2014) Specific humoral immune response induced by propionibacterium acnes can prevent Actinobacillus pleuropneumoniae infection in mice. Clin Vaccine Immunol 21(3):407–416. https://doi.org/10.1128/CVI.00667-13
Wang L, Qin W, Yang S, Zhai R, Zhou L, Sun C, Pan F, Ji Q, Wang Y, Gu J, Feng X, Du C, Han W, Langford PR, Lei L (2015) The Adh adhesin domain is required for trimeric autotransporter Apa1-mediated Actinobacillus pleuropneumoniae adhesion, autoaggregation, biofilm formation and pathogenicity. Vet Microbiol 177(1–2):175–183. https://doi.org/10.1016/j.vetmic.2015.02.026
Schwan WR, Huang XZ, Hu L, Kopecko DJ (2000) Differential bacterial survival, replication, and apoptosis-inducing ability of Salmonella serovars within human and murine macrophages. Infect Immun 68(3):1005–1013. https://doi.org/10.1128/IAI.68.3.1005-1013.2000
Herikstad H, Motarjemi Y, Tauxe RV (2002) Salmonella surveillance: a global survey of public health serotyping. Epidemiol Infect 129(1):1–8. https://doi.org/10.1017/epidmiolinfect.S0950268802006842
Penesyan A, Gillings M, Paulsen IT (2015) Antibiotic discovery: combatting bacterial resistance in cells and in biofilm communities. Molecules 20(4):5286–5298. https://doi.org/10.3390/molecules.20045286
Benincasa M, Pelillo C, Zorzet S, Garrovo C, Biffi S, Gennaro R, Scocchi M (2010) The proline-rich peptide Bac7(1-35) reduces mortality from Salmonella Typhimurium in a mouse model of infection. BMC Microbiol 10:178. https://doi.org/10.1186/1471-2180-10-178
Yoo JH, Ho S, Tran DH, Cheng M, Bakirtzi K, Kukota Y, Ichikawa R, Su B, Tran DH, Hing TC, Chang I, Shih DQ, Issacson RE, Gallo RL, Fiocchi C, Pothoulakis C, Koon HW (2015) Anti-fibrogenic effects of the anti-microbial peptide cathelicidin in murine colitis-associated fibrosis. Cell Mol Gastroenterol Hepatol 1(1):55–74.e1. https://doi.org/10.1016/j.jcmgh.2014.08.001
Dong N, Ma Q, Shan A, Lv Y, Hu W, Gu Y, Li Y (2012) Strand length-dependent antimicrobial activity and membrane-active mechanism of arginine- and valine-rich β-hairpin-like antimicrobial peptides. Antimicrob Agents Chemother 56(6):2994–3003. https://doi.org/10.1128/AAC.06327-11
Wu X, Wang Z, Li X, Fan Y, He G, Wan Y, Yu C, Tang J, Li M, Zhang X, Zhang H, Xiang R, Pan Y, Liu Y, Lu L, Yang L (2014) In vitro and in vivo activities of antimicrobial peptides developed using an amino acid-based activity prediction method. Antimicrob Agents Chemother 58(9):5342–5349. https://doi.org/10.1128/AAC.02823-14
Chen H, Su P, Chang Y, Wu S, Liao Y, Yu H, Lauderdale T, Chang K, Shih C (2013) Identification of a novel antimicrobial peptide from human hepatitis B virus core protein arginine-rich domain (ARD). PLoS Pathog 9(6):e1003425. https://doi.org/10.1371/journal.ppat.1003425
Lee E, Kim J, Jeon D, Jeong K, Shin A, Kim Y (2015) Functional roles of aromatic residues and helices of papiliocin in its antimicrobial and anti-inflammatory activities. Sci Rep 5:12048. https://doi.org/10.1038/srep12048
Lv Y, Wang J, Gao H, Wang Z, Dong N, Ma Q, Shan A (2014) Antimicrobial properties and membrane-active mechanism of a potential alpha-helical antimicrobial derived from cathelicidin PMAP-36. PLoS One 9(1):e86364. https://doi.org/10.1371/journal.pone.0086364
Xu W, Zhu X, Tan T, Li W, Shan A (2014) Design of embedded-hybrid antimicrobial peptides with enhanced cell selectivity and anti-biofilm activity. PLoS One 9(6):e98935. https://doi.org/10.1371/journal.pone.0098935
