Abstract
Nisin-, pediocin 34-, and enterocin FH99-resistant variants of Listeria monocytogenes ATCC 53135 were developed. In an attempt to clarify the possible mechanisms underlying bacteriocin resistance in L. monocytogenes ATCC 53135, sensitivity of the resistant strains of L. monocytogenes ATCC 53135 to nisin, pediocin 34, and enterocin FH99 in the absence and presence of different divalent cations was assessed, and the results showed that the addition of divalent cations significantly reduced the inhibitory activity of nisin, pediocin 34, and enterocin FH99 against resistant variants of L. monocytogenes ATCC 53135. The addition of EDTA, however, restored this activity suggesting that the divalent cations seem to affect the initial electrostatic interaction between the positively charged bacteriocin and the negatively charged phospholipids of the membrane. Nisin-, pediocin 34-, and enterocin-resistant variants of L. monocytogenes ATCC 53135 were more resistant to lysozyme as compared to the wild-type strain both in the presence as well as absence of nisin, pediocin 34, and enterocin FH99. Ultra structural profiles of bacteriocin-sensitive L. monocytogenes and its bacteriocin-resistant counterparts revealed that the cells of wild-type strain of L. monocytogenes were maximally in pairs or short chains, whereas, its nisin-, pediocin 34-, and enterocin FH99-resistant variants tend to form aggregates. Results indicated that without a cell wall, the acquired nisin, pediocin 34, and enterocin FH99 resistance of the variants was lost. Although the bacteriocin-resistant variants appeared to lose their acquired resistance toward nisin, pediocin 34, and enterocin FH99, the protoplasts of the resistant variants appeared to be more resistant to bacteriocins than the protoplasts of their wild-type counterparts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For the past few decades, food safety has been an important issue globally due to increasing food-borne diseases and changes in food habits. Illness caused due to the consumption of contaminated foods has a wide economic and public health impact worldwide. L. monocytogenes can be found in a wide variety of raw and processed foods. Milk and dairy products and various meats and meat products have been associated with Listeria contamination [26]. Foods such as soft cheeses, hot dogs, and seafood have been implicated in several outbreaks of human listeriosis.
Among the wide spectrum of antibacterial products released by microorganisms, the bacteriocins, especially those produced by lactic acid bacteria (LAB), have attracted the greatest attention as tools for food biopreservation. Bacteriocins are ribosomally synthesized peptides or proteins with antimicrobial activity. Several LAB bacteriocins with inhibitory activity offer potential applications in food biopreservation [10]. Food application of pediocins and enterocins can also provide a good alternative means in protecting food against food-borne pathogens. As products of lactic acid bacteria, they provide natural means of preservation and can be accepted by the consumers in the way nisin became accepted. As the trend of consumption of minimally processed and preserved foods is increasing, use of pediocins by the food industry could offer solutions and provide alternatives to conventional preservation means. Importantly, enterocins also show a strong activity against Listeria, which can be of practical use in the food industry [11, 13, 22]. Application of enterococcal bacteriocins on dairy foods has been the focus of many investigations [8, 13]. Enterococcus strains displaying a limited inhibitory spectrum due to the production of enterocins targeted toward Listeria and/or Clostridium [9, 13, 29] would be interesting as protective cultures for cheese manufacturers, given their very limited antagonistic activity toward dairy starter cultures such as Lactococcus and Streptococcus [8, 27].
However, similar to the use of antibiotics, the concern with the use of bacteriocins is the development of resistance in food-borne pathogens. The resistance mechanism is complex and may be due to three major factors: (1) variation of peptidoglycan composition [17], which should make it possible to increase the binding of divalent cations that should interact with the cationic peptide; (2) modification of the electric charge of the membrane by changes in the phospholipid content, thereby preventing pore formation [4, 20, 25]; and (3) increase in membrane rigidity, preventing peptide insertion and association [20]. However, resistance to bacteriocins has also been correlated with an altered fatty acid composition [18, 20] and an altered phospholipid composition [21]. Studies aimed at characterizing the resistance mechanisms of bacterial targets have revealed the stability of this phenomenon [7, 24], which occurs at either a low or a high level. In L. monocytogenes and E. faecalis, low-level resistance has been attributed to alterations in membrane lipid composition [31, 32] and high-level resistance has been attributed to the inactivation of the mptACD operon, which encodes the EII tMan mannose permease of the phosphotransferase system (PTS) [5, 15].
