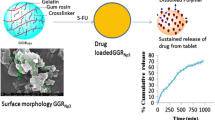
Abstract
The present study is utilizing the targeted therapeutic approach and antioxidant potential of selected probiotic biomass in mitigating toxic side effects of chemotherapeutic agents. Multicomponent carrier system consisting of 5-fluorouracil (5-FU) and selected probiotic strain with higher free radical scavenging activity was prepared using spray drying technique. Prepared spray dried microparticles were characterized for various physical, pharmaceutical, and biopharmaceutical properties including particle size, moisture content, entrapment efficiency, in vitro drug release, DSC, XRD, cell uptake, histopathology, and pharmacokinetic studies. In addition to the above, optimized formulation was subjected to in vivo targeting efficacy studies using radiographic technique. Optimized formulation meets the necessary physical requirement for pharmaceutical powder. X-ray studies revealed that the prepared spray dried formulations are able to target the colon. Pharmacokinetic endpoints with an extended t 1/2 and lower C max indicate lower systemic toxicity. Intact nature of colonic epithelium in experimental formulation clearly demonstrates the protective role of Lactobacillus rhamnosus in minimizing the harmful consequence induced by 5-FU. Existing outcomes provide the basis for a combination of targeted therapeutic approach with natural antioxidant capacity of potential probiotic strain which could help to mitigate the problems associated with traditional chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Probiotics are generally the live microorganisms that offer a number of health benefits to the host. In the past few decades, great progress in this area has been made. Much evidence has emphasized the beneficial role of probiotics in human health and disease. Clinical studies clearly suggest that the probiotic therapies help to treat gut abnormalities particularly effective in the treatment of acute and chronic inflammation. In a recent study, Loren et al. investigated the therapeutic benefits of Lactobacillus plantarum (CECT7484, CECT7485) and Pediococcus acidilactici (CECT7483)) in a murine model of colitis. Result suggests that selected probiotic group significantly reduces colitis severity compared to untreated controls [1]. Although the underlying mechanisms have not been fully elucidated, antioxidant activity of probiotics seems to play an important role in reducing inflammation. A number of studies have reported that probiotics particularly Lactobacillus species exhibit strong antioxidant activity [2]. Recently, Lactobacillus fermentum has been studied for use as a supplement in the treatment of inflammatory bowel disease (IBD). Reinforcing the effects of L. fermentum in inflammation, Chauhan and coworkers assessed its antioxidative efficacy in colitis mouse model. Results suggested that the selected strain of Lactobacillus exhibits significant antioxidant activity. In addition, probiotics also seems to upregulate the level of antioxidant enzymes [3]. Similarly, Jiali et al. investigated the antioxidant potential of different Lactobacillus species employing established in vitro systems, namely 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging, hydroxyl radical scavenging (HRS), reducing power (RP), and inhibition of linoleic acid peroxidation (ILAP). Result suggests that Lactobacillus rhamnosus CCFM 1107 displayed a high antioxidative effect similar to positive control [4]. Although the antioxidant potential of probiotics has not yet been fully understood, current literatures suggest polysaccharides, organic acids, and lipids are the key components involved in the antioxidant activity of probiotics [5]. Colon cancer is one of the best-understood neoplasms from a genetic perspective, yet it remains the second most common cause of death from cancer [6]. There are many chemotherapeutic agents used for the treatment of colon cancer, such as 5-fluorouracil (5-FU), cisplatin, mitomycin, etc. 5-FU is one of the widely used molecule in the treatment of colon cancer [7, 8]. Clinical benefits of 5-FU are greatly reduced due to its undesirable toxicity on normal cells and tissues. A recent study describes that local or systemic toxicity induced by 5-FU is mainly dose dependent. Therefore, controlled and targeted delivery of 5-FU ensures high level of drug concentration at the local tissue, which is not only essential to deduce full clinical benefits but also reduces systemic toxicity [9]. Recently, various drug delivery systems have been used to deliver drugs specifically to colon. Eudragit owing to its biodegradability and pH-dependent solubility has been successfully used as a polymeric material for colonic drug delivery. Accordingly, Rita et al. studied the release behavior of budesonide from spray dried Eudragit microparticles. Result suggests that Eudragit microparticles showed a better protection of the drug in gastric medium, whereas drug release was accelerated in intestinal fluid [10]. The results of the study indicated that Eudragit-based microparticulate systems could be a useful carrier for colon targeting of encapsulated bioactive. Accordingly, in the present study, Eudragit was selected as a polymeric constituent for its specific pH-dependent controlled delivery potential to achieve site-specific drug delivery. The recent literature suggests 5-FU-induced toxicity is often associated to free radical intermediates, resulting in lipid peroxidation and tissue damage. Therefore, a fine balance between free radicals and antioxidants is necessary to clarify the therapeutic activity of anticancer drugs. Considering the possible beneficial effects, probiotic supplement can help to reduce the side effects of traditional chemotherapy [11, 12].
