Abstract
Quorum sensing, bacterial cell-to-cell communication via small signaling molecules regulates virulence in many bacterial pathogens, and is a promising target for antivirulence therapy, which may inhibit virulence rather than cell growth and division. Herein, Bacillus strains capable of degrading QS molecules from freshwater environments were screened as potential aquaculture probiotics. A total of 34 Bacillus strains were isolated. Strain T-1 was selected with “H” streaking and double layer agar plate methods using Chromabacterium violaceum ATCC12472 as reporter, and eventually identified as Bacillus licheniformis based on biochemical and molecular identification. Quorum quenching by T-1 was confirmed using C. violaceum CV026. T-1 was non-hemolytic in vitro. In biocontrol experiments, T-1 reduced the pathogenicity of Aeromonas hydrophila cb15 in zebrafish co-injected intraperitoneally with both strains, achieving a relative percentage survival of 70%. Determination and analysis of the T-1 draft genome using the Illumina Hiseq 2500 platform identified the quorum quenching gene ytnP, encoding an acyl-homoserine lactone metallo-β-lactamase, as a potential QS quencher in T-1. In conclusion, B. licheniformis T-1 could be a safe and effective quorum quenching bacterium for protecting hosts against pathogenic bacterial infections in aquaculture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antibiotics are crucial for preventing, controlling, and treating diseases in aquatic animals, and many act by disrupting processes such as bacterial cell wall synthesis, DNA proliferation, and protein synthesis [1, 2]. However, excessive and inappropriate use of antibiotics has accelerated the evolution of bacteria that are resistant to antibiotics. As a consequence, many antibiotic treatments are no longer effective for treating humans and aquatic animals, which has implications for fish health [3]. Thus, developing novel strategies for controlling bacterial diseases without fear of further stimulating the evolution of antibiotic-resistant strains is important for human and veterinary medicine.
Aeromonas hydrophila, a Gram-negative bacterial pathogen found mainly in aquatic environments, causes a wide variety of symptoms in fish including tissue swelling, necrosis, ulceration, and hemorrhagic septicemia, and is strongly resistant to multiple antibiotics, resulting in significant economic losses to freshwater aquaculture [4]. The pathogenicity of A. hydrophila depends on the production of potential virulence factors, such as exoproteases and exotoxin [5]. Production of exoproteases is under the control of quorum sensing (QS). Evidence suggests that A. hydrophila harbors the AhyI/AhyR QS system, utilizes N-acyl-homoserine lactone (AHL)-dependent QS to regulate the expression of virulent genes [6, 7], and mediates microbial infection and colonization in the host, suggesting AHLs could be potential targets for controlling virulence. Therefore, inhibiting QS signals such as AHLs, a process referred to as quorum quenching (QQ), could decrease bacterial virulence, making it a promising strategy for fighting against pathogenic bacteria in aquaculture.
Quorum quenching mechanisms have been identified in many prokaryotic and eukaryotic organisms [8], where they regulate the antimicrobial activities of hosts by interfering with bacterial quorum sensing. The first quorum quenching gene, aiiA, was identified in Bacillus subtilis [9], and other quorum quenching genes have since been discovered, such as ahlD (Arthrobacteria sp.), aiiM (Microbacterium testaceum), attM (Agrobacterium fabrum), aidC (Chryseobacterium sp.), and ytnP (B. subtilis) [10,11,12,13]. Previous research indicates that QS inhibitors including bacteria or AHL-lactonase can reduce pathogenicity and protect aquatic animals against infection by Gram-negative pathogenic bacteria [14, 15]. Bacillus QSI-1 was isolated from the intestine of Carassius auratus gibelio, and fish fed a diet supplemented this organism displayed good survival [15]. Expression of the Bacillus cereus aiiA gene in Pichia pastoris can decrease mortality, and delay mortality in fish by quenching the QS signals of A. hydrophila [13]. Exactly how bacteria or AHL-lactonase disrupt QS is being investigated, and could provide new opportunities for therapeutic applications. Bacillus licheniformis produces a wide range of extracellular enzymes, and is reportedly a probiotic bacterium for animals, but little is known about the potential applications of B. licheniformis related to quorum sensing for the prevention and treatment of A. hydrophila.
Bacillus spp. are a natural resource bank for screening new quorum quenching bacteria, and are commonly regarded as safe strains for use in aquaculture as agents for improving water quality and preventing/controlling diseases [16]. However, recent studies indicate that Bacillus strains might contain toxin-producing genes [17]. Consequently, these results have given rise to concern about the safety of Bacillus products. A more rigorous selection process is thus required for Bacillus probiotic candidates.
The purpose of the present study was to identify safe and effective Bacillus spp. that degrade AHL molecules from freshwater culture ponds, and explore their potential mechanisms from the perspective of quorum sensing.