In order to assess the utility of pediocin 34 and enterocin FH99 as a food preservative against Listeria, it was important to determine their cellular target(s) and potential mechanisms of resistance to these bacteriocins. In this communication, we report on the isolation and characterization of nisin-, pediocin 34-, and enterocin FH99-resistant variants of L. monocytogenes. We propose that nisin, pediocin 34, and enterocin FH99 resistance in these variants is mediated by changes in their cell wall architecture limiting the access of these bacteriocins to a potential target in the cytoplasmic membrane and/or in the cytoplasm of the bacterium.
Materials and Methods
Bacterial Strains and Culture Conditions
Enterococcus faecium FH99 bacteriocinogenic strain was an isolate from human feces [14]. Pediococcus pentosaceous 34, a bacteriocinogenic strain isolated from cheddar cheese and Pediococcus acidilactici LB 42 (a sensitive strain used for detection of bacteriocin producers), was obtained from Prof Bibek Ray, Department of Animal Science, University of Wyoming, Laramie, Wyoming, USA. E. faecium FH 99, P. pentosaceus 34, and P. acidilactici LB 42 were maintained in MRS broth at 37 °C for 24 h. Listeria monocytogenes ATCC 53135 were procured from American Type Culture Collection (ATCC) and cultivated in Brain Heart Infusion (BHI) broth at 37 °C for 24 h.
Bacteriocins
One liter aliquots of MRS broth (pH 6.5) (HiMedia, Mumbai) were inoculated with active cultures of E. faecium FH 99 [14] and P. pentosaceus 34 (1% v/v) and incubated at 37 °C for 24 h. Cell-free culture supernatants (CFCS) were prepared by centrifugation of the cultures in refrigerated centrifuge (HANIL, Supra-30 K) at 7,000g for 10 min, filter sterilized by passing through a 0.2 μm, 45 mm diameter membrane filter and used for partial purification after neutralization. To 1,000 mL of cell-free culture supernatant, solid ammonium sulfate was added slowly with constant stirring to achieve 60% saturation and stirring was continued for another 1 h in a cold room at 5–7 °C. The mixture was then kept overnight in the cold room. It was then centrifuged at 7,000g for 20 min, and the precipitates were recovered. The supernatant was subsequently adjusted to 80% saturation levels by further addition of solid ammonium sulfate. The pellet in each case was dissolved in sterile Milli Q water. Nisin A (Nisaplin®) was procured from Danisco (Gurgaon, India). Nisin stock solutions were prepared from Nisaplin in 0.02 N HCl and autoclaved.
Antimicrobial Activity Assays
Measurement of Activity Units (AU)
The inhibitory spectrum of activity was obtained using the spot-on-lawn assay as described by Ulhman et al. [30], against P. acidilactici LB 42. Five microliters of the partially purified bacteriocin of E. faecium FH99 and P. pentosaceus 34 grown in MRS broth [6] was spotted on the tryptone glucose yeast extract (TGE) agar plates [2] (1.5% agar). Before spotting, TGE agar plates were overlaid with TGE soft agar (0.75%) seeded with actively growing cells of the test organism, P. acidilactici LB 42 (1% v/v). Plates were kept undisturbed for 3–4 h for diffusion of bacteriocin through agar and then incubated. The sensitivity of the strain in question was evaluated by checking for clear zones around the spots. Three independent replicates of experiment were done, and samples were tested in triplicate in each assay. The activity units of the partially purified bacteriocins were calculated using the following formula and expressed as activity units/mL: activity units/mL (AU/mL) = 200 × reciprocal of highest dilution that gave a clear zone.
The inhibitory spectrum of activity was obtained using the spot-on-lawn assay as described by Ulhman et al. [30] against L. monocytogenes ATCC 53135. Five microliters of nisin and the partially purified bacteriocin from E. faecium FH 99 and P. pentosaceus 34 grown in MRS broth [6] were spotted on TGE agar plates (1.5% agar) overlaid with TGE soft agar (0.75%) seeded with actively growing cells of the test organism [2]. Plates were kept undisturbed for 3–4 h for diffusion of bacteriocin through agar and then incubated. The sensitivity of the strain in question was evaluated by checking for clear zones around the spots. For MIC determinations, 5 μL of a 1:2 dilution series of a bacteriocin solution was spotted. The MIC was defined as the lowest concentration of bacteriocin that induced an inhibition zone. Three independent replicates of experiment were done.
Isolation of Spontaneous Bacteriocin-Resistant Variants
To obtain resistant strains, the wild-type strain of L. monocytogenes ATCC 53135 was cultured in BHI broth with increasing concentrations of bacteriocin corresponding to one-, three-, six- and then to 10- and 100-fold the MIC. The stability of these resistances in cultures without nisin, pediocin 34, and enterocin FH99 was checked and determined by MICs.