Therefore, an adjust therapy with potential probiotic strain could be useful in minimizing multiple toxicity during chemotherapy. An examination of the literature reveals that L. rhamnosus and L. plantarum, in addition to their potential health benefits, are known to produce specific exopolysaccharide and soluble protein which have potent free radical scavenging activity. Considering aforementioned benefits, the purpose of this study was to select potential probiotic strain in order to derive the greatest potential benefit from any cancer treatment [13]. Spray drying owing to its simple fabrication technology and single operational steps offers ample process flexibility to restore the maximum viable count of probiotics in the final formulation [14]. Therefore, the aim of the present study was to the develop and characterize spray dried formulation of 5-fluorouracil embedded with probiotic biomass. The objective is further extended to investigate the therapeutic relevance of the proposed strategies in appropriate animal model.
Material and Methods
Materials
The drug 5-FU was obtained from the United Biotech Pvt. Ltd., Baddi (India). Disodium hydrogen phosphate and potassium dihydrogen phosphate were purchased from SD Fine-Chem Limited, Mumbai (India). Eudragit S100 was purchased from Evonik Industries. l-Leucine was purchased from HiMedia Pvt. Ltd., Mumbai (India). COLO-205 cell line was purchased from NCCS Pune. L. plantarum (ATCC No. 8014) and L. rhamnosus (ATCC No. 8530) were purchased from IMTECH, Chandigarh, India. Sodium chloride was purchased from CDH, India. MTT dye was purchased from Loba Chemie Pvt. Ltd., Mumbai (India).
Determination of Antioxidant Potential of Probiotic Biomass Using DPPH Assay
The free radical scavenging activity of L. plantarum and L. rhamnosus was analyzed using method described by Fratianni et al. [15] with some modification. Freshly prepared probiotic biomass (0.3 mL) was mixed with 1 mL of ethanolic solution of DPPH and allowed to react for 30 min. Control was prepared by mixing 500 μl of ethanol with 1.5 mL DPPH solution in the dark and incubated for 30 min. Absorbance was recorded at 517 nm after 30 min of incubation with uninoculated MRS broth serving as blank.
IC50 values represent the concentration of sample, required to scavenge 50 % of DPPH free radicals. Radical scavenging activity was calculated by the following formula:
Preparation and Characterization of Spray Dried Microparticles
Polymeric microparticles of 5-FU embedded with probiotic biomass were prepared using spray dried technique [16]. Briefly, Eudragit S100, Leucine, 5-FU, and probiotic biomass were optimized, required quantity of each ingredients was taken, and dispersed into PBS (pH 7.4). Prepared dispersion was sonicated using bath sonicator for 3 min. The above dispersion was subjected for spray drying using lab spray drier (Labultima, LU-122 advanced spray dryer, India). Various process parameters including those that directly affected the final state of the product including temperature and flow rate were optimized. The powdered samples were stored at room temperature under vacuum conditions in a desiccator immediately after spray drying to limit the uptake of moisture. The yield was computed as a percentage of the mass of powder collected divided by the initial solid mass in the solution prior to spray drying. The percentage yield of spray dried microparticles was calculated by using Eq. (2):
Particle Morphology
Particle size of the prepared spray dried formulations was determined using Motic microscope. Briefly, a homogeneous dispersion of spray dried powder was prepared in cedarwood oil. A drop of suspension was spread on a glass slide and observed at ×1000 magnification using the ×100 objective installed with a digital camera and image analyzer software. About 100 particles were randomly snapped and used to determine the average particle size.
Karl Fischer Coulometric Titration
The moisture content of prepared spray dried powder was determined by Karl Fischer (KF) coulometric titration technique. The measurements were performed with a 737 KF Coulometer coupled with 703 Ti Stand (Metrohm Ltd., Antwerp, Belgium). Approximately 10 mg of spray dried powder was dissolved in anhydrous methanol in a 5-mL volumetric flask. The sample was injected into the reaction cell that contained KF reagent. The moisture content was then calculated.