Materials and Methods
Bacterial Strains and Culture Conditions
The quorum quenching strain T-1 was isolated from a freshwater culture pond sediment, and Escherichia coli and Bacillus subtilis were obtained from the microbiology lab of Shanghai Ocean University. Strains were cultivated in Luria-Bertani (LB) broth consisting of 1% tryptone, 0.5% yeast extract, and 0.5% NaCl (pH 7.4 ± 0.2), and incubated for 24 h at 30 °C. The target pathogen A. hydrophila cb15 was isolated from necrosis of Carassius auratus gibelio in Shanghai and cultivated in LB broth 150 rpm in a shaker overnight at 28 °C for 24 h. Chromabacterium violaceum ATCC12472 was used as a biosensor to detect potential QS inhibitors of C4-AHL and C6-AHL that are synthesized via the autoinducer synthase CviI, which binds to the receptor CviR to form complexes, resulting in the production of violacein [18]. Additionally, C. violaceum CV026 was used as an AHL biosensor to detect exogenous AHLs (C6-HSL). Both C. violaceum strains were cultured in LB broth at 26 °C for 24 h. Where necessary, growth media were supplemented with kanamycin (50 μg ml−1) [19].
Isolation of Bacillus spp. Candidates for Inhibiting Quorum Sensing
The isolation method was employed as described previously [12]. In brief, sediment from freshwater culture ponds was diluted to 10−5 with normal saline and incubated at 80 °C for 10 min, then 0.1 ml samples were plated in duplicate on LB agar and cultured at 28 °C for 24–48 h. Hemolytic testing of isolates was carried out on nutrient agar supplemented with Columbia CNA agar and 5% sheep blood. Isolates were inoculated onto hemolytic plates and incubated at 28 or 37 °C for 24 h. Plates were observed for hemolytic reaction, and strains not producing a change around colonies were considered to be non-hemolytic, while strains displaying a clean hemolysis zone around colonies were considered hemolytic [20]. Moreover, in order to test the safety of B. licheniformis T-1 on animals, and the impact on the aquatic environment, C. auratus gibelio, zebrafish, and mice were selected for safety evaluation. Chlorella vulgaris, Daphnia magna, and Brachionus calyciflorus were used as test organisms for ecological environment evaluation. Animal safety tests and ecology environment evaluation of B. licheniformis T-1 were conducted according to the National Standard (GB) Acute Toxicity methods [21].
Two methods of isolating Bacillus spp. for inhibiting QS were performed as described previously [22, 23]. Firstly, Bacillus strains were spread onto the center of an agar plate using a sterilized cotton swab, and C. violaceum ATCC12472 was spread in parallel along both sides where two strains formed an “H” shape, and plates were incubated overnight (24 h) at 26 °C. Next, 5 ml of molten soft LB agar was inoculated with 0.05 ml of C. violaceum ATCC12472 grown overnight in LB broth. The agar-culture solution was immediately poured onto LB agar and Bacillus spp. was spread onto the center of the plate. Plates were incubated overnight at 26 °C and examined for violacein pigment production. QS inhibition was detected by a colorless, opaque, but viable halo around discs (indicating loss of pigmentation).
To verify the AHL-degrading activity of T-1, C. violaceum CV026 was used as an AHL biosensor [24]. Agar plates were prepared by mixing overnight cultures of C. violaceum CV026 in 20 ml of LB agar which was then poured into petri dishes containing C6-HSL (200 μg ml−1). Four sterile Oxford cups were placed on LB agar plates, and 0.2 ml enrofloxacin (0.64 μg ml−1) was added to one cup as a negative control, while another received 0.2 ml sterile water as a blank control. The remaining two cups received 0.2 ml of strain T-1 as the experimental groups. Plates were cultivated for 24 h at 26 °C. The color of colonies around the Oxford cup zone was observed, and differences between the four cup zones were compared. All experiments were repeated twice.
To verify the potential bactericidal ability of T-1 against A. hydrophila, agar plates were prepared by mixing overnight cultures of A. hydrophila cb15 in 20 ml of LB agar, which was then poured into petri dishes. Five sterile Oxford cups were used, one receiving 0.2 ml sterile water (negative control), one receiving 0.2 ml enrofloxacin (0.64 μg ml−1) as a positive control, and the other three receiving 0.2 ml T-1 culture following centrifugation at 5000 rpm for 5 min and diluted at the concentration of 100, 10−1, and 10−2. Plates were cultivated for 24 h at 30 °C. Transparent circles around the Oxford cup zone were observed, and differences between the five Oxford cup zones were compared. All experiments were repeated twice.
Identification of T-1
Molecular identification of T-1 was performed by extracting genomic DNA and amplifying the 16S rDNA gene by PCR using universal primers 27 F (5’-AGAGTTTGATCATGGCTCAG-3′) and 1492 R (5’-GGTTACCTTGTTACGACTT-3′). Sequences were analyzed by Invitrogen Co. Ltd., and homology was compared using BLAST searches against the GenBank database. A phylogenetic tree was constructed with the Mega 5.05 software [25].
Physical and chemical identification was based on the Biolog method described previously [26]. Bacillus sp. was incubated on LB agar for 12 h, a single colony was transferred to GN/GP IFB inoculum with a sterile cotton swab, and the turbidity of the culture was adjusted to 92–96%. The sample was transferred into a GENIII Microplate 96-well plate with a pipette (0.1 ml per well) and incubated at 33 °C in a constant temperature incubator. Bacteria were identified by the Biolog method following culturing for 6 or 20 h.