Bacteriocin-Induced Changes in Bacterial Cell Morphology by Scanning Electron Microscopy
To visualize differences occurring in the morphology between wild-type L. monocytogenes ATCC 53135 and its nisin-, pediocin 34-, and enterocin FH99-resistant variants were grown overnight in BHI broth and incubated at 37 °C for 24 h. Bacterial cells were washed in 0.1 M phosphate-buffered saline (pH 7.0) and fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.0). After 2 h on a rotator (2 rpm) at room temperature, the samples were washed three times with the same buffer and postfixed with 2% osmium tetroxide in 0.1 M sodium cacodylate buffer (pH 7.0). After 1 h at room temperature, the samples were washed three times in the same buffer and for dehydration of cells, graded series of ethanol water mixtures of 30, 50, 70% and absolute alcohol were used. After dehydration in absolute alcohol, these were subsequently dehydrated in propylene oxide for 15 min and allowed to dry. Dried samples were mounted on aluminum stubs with silver paint and sputter coated with gold at approximately 200 Ǻ thickness. The samples were visualized with a Scanning Electron Microscope (SEM), Model 501, Philips-Holland with EDAX and EBIC attachments.
Divalent-Cation Requirement of Nisin-Resistant, Pediocin 34-Resistant, and Enterocin FH99-Resistant Variants
Divalent-cation requirement of nisin-resistant, pediocin 34-resistant, and enterocin FH99-resistant variants were determined by the method described by Crandall and Montville [4]. Overnight cultures of wild-type or nisin-, pediocin 34-, and enterocin FH99-resistant variants were harvested by centrifugation (7,000g; HANLIN, Supra-30 K), washed once with phosphate-buffered saline, and resuspended in 50 mM MES (morpholineethanesulfonic acid) buffer (pH 6.5) containing either divalent cations alone, divalent cations plus EDTA, EDTA alone, or none of these. The metal ions tested (final concentration of 10 mM) were MgSO4, MgCl2, CaCl2, and MnSO4 (Hi Media, Mumbai). The final concentration of EDTA was 20 mM. The cells were then treated with a final concentration of 50 IU of nisin, 500AU/mL of pediocin 34, and 120 AU/mL of enterocin FH99 for 20 min. Control samples were not treated with nisin, pediocin 34, or enterocin FH99. The cells were then diluted in saline and plated on BHI agar plates. The plates were incubated at 37 °C, and colonies were counted after 48 h. The results are reported as log reductions in cell viability relative to an untreated control. Three independent replicates of the experiment were done with each individual experiment consisting of triplicate reactions.
Lysozyme Sensitivity of Nisin-Resistant, Pediocin 34-Resistant, and Enterocin FH99-Resistant Variants
Overnight cultures of wild-type cells and nisin-, pediocin 34-, and enterocin FH99-resistant variants of L. monocytogenes ATCC 53135 were inoculated at 1% (v/v) into fresh BHI containing 2 mg of lysozyme (Sigma)/mL. The cultures were incubated at 37 °C, and samples were drawn at different time intervals (1, 2, 4, 6, and 24 h). The survivors at each time interval were enumerated on BHI agar medium after appropriate dilutions in saline, and colonies were counted after 24–48 h of incubation at 37 °C. Three independent replicates of experiment were done.
Role of the Cell Envelope of L. monocytogenes ATCC 53135 in the Acquisition of Bacteriocin Resistance
Protoplast Formation
Protoplasts of wild-type L. monocytogenes ATCC 53135 and its respective bacteriocin-resistant variants were prepared using the method of Ghosh and Murray [12]. L. monocytogenes ATCC 53135 and its nisin-, pediocin 34-, and enterocin FH99-resistant variants were grown in 100 mL BHI broth (pH 7.3) at 37 °C for 20 h to get 109 CFU/mL. Cell concentrations were determined using spread plates. Cells were harvested by centrifugation at 7,000g for 20 min (at 4 °C), washed with water, and weighed. The cell pellets were then resuspended in lysozyme incubation buffer (0.015 mol/L NaCl; 0.03 mol−1 Tris–HCl (pH 6.7) (Sigma) and 0.4 mol−1 sucrose at 20 mg/mL wet weight before addition of lysozyme (Sigma) solution (6 mg/mL) to give a final concentration of 600 μg/mL lysozyme. The samples were shaken for 15 min at 37 °C, and 1 mol/L MgCl2 was added to give a final concentration of 0.02 mol/L MgCl2. After a 45-min incubation at 37 °C without shaking, the samples were centrifuged at 1,500g for 15 min and the pellets washed in protoplast buffer (0.03 mol/L Tris–HCl (pH 6.7); 0.01 mol/L MgCl2 and 0.5 mol/L sucrose). The pellets were then resuspended to their original volume (100 mL) with protoplast buffer and stored briefly on ice before use.