In Vitro Drug Release Study
In vitro release of 5-FU from spray dried powder was evaluated using dialysis technique. 5-FU-loaded spray dried powder was dispersed in selected medium (0.1 N HCl as simulated gastric fluid and PBS 7.4 as simulated intestinal fluid without enzyme). The mixture was transferred into a dialysis bag with a molecular weight cutoff 10,000 Da. The dialysis bag was placed in a beaker containing the release medium at 37 °C under continuous orbital agitation of 100 rpm. At predetermined interval, 1 mL of sample was withdrawn and replaced with fresh medium at the same temperature to maintain the sink condition. The amount of drug release was quantified using UV spectroscopy. All the experiments were carried out in triplicate.
Entrapment Efficiency
Spray dried powder equivalent to 5 mg of drug was dissolved in suitable medium. The resulting solution was briefly vortex and was sonicated for 10 min using a bath sonicator. Then, it was centrifuged at 5000 rpm for 10 min. The amount of drug present in supernatant was determined using calibration curve using following Eq. (3):
X-Ray Diffraction
The physical state of 5-FU in prepared formulation was determined using X-ray diffractometer. The X-ray diffraction (XRD) patterns were determined for the 5-FU-loaded microparticles. Samples were exposed to a monochromatic nickel-filtered copper radiation (45 kV, 40 mA) in a wide-angle X-ray diffractometer (D8 Advance, BRUKER, Germany) with 2θ angle range of 3–40°.
Differential Scanning Calorimetry
DSC is a thermo-analytical technique, which is used to study degree of crystallinity and polymorphic transitions of drug in final formulation. The phase transition temperature of 5-FU in spray dried microparticles was analyzed by differential scanning calorimetry (SIIO 6300, SIIO, Japan) in perforated aluminum-sealed pans at a heating rate of 5 °C/min from 30 to 400 °C using nitrogen as blanket gas (50 mL/s).
Morphology
Surface characterization of spray dried powder was measured using SEM (JEOL-5400, JAPAN). Spray dried microparticles were analyzed without coating at 15–20 Kv. SEM images were obtained for all the runs to examine particle morphology. Particle size was determined by examining the diameter or digital photograph.
In Vitro Cytotoxicity Assay
Cytotoxicity of prepared formulations was determined against Colo-205 human epithelial colorectal adenocarcinoma cell lines purchased from National Center for Cell Sciences (NCCS), Pune, India. Colo-205 cells were seeded in 96-well microculture plates at 1 × 104 cells/well in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % heat-inactivated fetal bovine serum (FBS) and incubated for 24 h at 37 ± 0.5 °C in 5 % CO2 incubator. Tested formulations (5-FU and 5-FU–Eudragit S100–probiotic) were diluted to various concentrations in culture medium and incubated with Colo-205 cells for 24 h, respectively. The percentage cell growth inhibition was measured using MTT assay standard protocol [17].
Cell Uptake Studies
Cellular uptake of developed formulation was determined following the reported procedure [18] with slight modifications. Briefly, Colo-205 cells were incubated with tested formulations (5-FU and 5-FU–Eudragit S100–probiotic) at 37 °C with 5 % CO2 for 4 h. Then, the medium was removed and cells were washed three times with PBS (pH 7.4). The fluorescence due to uptake of formulation was visualized qualitatively (Olympus, CK X 41).
Antioxidant Potential of Spray Dried Formulation
The free radical scavenging activity of spray dried formulation was determined as described by Dawidowicz et al. [14, 19]. DPPH (4.3 mg) was dissolved in methanol (6.6 mL) to prepare 0.3 mM DPPH solution. DPPH (150 μl) added to 3 mL of methanol and the absorbance was measured at 516 nm for control reading. Spray dried powder containing an equivalent amount of drug (10 mg/kg) was diluted with methanol up to 3 mL. To this solution, 150 μl DPPH was added. The samples were kept in dark for 15 min, after which the optical density was observed at 516 nm using methanol as blank. The results were expressed as IC50 μg/mL, representing the substrate concentration required to produce 50 % reduction of the DPPH.
In Vivo Studies
Pharmacokinetic Study
Wistar rats (200–280 g) were used to study various pharmacokinetic parameters of tested formulations (plain drug, 5-FU–Eudragit S100 formulation and Eudragit S100–5-FU–probiotic formulations). Animals were housed under standard laboratory condition with free access to food and water. For pharmacokinetic study, animals were fasted overnight and for 8 h after the dosing. Animal protocol was approved by Institutional Animal Ethical Committee (IAEC) at ISF College of Pharmacy, Moga, India. Animal experiments were conducted as per Committee for Prevention, Control and Supervision of Experimental Animals (CPCSEA) guidelines as per approved protocol (ISFCP/IAEC/CPCSEA/Meeting No. 14/2015/ 269). Various pharmacokinetic parameters were obtained after oral administration of tested formulation. Total 27 animals were divided into three groups, each containing nine animals. Route of administration, dose, and sample used for preclinical study are presented in Table 1. For pharmacokinetic studies, blood from rat’s eye was collected followed by centrifugation at 5000 rpm for 30 min at a fixed temperature of 4 °C. Plasma (0.1 mL) was taken from the supernatant. Then, 0.8 mL acetonitrile was added. Of various drug concentrations, 0.1 mL was added in order to make 0.1–10 μg/mL concentrations. After brief vortexing for 5 min and centrifuging at 3000 rpm for 10 min, supernatant was collected. One milliliter of supernatant was withdrawn and was diluted up to 2 mL with buffer. Samples were analyzed by HPLC using the standard curve between AUC vs. time.