The morphology of T-1 was observed using a scanning electron microscope. T-1 was cultured in nutrient broth overnight, the culture was centrifuged at 4480 g for 2 min, the supernatant was discarded, and the cell pellet was washed three times with distilled water. Deposited Bacillus cells were fixed with 2.5% glutaraldehyde for 2–4 h; washed three times with distilled water; dehydrated for 15 min with 60, 70, 80, 95, and 100% ethanol in series; and the last two steps were repeated. A 0.05-ml sample of bacteria was placed on a coverslip and dried overnight. The sample was attached to the sample stage using silver conductive glue, coated with an ion sputter device, and observed and imaged with a scanning electron microscope [27].
Determination of the Protective Effects of T-1 on Zebrafish
Determination of the LD50 against A. hydrophila cb15 in Zebrafish
For 50% lethal dose (LD50) determination, A. hydrophila cb15 cells were cultured in LB broth at 28 °C for 24 h, and seven groups of 10 fish per group were challenged with 0.02 ml A. hydrophila cb15 at cell densities of 2.9 × 109, 2.9 × 108, 2.9 × 107, 2.9 × 106, 2.9 × 105, or 2.9 × 104 CFU ml−1. Controls were injected with normal saline. Fish were monitored for mortality for 96 h. During this period, activity and behavior were recorded at 1, 2, 4, 6, 8, 12, 24, 48, 72, and 96 h. LD50 values were calculated by linear interpolation [14].
Assaying the Protective Effects of T-1 on Zebrafish
The protective effects of T-1 on zebrafish were determined as described previously [25]. Healthy, energic zebrafish were divided into five groups (10 zebrafish per group). Group I was the blank control, and experimental groups II, III, and IV were separately co-injected intraperitoneally with 0.02 ml of A. hydrophila cb15 at a cell density of 7.2 × 107 CFU ml−1, or T-1 at a cell density of 2.6 × 108, 2.6 × 107, or 2.6 × 106 CFU ml−1. Group V was injected intraperitoneally with normal saline. Fish mortality was recorded at 1, 2, 4, 6, 8, 12, 24, 48, 72, and 96 h after injection.
Protective Effects of T-1 on Zebrafish at Different Timepoints after A. hydrophila Infection
A total of 60 zebrafish were divided into six groups, and groups I, II, III, IV, and V were injected intraperitoneally with 0.02 ml of A. hydrophila cb15 at a cell density of 7.2 × 107 CFU ml−1. Fish in groups I, II, III, and IV were then injected intraperitoneally with 0.02 ml of T-1 at a cell density of 2.6 × 108 CFU ml−1 at 0, 4, 8, or 16 h. Fish in group VI were injected with normal saline. Fish mortality was recorded at 1, 2, 4, 6, 8, 12, 24, 48, 72, and 96 h after injection.
Sequencing and Assembly of the T-1 Genome
Sequencing and assembly of the T-1 genome was completed by Shanghai Meiji Biological Company. Sequencing was performed using an Illumina Miseq 2500 sequencing platform to construct an Illumina PE library (400 bp library), and quality control was carried out using bioinformatics analysis following genome scans.
Sequence Analysis of the Quorum Quenching-Related T-1 Gene ytnP
Genomic DNA was extracted using a Bacterial Genome Extraction Kit (Tiangen Biotech, Beijing, China) according to the manufacturer’s instructions. The ytnP gene was amplified with forward and reverse primers F (5’-ATGAAGCTGATTCAGGTTGCATTT-3′) and R (5′- CTAGCGGCTTTCTCTTTTTACTGA-3′). Amplification involved denaturation for 5 min at 98 °C, followed by 34 cycles at 98 °C for 30 s, 55 °C for 30 s, and 72 °C for 1 min, and a final extension at 72 °C for 5 min. PCR products were purified using a TIANgel Midi Purification Kit (Tiangen Biotech) and cloned into the PMD19-T vector by overnight ligation at 16 °C. Ligated plasmids were transformed into competent E. coli cells using the heat-shock method, and 20 mg ml−1 5-bromo-4-chloro-indolyl-β-D-galactopyranoside (X-gal) and 50 mg ml−1 isopropyl β-D-1-thiogalactopyranoside (IPTG) were used to select positive clones. After PCR amplification, 1% agarose gel electrophoresis was carried out and products confirmed by comparison with DL2000 DNA markers. A positive clone was selected and sequenced by Sangon Biotech Co. Ltd. (Shanghai, China). The ytnP sequence was compared with closely related sequences using BLASTn and the NCBI website (http://blast.ncbi.nlm.nih.gov/). Neighbor-joining and bootstrap analysis with 1000 replicates were performed to construct a phylogenetic tree using MEGA 5.05 [25].
Statistical Analysis
Data were subjected to one-way analysis of variance (ANOVA) to confirm significant differences among experimental groups at p < 0.05.