Determination of the Efficiency of Protoplast Formation
The total number of CFU/mL present in each protoplast preparation was determined using spread plates. All enumerations were conducted using protoplast buffer as a diluent and BHI that had been hydrated with protoplast buffer. To determine the percentage of protoplasts present, a small volume of each protoplast suspension was centrifuged (1,500g for 15 min) and resuspended in an equal volume of water. The cells were incubated at 30 °C for 30 min with constant shaking to ensure lysis. Control whole cells were treated in the same manner.
Inactivation of Whole Cells and Protoplasts by Nisin, Pediocin 34, and Enterocin FH99
Overnight cultures of L. monocytogenes ATCC 53135 and its nisin-resistant, pediocin 34-resistant, and enterocin FH99-resistant variants were grown to 109 CFU/mL in BHI broth (10 mL) at 30 °C. After centrifugation (1,500g for 15 min), they were washed and resuspended to their original volume in protoplast buffer (0.03 mol/L Tris–HCl (pH 6.7); 0.01 mol/L MgCl2 and 0.5 mol/L sucrose). After 15 min equilibration at 30 °C, samples were removed and diluted in protoplast buffer, and cells were enumerated on BHI. Bacteriocin was added to the remaining cultures (MICs as shown in Table 1 were used) and samples taken for cell enumeration periodically over 3 h. Protoplasts were treated in exactly the same way as whole cells, except for the use of buffered diluents and agar. Three independent replicates were done.
Statistical Analysis
The data were subjected to various statistical analyses as and when needed, using SYSTAT 6.0.1 (Statistical Software Package, 1996, SPSS, Inc., USA), Microsoft R excel 2000 Software Package (Microsoft Corporation, USA) and GraphPad 3.02, 1999 (GraphPad Software Inc., San Diego CA).
Determination of Mean and Standard Error of the Mean (SEM)
The experimental data, as and when necessary, are presented as the mean and standard error of the mean (SEM) of different parameters studied in the present investigation. The mean and SEM were determined running Microsoft Excel 2000 Software Package, Microsoft Corporation, USA.
Graphical Presentation
The mean ± SEM of different parameters studied were graphically presented using Graph Pad 3.02, 1999, GraphPad Software Inc., San Diego CA.
Results
Table 1 shows the MICs of the wild-type strains L. monocytogenes ATCC 53135 as determined by the spot-on-lawn assay. The nisin, pediocin 34, and enterocin FH99 resistance phenotype in L. monocytogenes ATCC 53135 was stable during 60, 40, and 10 successive cultures without nisin, pediocin 34, and enterocin FH99, respectively. MICs of the wild-type and the nisin-, pediocin 34-, and enterocin FH99-resistant variants of Listeria monocytogenes ATCC 53135 were determined by the spot-on-lawn assay (data not shown). The MIC was determined as the minimal concentration giving a visible zone of inhibition after 24 h at 37 °C. Nisin-, pediocin 34-, and enterocin FH99-resistant variant of L. monocytogenes ATCC 53135 were 92, 64, and 1,250 fold more resistant to nisin, pediocin 34, and enterocin FH99, respectively, than its corresponding wild-type strain.
Bacteriocin-Induced Changes in Bacterial Cell Morphology by Electron Microscopy
In the present investigation, attempts were made to study ultra structural profiles of bacteriocin-sensitive L. monocytogenes ATCC 53135 and its bacteriocin-resistant counterparts. The cells of wild-type (bacteriocin sensitive) L. monocytogenes were maximally in pairs or short chains. On the other hand, nisin-, pediocin 34-, and enterocin FH99-resistant variants of L. monocytogenes ATCC 53135 tend to form aggregates (Fig. 1).
Divalent-Cation Requirement of Nisin-, Pediocin 34-, and Enterocin FH99-Resistant Variants
A major component of growth media, which could be required by the bacteriocin-resistant strain to resist bacteriocins, is divalent cations. Therefore, the nisin, pediocin 34, and enterocin FH99 sensitivity of the resistant strains of L. monocytogenes ATCC 53135 in the absence and presence of different divalent cations was assessed.