Histopathology
Prepared formulations were administered orally using a cannula. Animals were sacrificed 4 h after last administration and the colon was excised and placed in tubes. A portion (2 cm) of the colonic specimen from each rat (n = 6) was fixed in 10 % formalin, cut into 5-μm thickness, stained using hematoxylin–eosin, and histopathological observations were made. The stained sections of colon were examined for any inflammatory changes like infiltration of the cells, necrotic foci, and damage to tissue structures like lamina propria, villi, intestinal damage to nucleus, etc.
X-Ray Transmission Radiography to Determine the Targeting Potential
Targeting efficacy of optimized spray dried formulation in rat was determined using a radiographic imaging technique. It involves the use of radiopaque markers such as barium sulfate, incorporated in the formulation to determine the position of the microparticles. The amount of the barium sulfate was optimized for no effect on the physical characteristics of the optimized spray dried formulation. The animals (n = 2) were fasted overnight prior to the experiment, with free access to water. X-ray images of the colon of the rats were taken at various time points (2.0 and 4.0 h) to trace the in vivo movement and behavior of the microparticles in the GIT. X-ray images of the rabbits in prone position were captured using L&T Vision 100 (C-arm) X-ray machine, at 64 mAs and 63 kV techniques.
Statistical Analysis
The statistical analysis of data was performed using analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. The results were expressed as mean ± standard deviation and showed the number of repeats. A difference of p < 0.05 was considered as statistically significant. The survival analysis was done by Kaplan−Meier method using GraphPad Prism V5.01.
Results
Determination of Antioxidant Potential of Probiotic Biomass Using DPPH Assay
The results were expressed as the dose required to cause 50 % inhibition by the bacteria (IC50), and the results are depicted in Table 2. The higher antioxidant activity was observed with L. rhamnosus (63.57 μg/mL). L. plantarum showed free radical scavenging activity in the range of 70–72 μg/mL. The results of the study are consistent with the observation of Bae and his coworkers [20].
Formulation Optimization
Formulation variables, i.e., weight ratio of Eudragit S100, leucine, 5-FU, and probiotic biomass, were optimized on the basis of their effect on percent yield, particle size, moisture content, and percent entrapment as shown in Table 3. At the initial stage, the concentration of polymer and leucine was optimized under a defined spray drying parameter (inlet air temperature 140 °C and flow rate 5 mL/min). Results show that as concentration of polymer increases from 0.3 to 1.2 % w/v against a fixed concentration leucine, it affects the particle size and percentage yield. However, percent yield was found to decrease with an increase in particle size above polymer concentration 1 % w/v that could be related to high viscosity of the slurry. In addition to above, high solid content increases interparticular attractive force, enough to cause the aggregation of particle. Finally, it was evident from the results that 1 % w/v concentration of polymer produced effective dry powder. Keeping the polymer concentration constant, the effect of the anti-adherent leucine was investigated. Leucine concentration at 0.2 % w/v shows highest product yield with minimum moisture content. As the leucine concentration increases further, there is a linear increment in moisture content with an increase in particle size. Of leucine, 0.2 % w/v percentage was considered to be the optimized anti-adherent concentration. Further, the formulation was screened for drug concentrations on the basis of entrapment efficiency keeping the concentration of polymer and leucine constant. Results suggested that the entrapment efficiency was significantly reduced above a total drug concentration of 0.2 % w/v which could be related to saturated solubility of drug in the selected medium, where equilibrium of drug was established between polymer and medium. Further increase in drug concentration causes the extra drug to precipitate resulting in a decrease in entrapment efficiency. Therefore, 0.2 % w/v concentration of drug was considered the optimized drug concentration for further study. Lastly, the concentration of probiotic biomass was optimized keeping the concentration of polymer, leucine, and drug constant. Selection of final formulation was based on particle size, moisture content, and percentage yield. It has been observed that the moisture content was increased with increasing concentration of probiotic biomass. Probiotic biomass at a CFU level of 1013 was selected as optimized formulation parameter obtaining spray dried powder with desirable characteristics. The results of optimized process suggested that formulation F19 (1 % w/v of Eudragit, 0.2 % w/v of leucine, 0.2 % of drug, and 1013 of probiotic mass) demonstrated all the required characteristic and were therefore selected for formulation development.