Results
Isolation of Candidate Bacillus spp. Strains for Inhibiting Quorum Sensing
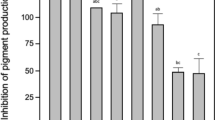
C. violaceum synthesizes the violet pigment as a result of QS. Loss of purple pigmentation in C. violaceum ATCC12472 in the vicinity of Bacillus spp. was indicative of QS inhibition (Fig. 1). Where Bacillus inhibited the growth of C. violaceum, clear inner zones of inhibition were observed. The outer colorless zone of inhibition was opaque and not transparent, indicating that the halos around discs were caused by inhibition of QS, rather than inhibition of cell growth (Fig. 1). A total of 34 Bacillus spp. strains were isolated from river water, sediment, fish intestinal tract, or strains preserved in the lab (Table 1). The results of hemolysis experiments indicated that Bacillus strains T-1, D-1, Y-1, DY-1, and Y-9 lacked hemolytic properties, while Bacillus strains FF1-2 and DS-2 possessed hemolytic activity (Fig. 1a). T-1 displayed the strongest ability to inhibit quorum sensing among isolates identified by the “H” streaking method (Fig. 1b), and the results of double layer agar experiments further confirmed the inhibitory effects of T-1 (Fig. 1c).
Quorum quenching of Bacillus sp. strain T-1. a Hemolytic analysis of different Bacillus strains: a, b: hemolytic analysis at 28 °C; c, d: hemolytic analysis at 37 °C. b QQ effects of T-1 by method of ‘H’ streaking: a: negative control; b: Y-1; c: D-1; d: Y-9; e: DY-1; f: T-1. c QQ effects of T-1 by method of double layer agar. a: blank control, b: T-1; c: Y-1; d: D-1. The arrows showed the ability of the isolated quorum quenching strains interfered with violacein of Chromabacterium violaceum ATCC12472
To verify the AHL-degrading activity of T-1, C. violaceum CV026 was used as an indicator strain. After incubation with T-1, the purple pigment around the Oxford cup zone on the plate disappeared (Fig. 2), while the area around the Oxford cup zone on the sterile water negative control plate remained purple. Additionally, there were no bacteria in the area around the Oxford cup zone on the plate treated with enrofloxacin. These results demonstrate that C6-HSL was degraded by T-1, and it did not affect the growth of C. violaceum CV026, which indicates that T-1 has the ability to degrade AHLs. Moreover, the results of animal safety and ecological environment evaluation experiments indicate that B. licheniformis T-1 inhibits quorum sensing and is nontoxic to C. auratus gibelio, zebrafish, and mice, and has no adverse effects on plankton in a water ecological environment. Furthermore, the results in Fig. 2b showed that there were no bacteria in the area around the Oxford cup zone on the plate treated with enrofloxacin, but A. hydrophila cb15 cells grew well in the area around the Oxford cup treated with T-1, comparable with the negative control plate.
a AHL-degradation assay on Luia-Bertani agar. (1. blank control: sterile water; 2. negative control: enrofloxacin; 3 and 4. experimental groups: strain T-1. The blank arrow showed that there was no bacteria in the area of C. violaceum CV026 around Oxford Cup and it inhibits the growth of bacteria. The white arrow around 3 and 4 showed the bacteria appeared and the area near T-1 of C. violaceum CV026 has no purple pigment indicates the ability to degrade AHL molecules). b Antibacterial effect of T-1 on Aeromonas hydrophila (1. positive control: enrofloxacin; 2. blank control: sterile water; 3. 100 of T-1; 4. 10−1 of T-1; 5. 10−2 of T-1. The blank arrow showed that there was no bacteria in the area of enrofloxacin around Oxford Cup and it inhibits the growth of bacteria, and the A. hydrophila cells grew well in the presence of T-1 of 3, 4, and 5)
Identification of T-1
Analysis of 16S rDNA Sequences
The 16S rDNA sequences of T-1 were compared with homologous sequences in GenBank. The phylogenetic tree based on 16S rDNA sequences of T-1 revealed B. licheniformis 55P3-1 as the closest homolog (accession number JN366777.1). Thus, T-1 was preliminarily identified as B. licheniformis (GeneBank accession number KP117098).
Analysis of T-1 Using the Biolog Microbial System
According to the Biolog system, if a bacterial strain incubated at 33 °C for 4–6 h has a similarity (SIM) ≥ 0.75, and a SIM ≥ 0.50 when incubated for 16–24 h, the species can be considered identified (SIM indicates the reliability, and a larger value represents greater reliability). T-1 was incubated at 33 °C for 20 h, and the SIM value was 0.610, confirming T-1 as B. licheniformis (Table 2).