Figure 2 shows the effect of MgSO4, MgCl2, CaCl2, and MnSO4 on the sensitivity of nisin-, pediocin 34-, and enterocin FH99-resistant L. monocytogenes ATCC 53135 variants to nisin, pediocin 34, and enterocin FH99 in the presence or absence of EDTA. In case of L. monocytogenes ATCC 53135, the viability of the nisin-resistant cells suspended in MES buffer supplemented with 10 mM MgSO4 and treated with 50 IU of nisin/mL was reduced by 0.50 log cycles only. The effect was confirmed to be due to the divalent cation by involving EDTA, a chelator of divalent cations. A significant difference (P < 0.05) in viable count of nisin-, pediocin 34-, and enterocin FH99-resistant variants of L. monocytogenes ATCC 53135 was observed when treated with nisin, pediocin 34, and enterocin FH99 alone or in combination with EDTA. Inclusion of 20 mM EDTA in the system containing MgSO4 increased the lethality caused by nisin to a 2.51 log reduction. The inhibition of pediocin 34- and enterocin FH99-resistant variants was also affected by the addition of Mg2+ ions. In the presence of 10 mM MgSO4, a reduction of 0.35 and 0.08 log cycles was caused by pediocin 34 and enterocin FH99, but the inclusion of 20 mM EDTA increased the lethality caused by pediocin 34 by 2.52 log cycles and enterocin FH99 by 3.87 log cycles (Fig. 2a). For the nisin- and pediocin-resistant variants, in the presence of MgCl2, inclusion of EDTA resulted in the increase of lethality caused by nisin and pediocin 34 to about 1.40 and 1.63 log cycles, respectively. However, for the enterocin-resistant variant, a reduction of about 1.94 log cycles was observed after inclusion of EDTA in the system (Fig. 2b).
For the nisin- and pediocin-resistant variants, in the presence of CaCl2, inclusion of EDTA resulted in the increase of lethality caused by nisin and pediocin 34 to about 3.17 and 3.50 log cycles, respectively. However, for the enterocin-resistant variant, reduction of about 2.21 log cycles was observed after inclusion of EDTA in the system (Fig. 2c). In the presence of MnSO4, inclusion of EDTA resulted in the increase of lethality by nisin and pediocin 34 to about 3.65 and 3.02 log cycles, respectively, for the nisin- and pediocin-resistant variants. However, for the enterocin-resistant variant, reduction of about 3.19 log cycles was observed after inclusion of EDTA in the experimental system (Fig. 2d).
Lysozyme Sensitivity of Nisin-, Pediocin 34-, and Enterocin FH99-Resistant Variants
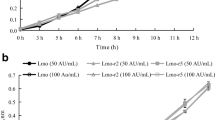
In the presence of lysozyme, the nisin-resistant variant showed an increase of 0.62 log cycles after 4 h, whereas the wild-type strain showed a decrease of about 2.5, 2.9, and 2.08 log cycles after 4, 6, and 8 h, respectively. Pediocin 34-resistant variant of L. monocytogenes ATCC 53135 depicted a log reduction of 0.58, 2.88, and 2.04 log cycles after 4, 6, and 8 h, respectively. Enterocin FH99-resistant variant showed an increase of 0.44 log cycles after 4 h. However, after 6 and 8 h, it showed a decrease of approximately 0.57 and 0.82 log cycles, respectively (Fig. 3a).
Growth of wild-type (WT), nisin-resistant (Nr), pediocin 34-resistant (Pr), and enterocin FH99-resistant (Er) variants of L. monocytogenes ATCC 53135 in presence of (a) lysozyme, (b) growth of WT and Nr variant in presence of both lysozyme and nisin, (c) Growth of WT and Pr variant in presence of both lysozyme and pediocin 34 and (d) Growth of WT and Er variant in presence of both lysozyme and enterocin FH99
The effect of lysozyme on the wild-type and nisin-, pediocin 34-, and enterocin FH99-resistant variants of L. monocytogenes ATCC 53135 in the presence as well as in the absence of nisin, pediocin 34, and enterocin FH99 was determined. A significant differences (P < 0.05) in viable count of wild-type L. monocytogenes and its nisin-, pediocin 34-, and enterocin FH99-resistant variants were observed when treated with nisin, pediocin 34, and enterocin FH99 alone and in combination with lysozyme. Wild-type L. monocytogenes ATCC 53135 showed a log reduction of 3.07 and 3.86 log cycles after 1- and 2-h and no growth after 2-h incubation with lysozyme and nisin. On the other hand, the nisin-resistant variant showed a log reduction of about 0.57, 1.09, and 1.64 log cycles after 2, 6, and 24 h, respectively, in the presence of lysozyme and nisin (Fig. 3b). In the presence of pediocin 34 and lysozyme, wild-type L. monocytogenes ATCC 53135 showed a log reduction of 1.9 and 3.6 log cycles after 4 and 8 h, respectively, and no growth was observed after 24 h, whereas, pediocin 34-resistant variant showed a log reduction of 3.11 after 6 h and no growth after 8 and 24 h (Fig. 3c). Wild-type L. mononocytogenes ATCC 53135 in the presence of both lysozyme and enterocin FH99 showed a log reduction of 0.35 and 4.0 log cycles after 4 and 8 h, respectively, and no growth after 24-h incubation. Enterocin-resistant variant showed an increase of approximately 0.89 log cycles after 6 h whereas a log reduction of 1.27, 1.23, and 0.09 log cycles was observed after 6-, 8-, and 24-h incubation (Fig. 3d). In conclusion, it was observed that L. monocytogenes ATCC 53135 nisin-, pediocin 34-, and enterocin-resistant variants were more resistant to lysozyme when compared with the wild-type strain both in the presence as well as absence of either nisin, pediocin 34, and enterocin FH99.