In Vitro Characterization of Spray Dried Formulation
In vitro characterization of spray dried formulations includes particle morphology, moisture content, in vitro drug release, entrapment efficiency, X-ray diffraction studies, differential scanning calorimetry, and morphology.
Moisture Content
The effects of independent variable on moisture content of spray dried formulations are shown in Table 3. The moisture content of optimized formulation (F19) was found to be 4.56 % under the defined spray drying condition. Moisture content increased linearly with the increase in polymer concentration.
In Vitro Drug Release
The drug release data obtained for spray dried powder with and without probiotic are shown in Figs. 1 and 2. The plot represents cumulative percent drug release as a function of time. The results of dissolution studies indicated sustained release behavior of 5-FU in simulated gastric and intestinal fluid over a period of 5 h. Eudragit by virtue of its poly anionic nature inhibits the gastric burst release. Spray dried formulations with Eudragit S100 showed a steady drug release which approximated zero-order release.
Entrapment Efficiency
Entrapment efficiency was determined for all prepared formulations. There was no significant difference in entrapment efficiency of drug observed pertaining to weight ratio of 0.05 to 2 % w/v. However, a significant decrease in entrapment efficiency was observed at drug concentration above 0.2 % w/v which could be related to equilibrium solubility suggesting the saturation capacity of polymer.
X-Ray Diffraction
Diffractogram of 5-FU in Eudragit S100–probiotic formulation had shown strong peaks at about 18° 2.theta (Fig. 3). The X-ray diffractogram of enteric-coated spray dried microparticles was found to be different from the pure drug, presence of small number of crystalline peaks with much reduced intensity compare to pure drug indicating that change of crystalline state of drug.
Differential Scanning Calorimetry
The DSC curve of spray dried microparticles shows peaks at 97.47, 137.92, 219.54, and 307.74 °C and a minor peak at 219 °C due to the existence of drug crystals (Fig. 4). These studies further strengthened the evidence that there is compatibility between the drug and the polymer and also confirmed the presence of drug within polymeric microparticles. Presence of broad exothermic peak at 219 °C with reduced peak intensity suggests the change in physical state of drug in formulation which is also supported by XRD study. The change in physical state could be related to rapid drying during spray drying process causing phase transformation of drug.
Morphology
Surface morphology of spray dried powder was analyzed by SEM. Results suggested that spray dried particles of 1 % w/v polymer appear to be spherical with relatively wrinkled surface (Fig. 5). The wrinkled morphology could be due to flash evaporation leaving a relatively rough surface. Particle size was increased on increasing polymer concentration from 0.3 to1.2 % w/v.
In Vitro Cytotoxicity Assay
The cytotoxic activity of drug-loaded microparticles was determined using MTT assay after 24 h incubation of formulation with Colo-205 cells. Cytotoxicity of 5-FU and 5-FU–Eudragit S100–probiotic formulations was investigated with different 5-FU concentrations to elucidate the dose- and time-dependent anticancer activity of developed formulation against plain 5-FU. All the formulations revealed significant increase in cytotoxicity with an increase in concentration and time; hence, a positive correlation was observed between the concentration/time and cytotoxicity. At the same concentration of 5-FU and incubation time, 5-FU–Eudragit S100–probiotic formulation showed higher anticancer activity. For instance, at a highest tested dose of 100 μg/mL, 5-FU showed 20.53 ± 3.20 % cell viability after 24 h. However, the prepared formulation shows a significantly higher cytotoxicity of 14.53 ± 2.90 % (Fig. 6) towards the target Colo-205 cells than that of an equivalent concentration of free drug.
Cell Uptake Studies
Cellular uptake assay was performed to assess the intracellular fate of microparticles. Cellular uptake of formulations was determined after 4 h (Fig. 7). Result exhibits enhanced cellular uptake of 5-FU–Eudragit S100–probiotic formulation. Additionally, on the basis of results of fluorescence microscopy, cells incubated with 5-FU–Eudragit S100–probiotic formulation showed greater fluorescence in comparison with Colo-205 cells incubated with 5-FU.