Analysis of T-1 by Scanning Electron Microscopy
Scanning electron microscopy (SEM) analysis of T-1 in solid and liquid mediums revealed short rods with dimensions of 3 ± 0.21 μm × 10 ± 0.34 μm, arranged in a linear manner, and displaying morphological characteristics typical of Bacillus spp. (Fig. 3).
a Phylogenic relationships based on 16S rDNA sequences of T-1.The branching pattern was generated by the Neighbor-joining method. Bootstrap values of 1000 or more are indicated at the nodes. Sequence accession numbers are listed in parentheses. b SEM of Bacillus sp. strain T-1. a, b on solid medium (20 h) (a × 15,000; b × 25,000); c, d on liquid medium (20 h) (c × 15,000; d × 25,000)
The LD50 of A. hydrophila cb15 in Zebrafish
The LD50 of A. hydrophila cb15 in zebrafish was 1.45 × 107 CFU ml−1.
Protective Effects of T-1 on Zebrafish Challenged with A. hydrophila
Experimental analysis of protection ability showed that no fish died after being injected with normal saline, and the percentage survival of the group injected with 7.2 × 107 CFU ml−1A. hydrophila cb15 was below 20%. The results also showed that the protective effects of T-1 on zebrafish challenged with A. hydrophila cb15 were dependent on the dose of T-1 (Fig. 4a); survival of zebrafish reached 70% when co-injected with T-1 at a dose of 2.6 × 108 CFU ml−1 at 96 h after injection, which was significantly higher than control and other groups (p < 0.05). T-1 significantly attenuated A. hydrophila cb15 infection. Besides, at the same injected T-1 dose, the protective effects were correlated with injection time. Survival of zebrafish was beyond 60% following injection with T-1 at 0 and 4 h after A. hydrophila cb15 infection, and 4 h was the optimal injection time. Compared with survival of zebrafish in the positive control (only A. hydrophila cb15) and other experimental groups (8 and 16 h), the protective effects were better after injection at 4 h after infection (Fig. 4b).
a Protective effects of T-1 on zebrafish with A. hydrophila cb15. Abdominal injections of A. hydrophila cb15 result in significant levels of mortality in zebrafish reared in a closed aquaculture system. However, co-injection with T-1 significantly increases the survival over a period of 96 h. Shown are the cumulative mortalities for replicate groups of zebrafish injected with different concentrations of T-1. b Protective effects of T-1 on zebrafish at different timepoints after A. hydrophila infection. Survival of zebrafish was beyond 60% when injected T-1 at the 0 and 4 h and survival of zebrafish was less than 40% when injected T-1 at the 8 and 16 h
Prediction and Analysis of the T-1 Quorum Quenching Gene
Sequencing of the T-1 genome was performed using an Illumina Miseq 2500 sequencing platform, an Illumina PE library (400 bp library) was constructed, and quality control was carried out using bioinformatics analysis following genome scanning.
Four AHL lactonase metallo-β-lactamase genes (ytnP, yycJ, yobT, and yrkH), were predicted from the T-1 draft genome framework. A BLAST search using the AHL-lactonase metallo-β-lactamase gene revealed 99% sequence identity between the ytnP gene of T-1 and its homolog in B. subtilis subsp. BSP1 (Fig. 5a), but lower homology with yycJ, yobT, and other metallo-β-lactamases. As shown in Fig. 5b, proteins encoded by ytnP and yobT contain the conserved HXHXDH motif that is essential for AHL lactone enzyme activity. However, proteins encoded by yycJ and yrkH lack key histidine residues, which likely render them unable to perform this function.
Evolutionary tree with acyl-homoserine lactone (AHL) metallo-β-lactamase gene and protein sequence analysis of AHL-lactonase metallo-β-lactamase, yycJ (T-1-1), yrkH (T-1-4), and yobT (T-1-3) (Fig. 5a). The similar and identical residues are shaded in gray and black, respectively. The conserved motif HXHXDH is boxed (Fig. 5b).
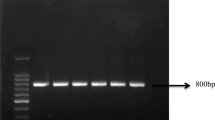
PCR Amplification of the ytnP Gene from T-1
An autoinducer inactivation gene (ytnP) has been identified previously in Gram-positive Bacillus sp. [12]. T-1, D-1, B. subtilis, and E. coli were tested for the presence of a ytnP homolog by PCR, and three of the four bacteria were positive for amplification of ytnP (the expected amplicon was ~ 900 bp), while E. coli was negative. A positive clone from T-1 was selected, subjected to PCR amplification, and sequenced by Sangon Biotech Co. Ltd. The results revealed 100% sequence identity, confirming the presence of ytnP in T-1.
Discussion
In the present study, we isolated the novel B. licheniformis strain T-1 from a freshwater environment that can effectively degrade AHLs (Figs. 1 and 2). AHL-producing and AHL-degrading bacteria co-exist in ecosystems, and have developed different strategies to gain a competitive advantage [28, 29]. Recently, the ability to degrade AHLs has been identified in several Bacillus genera, including B. subtilis, B. cereus, and Bacillus thuringiensis [30,31,32]. B. licheniformis is a Gram-positive, oxidase-positive, and catalase-positive endospore bacterium belonging to the genus Bacillus [33, 34]. It produces a wide range of extracellular enzymes and has been reported as a probiotic bacterium for animals [35, 36]. Although research is in progress, there are few reports on the quorum quenching of B. licheniformis. In addition, recent studies indicate that Bacillus might contain toxin-producing genes [17], which could impact their food, environmental, and clinical applications [16, 37]. Herein, we screened B. licheniformis T-1, which can be considered non-hemolytic (Fig. 1) and is nontoxic to C. auratus gibelio, zebrafish, and mice, and has no adverse effects on plankton in a water ecological environment [21]. Thus, T-1 could have potential as a safe probiotic for use in aquaculture.