Role of the Cell Envelope in the Acquisition of Bacteriocin Resistance
The role of the cell wall in the acquisition of bacteriocin resistance in strains of wild-type and resistant variants was investigated. For this, protoplasts were prepared using the method of Ghosh and Murray [12]. Efficiency of protoplast formation and inactivation of whole cells and protoplasts by bacteriocins was determined (Fig. 4). The efficiency of protoplast formation in those cells surviving lysozyme treatment as determined by lysis in water was high (>90%). Control cells were unaffected by suspension in water.
The effect of (a) nisin (N) on whole cells and protoplast cells of WT and nisin-resistant variant cells (Nr), (b) pediocin 34 (P) on WT protoplast cells and protoplast of pediocin 34-resistant variant, (c) enterocin FH99 (FH99) on whole cells and protoplast cells of WT and enterocin FH99-resistant variant cells(Er). Mean and range of triplicate experiments indicated
The inactivation of whole cells and protoplasts of L. monocytogenes ATCC 53135 and its nisin-, pediocin 34-, and enterocin FH99-resistant variants was studied using nisin, pediocin 34, and enterocin FH99 (MICs of each bacteriocin as given in Table 1 were used).
Significant differences (P < 0.05) in viable count of whole cells of wild-type L. monocytogenes and whole cells of its nisin-, pediocin 34-, and enterocin FH99-resistant variants were observed when treated with nisin, pediocin 34, and enterocin FH99, respectively. Also, significant differences (P < 0.05) in viable count of protoplasts of wild-type L. monocytogenes and protoplasts of its nisin-, pediocin 34-, and enterocin FH99-resistant variants were observed when treated with nisin, pediocin 34, and enterocin FH99, respectively. In the presence of nisin, the number of whole cells of wild-type L. monocytogenes ATCC 53135, decreased by 1.6 log cycles over 3 h compared with a decrease of 0.5 log in the nisin-resistant variant whole cell population. After a 3-h incubation with nisin, the nisin-resistant L. monocytogenes ATCC 53135 protoplasts had decreased in concentration by 3.09 log cycles compared with a 0.50 log cycle reduction of the nisin-resistant L. monocytogenes ATCC 53135 whole cells. In contrast, the reduction in concentration of the L. monocytogenes ATCC 53135 wild-type protoplasts was about a 5.23 log cycles after 3 h (Fig. 4a). After a 3-h incubation with pediocin 34-, the pediocin-resistant L. monocytogenes ATCC 53135 protoplasts had decreased in concentration by 2.00 log cycles compared with a 0.44 log cycle reduction of the pediocin 34-resistant L. monocytogenes ATCC 53135 whole cells. In contrast, the reduction in concentration of the L. monocytogenes ATCC 53135 wild-type protoplasts was about 4.55 log cycles compared to a reduction of 1.20 log cycles of wild-type whole cells after 3 h (Fig. 4b). After a 3-h incubation with enterocin FH99, the enterocin-resistant L. monocytogenes ATCC 53135 protoplasts decreased in concentration by 2.06 log cycles compared to only 0.24 log cycle reduction of the enterocin FH99-resistant L. monocytogenes ATCC 53135 whole cells. In contrast, the reduction in concentration of the L. monocytogenes ATCC 53135 wild-type protoplasts was about 4.85 log cycles compared to a reduction of 2.24 log cycles of wild-type whole cells after 3 h (Fig. 4c).
Discussion
Bacteriocin-Induced Changes in Bacterial Cell Morphology by Electron Microscopy
The formation of bacterial cell aggregates observed in resistant cells may be the prime mechanism of resistance because overall, a smaller cell surface in aggregated cells is exposed to bacteriocins. Similar results have been reported by Mehla and Sood [19].