Determination of Antioxidant Potential of Spray Dried Formulation Using DPPH Assay
The results were expressed as the dose required to cause 50 % inhibition by the bacteria (IC50) and are depicted in Table 4. The IC50 value for the spray dried product, i.e., F19, was 63.73 μg/mL. Results of free radical scavenging assay inferred that the spray dried formulation exhibited a comparable antioxidant activity to standard (47.37 μg/mL). Further optimization of multiple responses involved in methodology adopted restores the antioxidant potential of native L. rhamnosus. Thus, Eudragit appears to be an ideal matrix for immobilization of cells.
In Vivo Studies
Pharmacokinetic Study
Plasma drug concentration–time profiles of 5-FU, 5-FU–Eudragit S100 formulation, and 5-FU–Eudragit S100-probiotic formulation administered orally in Wistar rats are presented in Table 5 and Fig. 8. From the given results, it was observed that the developed optimized formulation showed extended plasma concentration with higher t 1/2 over the plain drug t 1/2 (5.60 h). Further, the plasma concentration takes longer time to reach its maximum value in case of 5-FU–Eudragit formulation followed by 5-FU–Eudragit S100–probiotic formulation and plain drug, indicating sustained release behavior of experimental formulation.
Histopathology
5-FU at a dose of working in probiotic-assisted formulation minimizes tissue damage when compared with plain and 5-FU-loaded Eudragit formulation. Abnormalities of tissue function were characterized by distorted lamina propria infiltrated by dense polymorphonuclear cells with scattered zones of congestion and hemorrhage as shown in Fig. 9. Further, the villous epithelial cell hyperplasia was clearly observed in 5-FU-treated groups. These observations clearly demonstrate prominent lamina propria was observed with scattered number of PMN cells in probiotic-assisted formulation clearly evident of the protective effects of probiotics. Protective action of probiotic biomass could be possibly related to its anti-inflammation, antioxidant, homeostatic, and immunomodulator activity. As already reported, 5-FU-induced tissue damage may be due to generation of free radical causing lipid peroxidation resulting in tissue damage.
X-Ray Transmission Radiography
X-ray studies shows that the spray dried formulation successfully targeted to the colon (Fig. 10). Polyanionic nature of Eudragit retarded the release of encapsulated material in stomach. pH-dependent drug release behavior of Eudragit ensures colon-specific drug delivery. It can be concluded from the X-ray images that the enteric-coated microparticles release the drug in the colon. Hence, it proves that the formulation is ideal for colon targeting.
Discussion
In order to understand the variation of antioxidant activities of different Lactobacillus strains, selected Lactobacillus strains were evaluated for their antioxidant potential, key to reducing inflammation associated with chemotherapy. Free radical scavenging activity of L. rhamnosus was found to be significantly higher than L. plantarum. Higher scavenging activity of L. rhamnosus could be related to the ability of L. rhamnosus to produce unusually high proportion of soluble protein and exopolysaccharide. Similar observations were also reported by Bae et al. [20].
In the present study, spray dried technique was used to develop dry powder formulation with desirable features. The selected formulation was characterized for various in vitro and in vivo properties. The moisture content of optimized formulation (F19) was found to be 4.56 %. Moisture content of spray dried powder is related to the particle size and the amount of absorbed water, as the rate of evaporation is directly related to surface area. Larger particles have less available surface area for evaporation causing an increase in moisture content. Spray dried formulations with Eudragit S100 showed a steady drug release behavior which approximated zero-order release. The rate of drug release depends on the swelling of Eudragit in alkaline pH. The degree of swelling deteriorates the pore size and pore density which in turn influences the release rate. There was no significant difference in drug release pattern observed from spray dried power with and without probiotics.
Entrapment efficiency was found to be decreased at drug concentration above 0.2 % w/v which could be related to equilibrium solubility suggesting the saturation capacity of polymer. This finding has been reported by several authors. High water solubility of 5-FU, polymer concentration, and physicochemical proportion of polymer play a key role in achieving high entrapment efficiency in experimental formulation. The physical state of the drug in prepared formulations was determined by using XRD and DSC. The X-ray diffractogram and DSC thermogram of spray dried microparticles were found to be different from the pure drug. Reduced intensity indicating transition of crystalline state of 5-FU could be attributed to flash evaporation involved in spray drying process and could prevent the formation of stable drug crystal. Surface morphology of spray dried powder was analyzed by SEM. Particle size was increased on increasing polymer concentration from 0.3 to 1.2 % w/v and due to the increase in particle density increases hydrophobic interaction known to promote particle aggregation which markedly increases particle size. Further, the higher particle size is connected with the high surface energy of spray dried microparticles and high molecular weight of polymer.