B. licheniformis is used as a dietary supplement to significantly increase the growth rate of animals. Administration of B. licheniformis can inhibit Vibrio spp. by competitive exclusion, and improve the intestinal microflora and immune ability of Litopenaeus vannamei [38]. Similar improvement in immune parameters and reduction in gut pathogenic bacteria have been reported for Penaeus japonicus when B. licheniformis was fed in combination with B. subtilis and isomaltooligosaccharide [39]. Additionally, B. licheniformis KADR5 has been isolated from the gut of healthy Labeo rohita fish injected with A. hydrophila (in the case of subcellular components) and orally infected (in case of live cells, at a cell density of 108 CFU g−1 of feed) [40]. In the present study, B. licheniformis T-1 significantly decreased the mortality of zebrafish infected with A. hydrophila cb15, and increased survival to 70%. Compared with the results of AHL-lactonase AiiA expressed in E. coli or P. pastoris, supplementation of AiiAAI96 into fish feed by oral administration significantly attenuated A. hydrophila infection in zebrafish, with survival < 60% [14, 41]. In addition, survival of zebrafish was > 60% at 0 and 4 h after A. hydrophila injection, but lower at 8 and 16 h (Fig. 4). This suggests that T-1 might have much stronger prophylactic and therapeutic effects during the early phase of A. hydrophila infection. Thus, these results indicate that T-1 is safe and effective as a potential probiotic and biocontrol agent for disease prevention and control in aquaculture.
Quorum quenching is considered a promising strategy for controlling bacterial diseases as an alternative to antibiotics to reduce the risk of drug resistance [42, 43]. There are several kinds of hydrolytic enzymes in Bacillus spp. that degrade AHLs and block pathogenicity. However, in the case of B. licheniformis, there are few studies on the quorum quenching mechanism or quorum quenching genes. One B. licheniformis strain isolated from seawater that can degrade AHLs possesses the aiiA gene [44]. In the present study, the T-1 draft genome was determined and analyzed using the Illumina Hiseq 2500 platform (unpublished). We annotated a typical AHL lactonase gene, ytnP, encoding an AHL lactonase metallo-β-lactamase, and three other potential genes (yobT, yrkH, and yycJ; Fig. 5). Gene ytnP was subsequently successfully amplified, but attempts to amplify aiiA were unsuccessful (Fig. 6), and aiiA was not found in the T-1 genome. Analysis of protein sequences corresponding to ytnP and comparison with homologs aiiA (B. thuringiensis), ahlD (Arthrobacteria sp.), aiiM (Microbacterium testaceum), attM (Agrobacterium fabrum), and aidC (Chryseobacterium sp.) [10,11,12,13] revealed the presence of a typical zinc finger motif, including the HXHXDH consensus sequence containing key histidine residues, in the protein encoded by ytnP, confirming the presence of the necessary lactonase architecture. Meanwhile, Schneider and colleagues reported that addition of 20 nM purified Ytnp, encoded by the quorum quenching gene ytnP, to cultures of Pseudomonas aeruginosa inhibited biofilm formation, which indicates that Ytnp may block the signaling pathways controlled by quorum sensing in P. aeruginosa [12]. Thus, ytnP might be an important gene for quorum quenching in T-1. Besides, we speculated that B. licheniformis strains isolated from different environments might possess different quorum quenching systems. T-1 was isolated from freshwater, whereas the strain in the previous study was from seawater [44]. The environments of bacteria in freshwater and seawater are clearly different, and this could affect microbial growth and genetic variation, but this hypothesis needs further investigation.
In conclusion, the present study revealed that B. licheniformis T-1 is a safe quorum quenching bacterium and a potential probiotic. Survival of zebrafish challenged with A. hydrophila reached 70% when intraperitoneally co-injected with T-1, demonstrating its potential as a biocontrol agent for disease prevention and control in aquaculture. The ytnP gene might be important in quenching QS, but further studies on the quorum quenching mechanism of T-1 are needed.