Hing TC, Ho S, Shih DQ, Ichikawa R, Cheng M, Chen J, Chen X, Law I, Najarian R, Kelly CP, Gallo RL, Targan SR, Pothoulakis C, Koon HW (2013) The antimicrobial peptide cathelicidin modulates Clostridium difficile-associated colitis and toxin A-mediated enteritis in mice. Gut 62(9):1295–1305. https://doi.org/10.1136/gutjnl-2012-302180
Kang JK, Hwang JS, Nam HJ, Ahn KJ, Seok H, Kim S, Yun EY, Pothoulakis C, Lamont JT, Kim H (2011) The insect peptide coprisin prevents Clostridium difficile-mediated acute inflammation and mucosal damage through selective antimicrobial activity. Antimicrob Agents Chemother 55(10):4850–4857. https://doi.org/10.1128/AAC.00177-11
Kim DH, Hwang JS, Lee IH, Nam ST, Hong J, Zhang P, Lu LF, Lee J, Seok H, Pothoulakis C, Lamont JT, Kim H (2016) The insect peptide CopA3 increases colonic epithelial cell proliferation and mucosal barrier function to prevent inflammatory responses in the gut. J Biol Chem 291(7):3209–3223. https://doi.org/10.1074/jbc.M115.682856
Czyzewski AM, Jenssen H, Fjell CD, Waldbrook M, Chongsiriwatana NP, Yuen E, Hancock REW, Barron AE (2016) In vivo, in vitro, and in silico characterization of peptoids as antimicrobial agents. PLoS One 11(2):e0135961. https://doi.org/10.1371/journal.pone.0135961
Schlusselhuber M, Torelli R, Martini C, Leippe M, Cattoir V, Leclercq R, Laugier C, Groetzinger J, Sanguinetti M, Cauchard J (2013) The equine antimicrobial peptide eCATH1 is effective against the facultative intracellular pathogen Rhodococcus equi in mice. Antimicrob Agents Chemother 57(10):4615–4621. https://doi.org/10.1128/AAC.02044-12
Papareddy P, Kalle M, Bhongir RKV, Moergelin M, Malmsten M, Schmidtchen A (2014) Antimicrobial effects of helix D-derived peptides of human antithrombin III. J Biol Chem 289(43):29790–29800. https://doi.org/10.1074/jbc.M114.570465
Acknowledgments
Lei Wang and Xueqin Zhao contributed equally to this work. We thank Xiaojing Xia, Chunling Zhu, Wanhai Qin and Hanna Fotina for providing language assistance. We thank Yanzhao Xu, Bolin Hang, Yawei Sun, Shijun Chen, Huihui Zhang and Jinqing Jiang for writing and editing assistance. We also thank Jianhe Hu and Gaiping Zhang for guidance and for help designing the article.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Nos. 31672559, 31702259 and 31372447), the National Key Research and Development Program of China (2016YFD0500708-04), the Program for Science Technology Innovation Talents in Universities of Henan Province (14HASTIT026), and the Excellent Youth Foundation of Henan Scientific Committee (174100510005).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Female BALB/c mice (6 to 8 weeks old, body weight of 18 to 22 g) were purchased from the Animal Center of Zhengzhou University (No. 41003100003648). All animal studies were conducted according to the experimental practices and standards of the Animal Welfare and Research Ethics Committee at Zhengzhou University. The study was approved by the Animal Centre of Zhengzhou University.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, L., Zhao, X., Xia, X. et al. Antimicrobial Peptide JH-3 Effectively Kills Salmonella enterica Serovar Typhimurium Strain CVCC541 and Reduces Its Pathogenicity in Mice. Probiotics & Antimicro. Prot. 11, 1379–1390 (2019). https://doi.org/10.1007/s12602-019-09533-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-019-09533-w