Apparently, higher cytotoxic activity in probiotic-based formulation could be related to antitumor effects of probiotic. The experimental evidence supports earlier finding, where the exopolysaccharide produced by L. acidophilus and L. rhamnosus exhibits antitumorigenic activity against HT-29 colon cancer cells. This activity was due to the activation of autophagic cell death promoted by induction of Beclin-1 and GRP 78. Further, the controlled drug-released behavior of experimental formulation improves drug permeability improving antitumor activity. Moreover, the combined benefits of extended release with probiotic-assisted tumor suppress the capacity of 5-FU in colorectal cell line, suggesting that this can be used as adjuvant on chemotherapy [21, 22]. Enhanced cytotoxicity could be related to increased cell permeability, which is evident from cell uptake results. Enhanced cell permeability elucidates the possible role of biosurfactant produced by L. rhamnosus in helping to increase the membrane permeability thereby increasing in vitro antitumor activity. Such explorations along with enhanced cytotoxicity against cancer cells and improved drug release profile could support the therapeutic potential of developed microparticles. It is well known that each microorganism has critical preservation activities below which limiting their activity. Results of antioxidant activity suggested that spray drying of probiotics with Eudragit S100 under optimal processing conditions resulted in the preservation of the antioxidant activity of probiotics.
Assessment of pharmacokinetic properties is useful not only to determine the effect of formulation but also to ensure product safety. Observed mean C max (27.25 μg/mL) value and longer half-life of probiotic-anchored formulation largely contribute to the interpretation of systemic toxicity. Pharmacokinetic finding established the controlled drug release behavior of experimental formulation which offers more effective modality of chemotherapy by providing higher local concentration of the drug at the target sites and minimizing systemic drug absorption. Histogram revealed probiotic-treated groups show intact tissue structure with no sign of tissue abnormality. Probiotics, due to its tendency to exhibit free radical scavenging activity and it is considered to be associated with exopolysaccharide and soluble protein, provide adequate protection to local tissue injury induced by 5-FU. X-ray transmission radiography was used to determine the targeting potential of the prepared formulation. X-ray studies revealed that the spray dried formulation successfully targeted to the colon. Further pH-dependent drug release behavior of Eudragit ensures colon-specific drug delivery.
Conclusion
Chemotherapy is undoubtedly the only effective treatment option for cancer patients. However, chemotherapy side effects can greatly reduce the therapeutic efficacy and alter quality of life of a patient. Despite extensive research efforts to reduce off-target side effects, very few treatment approaches are found to be clinically effective. Recently, probiotics are found to reduce the side effect of antimicrobials and chemotherapeutic drugs. We present here multifunctional constructs combining targeted drug delivery approach and probiotics to circumvent the side effects of chemotherapy. Developed 5-FU–Eudragit S100–probiotic formulation seems ideal for colon-targeted drug delivery by virtue of its pH-dependent drug release behavior. L. rhamnosus due to its unique free radical scavenging activity along with other possible mechanism significantly alternates local tissue damage induced by 5-FU. Therefore, the therapeutic approach combining targeted drug delivery strategy and beneficial health effect of selected probiotic strain could be successfully used to mitigate the systemic and local toxicity of chemotherapy.
References
Lorén V, Manyé J, Fuentes MC, Cabré E, Ojanguren I, Espadaler J (2016) Comparative effect of the I3.1 probiotic formula in two animal models of colitis. Probiotics and Antimicrobial Proteins 10:1-0
Wang BG, Xu HB, Xu F, Zeng ZL, Wei H (2015) Efficacy of oral Bifidobacterium bifidum ATCC 29521 on microflora and antioxidant in mice. Can J Microbiol 62(3):249–262
Chauhan R, Sudhakaran Vasanthakumari A, Panwar H, Mallapa RH, Duary RK, Batish VK, Grover S (2014) Amelioration of colitis in mouse model by exploring antioxidative potentials of an indigenous probiotic strain of Lactobacillus fermentum Lf1. Biomed Res Int 2014:206732