References
Defoirdt T, Boon N, Bossier P, Verstraete W (2004) Disruption of bacterial quorum sensing: an unexplored strategy to fight infections in aquaculture. Aquaculture 240(1):69–88
Suga H, Smith KM (2003) Molecular mechanisms of bacterial quorum sensing as a new drug target. Curr Opin Chem Biol 7(5):586–591
He S, Wang Q, Li S, Chao R, Guo X, Zhen Z et al (2017) Antibiotic growth promoter olaquindox increases pathogen susceptibility in fish by inducing gut microbiota dysbiosis. Sci China Life Sci 60(11):1–11
Defoirdt T, Thanh LD, Van DB, De SP, Sorgeloos P, Boon N et al (2011) N-acylhomoserine lactone-degrading Bacillus strains isolated from aquaculture animals. Aquaculture 311(1–4):258–260
Sha J, Pillai L, Fadl AA, Galindo CL, Erova TE, Chopra AK (2005) The type III secretion system and cytotoxic enterotoxin alter the virulence of Aeromonas hydrophila. Infect Immun 73(10):6446–6457
Ponce-Rossi ADR, Pinto UM, Ribon ADOB, Bazzolli DMS, Vanetti MCD (2016) Quorum sensing regulated phenotypes in Aeromonas hydrophila ATCC 7966 deficient in AHL production. Ann Microbiol 66(3):1–10
Garde C, Bjarnsholt T, Givskov M, Jakobsen TH, Hentzer M, Claussen A, Sneppen K, Ferkinghoff-Borg J, Sams T (2010) Quorum sensing regulation in aeromonas hydrophila. J Mol Biol 396(4):849–857
Dong YH, Wang LH, Zhang LH (2007) Quorum-quenching microbial infections: mechanisms and implications. Philos Trans R Soc B 362(1483):1201–1211
Dong YH, Xu JL, Li XZ, Zhang LH (2000) AiiA an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc Natl Acad Sci U S A 97(7):3526–3531
Khan SR, Farrand SK (2009) The Blcc (AttM) lactonase of Agrobacterium tumefaciens does not quench the quorum-sensing system that regulates ti plasmid conjugative transfer. J Bacteriol 191(4):1320–1329
Liu CF, Liu D, Momb J, Thomas PW, Lajoie A, Petsko GA, Fast W, Ringe D (2013) A phenylalanine clamp controls substrate specificity in the quorum-quenching metallo-γ-lactonase from Bacillus thuringiensis. Biochemistry 52(9):1603–1610
Schneider J, Yepes A, Garcia-Betancur JC, Westedt I, Mielich B, López D (2012) Streptomycin-induced expression in Bacillus subtilis of ytnP a lactonase-homologous protein that inhibits development and streptomycin production in Streptomyces griseus. Appl Environ Microbiol 78(2):599–603
Wang WZ, Morohoshi T, Ikenoya M, Someya N, Ikeda T (2010) AiiM a novel class of N-acylhomoserine lactonase from the leaf-associated bacterium Microbacterium testaceum. Appl Environ Microbiol 76(8):2524–2530
Chen RD, Zhou ZG, Cao YN, Bai YG, Yao B (2010) High yield expression of an AHL-lactonase from Bacillus sp. b546 in Pichia pastoris and its application to reduce Aeromonas hydrophila mortality in aquaculture. Microb Cell Factories 9:39
Chu W, Lu F, Zhu W, Kang C (2011) Isolation and characterization of new potential probiotic bacteria based on quorum-sensing system. J Appl Microbiol 110(1):202–208
Hong HA, Huang J, Khaneja R, Hiep LV, Urdaci MC, Cutting SM (2010) The safety of Bacillus subtilis and Bacillus indicus as food probiotics. J Appl Microbiol 105(2):510–520
Duc HL, Hong HA, Barbosa TM, Henriques AO, Cutting SM (2004) Characterization of Bacillus probiotics available for human use. Appl Environ Microbiol 70(4):2161–2171
Morohoshi T, Kato M, Fukamachi K, Kato N, Ikeda T (2010) N-acylhomoserine lactone regulates violacein production in Chromobacterium violaceum type strain atcc 12472. FEMS Microbiol Lett 279(1):124–130
Chenia HY (2013) Anti-quorum sensing potential of crude Kigelia Africana fruit extracts. Sensors-Basel 13(3):2802–2817
Kang L, Han S, Wang J, Bo X, Ma X (2008) Detecting hematolysis of Enterococcus from sheep. Acta Microbiol Sin 48(7):924
Song ZF, Chen B, Guo J, Xu HD, Ren JF, Zhang QH (2015) Animal safety and ecological evaluation of Bacillus licheniformis T-1 with quorum sensing inhibitory effect. J Fish China 39(9):1395–1404
Rasmussen TB, Bjarnsholt T, Skindersoe ME, Hentzer M, Kristoffersen P, Köte M et al (2005) Screening for quorum-sensing inhibitors (QSI) by use of a novel genetic system the QSI selector. J Bacteriol 187(5):1799–1814
Riquelme C, Araya R, Vergara N, Vergara N, Rojas A, Guaita M, Candia M (1997) Potential probiotic strains in the culture of the Chilean scallop Argopecten purpuratus (lamarck 1819). Aquaculture 154(1):17–26
McClean KH, Winson MK, Fish L, Taylor A, Chhabra SR, Camara M et al (1997) Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143(12):3703