Divalent-Cation Requirement of Nisin-, Pediocin 34-, and Enterocin FH99-Resistant Variants
Supplementation of 10 mM MgSO4, MgCl2, CaCl2, and MnSO4 reduced the lethality caused by nisin, pediocin 34, and enterocin FH99. In the absence of bacteriocins, the divalent cations had no effect on cell viability. The effect due to divalent cations was further confirmed by experiments involving EDTA, a chelator of divalent cations. Inclusion of 20 mM EDTA in any of the systems containing divalent cations increased the lethality caused by nisin, pediocin 34, and enterocin FH99. Divalent cations did not prevent nisin, pediocin 34, and enterocin FH99 from killing wild-type cells (data not shown). The EDTA, either alone or in combination with divalent cations, had no effect on the viability of nisin-, pediocin 34-, and enterocin FH99-resistant variants (data not shown).
The addition of divalent cations significantly reduced the inhibitory activity of nisin, pediocin 34, and enterocin FH99 against cells of resistant variants of test culture L. monocytogenes ATCC 53135. Similar results have earlier been reported by Abee et al. [1] who found that di- and trivalent cations (Mg2+, Ca2+, and Gd3+) decreased the nisin Z-induced rate of K1+ efflux from whole cells of L. monocytogenes Scott A. They suggested that di- and trivalent cations might inhibit the electrostatic interactions between the positive charges on the nisin molecule and negatively charged phospholipids head groups. Alternatively or additionally, the neutralization of the negative head group charges may induce a condensation of these phospholipids, resulting in a more rigid membrane [1]. In conclusion, the impact of divalent cations on bactericidal activity of nisin, pediocin 34, and enterocin FH99 revealed that Mg2+, Mn2+, and Ca3+ cations were able to reduce the binding of antimicrobial peptide to the cell membrane. Divalent and trivalent cations seem to affect the initial electrostatic interaction between the positively charged bacteriocin and the negatively charged phospholipids of the membrane [23].
Lysozyme Sensitivity of Nisin-, Pediocin 34-, and Enterocin FH99-Resistant Variants
Lysozyme had two modes of action: (1) enzymatic lysis of the bacterial cell wall and (2) membrane perturbation inducing cell death [16]. The efficacy of lytic activity of lysozyme is preferentially directed to Gram-positive bacteria because of the cell wall composition (peptidoglycan, the target of lysozyme). Nisin-, pediocin 34-, and enterocin-resistant variants of L. monocytogenes ATCC 53135 were more resistant to lysozyme as compared to the wild-type strain both in the presence as well as absence of nisin, pediocin 34, and enterocin FH99. These results suggest that certain cell wall-associated modifications might have occurred in these resistant variants that made them resistant toward the lytic effect of lysozyme and bacteriocins. The results of our study are in contrast (no additive effect between lysozyme and bacteriocins was observed) to the studies conducted by Calvez et al. [3] where they showed that the resistant variant to divercin RV41 did not confer any cross-resistance but exhibited an additive effect ascribed to the combined action of lysozyme and (P)-DvnRV41. This phenomenon could be due to the mode of action of each substance. The lysozyme, which causes cell wall disruption and stress in the cell, was unable to do so due to some modification in the cell wall architecture. Therefore, there might be a possibility that the access to IIC subunit encoded by the mptC gene of the mannose transport system might be hindered, which thereby might constitute a putative target of pediocin 34, and enterocin FH9 [3]. With wild-type cells, nisin passes through the cell wall, binds to the cytoplasmic membrane, probably via electrostatic interactions with the anionic phospholipids (phosphatidylglycerol and cardiolipin), and disrupts the membrane through the formation of pores. In the nisin-resistant variant, cell wall alterations may prevent nisin from interacting with the cytoplasmic membrane. Nisin, which does reach the membrane, interacts with it to induce the changes in fatty acid and phospholipid composition. Nisin’s ability to bind to the cytoplasmic membrane of the nisin-resistant variant may be hindered by the decrease in the net negative charge of the membrane surface, and its ability to insert may be hampered by a decrease in membrane fluidity [4].
Role of the Cell Envelope in the Acquisition of Bacteriocin Resistance
Results indicated that without a cell wall, the acquired nisin, pediocin 34, and enterocin FH99 resistance of the variants were lost. When the cell wall was removed from the wild-type strain, the nisin-, pediocin 34-, and enterocin FH99-resistant variants of L. monocytogenes ATCC 53135 showed sensitivity to the three bacteriocins. Although the bacteriocin-resistant variants appeared to lose their acquired resistance toward nisin, pediocin 34, and enterocin FH99, the protoplasts of the resistant variants appeared to be more resistant to bacteriocins than the protoplasts of their wild-type counterparts. This may be due to non-specific adsorption of the bacteriocins on to freshly exposed hydrophobic sites on the protoplast. These results concur with Schved et al. [28] who attributed the resistance of Lactobacillus plantarum strains to pediocin SJ-1, to the barrier properties of the cell wall. However, our results are in contrast to the observation of Zajdel et al. [33] who in their study stated that the bacteriocin lactostrepsin (Las) 5 did not kill protoplasts prepared from either sensitive or resistant bacterial cells. The authors suggested that interaction with the cell wall is a condition necessary for Las 5 activity. The observations with protoplasts, however, indicate that the cell wall architecture plays a major role in the development of bacteriocin resistance.