Xing J, Wang G, Zhang Q, Liu X, Gu Z, Zhang H, Chen YQ, Chen W (2015) Determining antioxidant activities of lactobacilli cell-free supernatants by cellular antioxidant assay: a comparison with traditional methods. PLoS One 10(3):e0119058
Polak-Berecka M, Wasko A, Szwajgier DO, Choma A (2013) Bifidogenic and antioxidant activity of exopolysaccharides produced by Lactobacillus rhamnosus E/N cultivated on different carbon sources. Pol J Microbiol 62(2):181–189
Hofman M, Morrow GR, Roscoe JA, Hickok JT, Mustian KM, Moore DF, Wade JL, Fitch TR (2004) Cancer patients’ expectations of experiencing treatment-related side effects. Cancer 101:851–857. doi:10.1002/cncr.20423
O’Brien CA, Pollett A, Gallinger S, Dick JE (2007) A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 445:106–110. doi:10.1038/nature05372
Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R (2007) Identification and expansion of human colon-cancer-initiating cells. Nature 445:111–115. doi:10.1038/nature05384
Tacar O, Sriamornsak P, Dass CR (2013) Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Phar 65:157–170. doi:10.1111/j.2042-7158.2012.01567.x
Cortesi R, Ravani L, Menegatti E, Esposito E, Ronconi F (2012) Eudragit® microparticles for the release of budesonide: a comparative study. Indian J Pharm Sci 74(5):415–421
Jain K (2005) Nanotechnology-based drug delivery for cancer. Technol Cancer Res Treat 4:407–416. doi:10.1177/153303460500400408
Paharia A, Yadav AK, Rai G, Jain SK, Pancholi SS, Agrawal GP (2007) Eudragit-coated pectin microspheres of 5-fluorouracil for colon targeting. AAPS Pharm Sci Tech 8:E87–E93
Kumar K, Sastry N, Polaki H, Mishra V (2015) Colon cancer prevention through probiotics: an overview. J Cancer Sci Ther 7:081–092. doi:10.4172/1948-5956.1000329
Nagpal R, Kumar A, Kumar M, Behare PV, Jain S, Yadav H (2012) Probiotics, their health benefits and applications for developing healthier foods: a review. FEMS Microbiol Lett 334:1–15. doi:10.1111/j.1574-6968.2012.02593.x
Fratianni F, Pepe S, Cardinale F, Granese T, Cozzolino A, Coppola R, Nazzaro F (2014) Eruca sativa ,might influence the growth, survival under simulated gastrointestinal conditions and some biological features of Lactobacillus acidophilus, Lactobacillus plantarum and Lactobacillus rhamnosus strains. Int J Mol Sci 15:17790–17805. doi:10.3390/ijms151017790
Tobar-Grande B, Godoy R, Bustos P, von Plessing C, Fattal E, Tsapis N, Olave C, Gómez-Gaete C (2013) Development of biodegradable methylprednisolone microparticles for treatment of articular pathology using a spray-drying technique. Int J Nanomedicine 8:2065. doi:10.2147/IJN.S39327
Bansal S, Kumar S, Choudhary A, Tiwari A, Aggarwal V, Joseph A (2014) Synthesis, docking study & anticancer evaluation of novel 3-phenyl-2-(3-aryl-1-phenyl-1H-pyrazol-4-yl) thiazolidine-4-ones. Ind Glo J Pharm Sci 4:18–24
Xiao B, Zhang M, Viennois E, Zhang Y, Wei N, Baker MT, Jung Y, Merlin D (2015) Inhibition of MDR1 gene expression and enhancing cellular uptake for effective colon cancer treatment using dual-surface-functionalized nanoparticles. Biomaterials 48:147–160. doi:10.1016/j.biomaterials.2015.01.014
Dawidowicz AL, Wianowska D, Olszowy M (2012) On practical problems in estimation of antioxidant activity of compounds by DPPH method (problems in estimation of antioxidant activity). Food Chem 131:1037–1043. doi:10.1016/j.foodchem.2011.09.067
Bae SH, Jung EY, Kim SY, Shin KS, Suh HJ (2010) Antioxidant and immuno-modulating activities of Korean traditional rice wine, Takju. J Food Biochem 34:233–248. doi:10.1111/j.1745-4514.2009.00327.x
Baldwin C, Millette M, Oth D, Ruiz MT, Luquet FM, Lacroix M (2010) Probiotic Lactobacillus acidophilus and L. casei mix sensitize colorectal tumoral cells to 5-fluorouracil-induced apoptosis. Nutr Cancer 62:371–378. doi:10.1080/01635580903407197
Kim HJ, Lee JH, Kim SJ, Oh GS, Moon HD, Kwon KB, Park C, Park BH, Lee HK, Chung SY (2010) Roles of NADPH oxidases in cisplatin-induced reactive oxygen species generation and ototoxicity. J Neurosci 30:3933–3946
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, A., Arora, M., Goyal, A.K. et al. Spray Dried Formulation of 5-Fluorouracil Embedded with Probiotic Biomass: In Vitro and In Vivo Studies. Probiotics & Antimicro. Prot. 9, 310–322 (2017). https://doi.org/10.1007/s12602-017-9258-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-017-9258-x