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) Mega5: molecular evolutionary genetics analysis using maximum likelihood evolutionary distance and maximum parsimony methods. Mol Biol Evol 28(10):2731–2739
Klingler JM, Stowe RP, Obenhuber DC, Groves TO, Mishra SK, Pierson DL (1992) Evaluation of the biolog automated microbial identification system. Appl Environ Microbiol 58(6):2089–2092
Mrvar-Brecko A, Sustar V, Jansa V, Stukelj R, Jansa R, Mujagić E et al (2010) Isolated microvesicles from peripheral blood and body fluids as observed by scanning electron microscope. Blood Cell Mol Dis 44(4):307–312
Membrebe JD, Yoon NK, Hong M, Lee J, Lee H, Park K, Seo SH, Yoon I, Yoo S, Kim YC, Ahn J (2016) Protective efficacy of Streptococcus iniae derived enolase against Streptococcal infection in a zebrafish model. Vet Immunol Immunopathol 170:25–29
Wang WZ, Morohoshi T, Someya N, Ikeda T (2012) AidC a novel N-acylhomoserine lactonase from the potato root-associated cytophaga-flavobacteria-bacteroides (cfb) group bacterium Chryseobacterium sp. strain strb126. Appl Environ Microbiol 78(22):7985–7992
Kalia VC (2013) Quorum sensing inhibitors: an overview. Biotechnol Adv 31(2):224–245
Tang K, Zhang Y, Yu M, Shi X, Coenye T, Bossier P, Zhang XH (2013) Evaluation of a new high-throughput method for identifying quorum quenching bacteria. Sci Rep-UK 3(10):2935
Zhou Y, Choi YL, Sun M, Yu Z (2008) Novel roles of Bacillus thuringiensis to control plant diseases. Appl Microbiol Biotechnol 80(4):563–572
Boer ASD, Priest F, Diderichsen B (1994) On the industrial use of Bacillus licheniformis: a review. Appl Microbiol Biotechnol 40(5):595–598
Pasnik DJ, Evans JJ, Klesius PH (2010) Bacillus licheniformis isolated during a fish kill is non-pathogenic. Fish Sci 74(6):1351–1353
Alexopoulos C, Georgoulakis IE, Tzivara A, Kyriakis CS, Govaris A, Kyriakis SC (2010) Field evaluation of the effect of a probiotic-containing Bacillus licheniformis and Bacillus subtilis spores on the health status performance and carcass quality of grower and finisher pigs. Transbound Emerg Dis 51(6):306–312
Deng W, Dong XF, Tong JM, Zhang Q (2012) The probiotic Bacillus licheniformis ameliorates heat stress-induced impairment of egg production gut morphology and intestinal mucosal immunity in laying hens. Poult Sci 91(3):575–582
Endres JR, Clewell A, Jade KA, Farber T, Hauswirth J, Schauss AG (2009) Safety assessment of a proprietary preparation of a novel probiotic Bacillus coagulans as a food ingredient. Food Chem Toxicol 47(6):1231–1238
Li K, Zheng T, Tian Y, Xi F, Yuan J, Zhang G, Hong H (2007) Benificial effects of Bacillus licheniformis on the intestinal microflora and immunity of the white shrimp Litopenaeus vannamei. Biotechnol Lett 29(4):525–530
Zhang Q, Tan B, Mai K, Zhang W, Ma H, Ai Q, Wang X, Liufu Z (2011) Dietary administration of Bacillus (B. licheniformis and B. subtilis) and isomaltooligosaccharide influences the intestinal microflora immunological parameters and resistance against Vibrio alginolyticus in shrimp Penaeus japonicus (decapoda: penaeidae). Aquac Res 42(7):943–952
Ramesh D, Vinothkanna A, Rai AK, Vignesh VS (2015) Isolation of potential probiotic Bacillus spp. and assessment of their subcellular components to induce immune responses in Labeo rohita against Aeromonas hydrophila. Fish Shellfish Immunol 45(2):268–276
Bai F, Yin H, Chen J, Zhang XH (2008) Disruption of quorum sensing in Vibrio harveyi by the AiiA protein of Bacillus thuringiensis. Aquaculture 274(1):36–40
Abed RM, Dobretsov S, Al-Fori M, Gunasekera SP, Sudesh K, Paul VJ (2013) Quorum-sensing inhibitory compounds from extremophilic microorganisms isolated from a hypersaline cyanobacterial mat. J Ind Microbiol Biotechnol 40(7):759–772
Schuster M, Sexton DJ, Diggle SP, Greenberg EP (2013) Acyl-homoserine lactone quorum sensing: from evolution to application. Annu Rev Microbiol 67(1):43–63
Vinoj G, Vaseeharan B, Thomas S, Spiers AJ, Shanthi S (2014) Quorum-quenching activity of the AHL-lactonase from Bacillus licheniformis DAHB1 inhibits Vibrio biofilm formation in vitro and reduces shrimp intestinal colonisation and mortality. Mar Biotechnol 16(6):707–715
Acknowledgments
This work was supported by the Innovation Fund of Nanjing Government Jiangsu Province, China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, B., Peng, M., Tong, W. et al. The Quorum Quenching Bacterium Bacillus licheniformis T-1 Protects Zebrafish against Aeromonas hydrophila Infection. Probiotics & Antimicro. Prot. 12, 160–171 (2020). https://doi.org/10.1007/s12602-018-9495-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-018-9495-7