Conclusions
Since bacteriocins are considered as potential tools for biopreservation, more study is needed to determine the distribution of bacteriocin-resistance phenomena among microorganisms that cause food spoilage. Among the food-borne pathogens, knowledge of the characteristics of bacteriocin-resistant variants and the conditions that prevent their emergence will help in determining the optimal conditions for application of bacteriocins in foods and minimize the incidence of resistance. Insight to the mechanism of action of nisin, pediocin 34, and enterocin FH99 suggests that nisin, pediocin 34, and enterocin FH99 resistance in L. monocytogenes is linked to a modification in the cell wall that might limit the diffusion of these bacteriocins into the cell. This, in turn, suggests that nisin, pediocin 34, and enterocin FH99 require access to the cytoplasmic membrane and/or the cytoplasm to exert their antimicrobial activity.
References
Abee T, Rombouts FM, Hugenholtz J, Guihard G, Letellier L (1994) Mode of action of nisin Z against Listeria monocytogenes Scott A grown at high and low temperatures. Appl Environ Microbiol 60:1962–1968
Biswas SR, Ray P, Johnson MC, Ray B (1991) Influence of growth conditions on the production of a bacteriocin AcH by Pediococcus acidilactici H. Appl Environ Microbiol 5:1265–1267
Calvez S, Kohler A, Prévost H, Møretrø T, Drider D (2010) Physiological and structural differences between enterococcus faecalis JH2–2 and mutant strains resistant to (P)-divercin RV41. Probiotics Antimicrob Prot 2:226–232
Crandall AD, Montville TJ (1998) Nisin resistance in Listeria monocytogenes ATCC 700302 is a complex phenotype. Appl Environ Microbiol 64:231–237
Dalet K, Cenatiempo Y, Cossart P, Héchard Y (2001) A σ54-dependent PTS permease of the mannose family is responsible for sensitivity of Listeria monocytogenes to mesentericin Y105. Microbiolgy 147:3263–3269
De Man JC, Rogosa M, Sharpe ME (1960) A medium for the cultivation of lactobacilli. J Appl Bacteriol 2:130–135
Dykes GA, Hastings JW (1998) Fitness costs associated with class IIa bacteriocin resistance in Listeria monocytogenes B73. Lett Appl Microbiol 26:5–8
Foulquie Moreno MR, Sarantinopoulos P, Tsakalidou E, De Vuyst L (2006) The role and application of enterococci in food and health. Int J Food Microbiol 106:1–24
Franz CMAP, Schillinger U, Holzapfel WH (1996) Production and characterization of enterocin 900, a bacteriocin produced by Enterococcus faecium BFE 900 from black olives. Int J Food Microbiol 29:255–270
Galvez A, López RL, Abriouel H (2008) Application of bacteriocins in the control of food borne pathogenic and spoilage bacteria. Crit Rev Biotech 28:125–152
Galvez A, Valdivia E, Abriouel H, Camafeita E, Mendez E, Martinez-Bueno M, Maqueda M (1998) Isolation and characterization of enterocin EJ97, a bacteriocin produced by Enterococcus faecalis EJ97. Arch Microbiol 171:59–65
Ghosh HK, Murray RGE (1967) Fine structure of Listeria monocytogenes in relation to protoplast formation. J Bactreriol 93:411–426
Giraffa G (1995) Enterococcal bacteriocins: their potential as anti-Listeria factors in dairy technology. Food Microbiol 12:291–299
Gupta H, Malik RK, De S, Kaushik JK (2010) Purification and characterization of enterocin FH 99 produced by a faecal isolate Enterococcus faecium FH 99. Indian J Microbiol 50:145–155
Héchard Y, Pelletier C, Cenatiempo Y, Frére J (2001) Analysis of σ54 dependent genes in Enterococcus faecalis: a mannose PTS permease (EIIMan) is involved in sensitivity to a bacteriocin, mesentericin Y105. Microbiology 147:1575–1580
Ibrahim HR, Aoki T, Pellegrini A (2002) Strategies for new antimicrobial proteins and peptides: lysozyme and aprotinin as model molecules. Curr Pharm Des 8:671–693
Maisnier-Patin S, Richard J (1996) Cell wall changes in nisin-resistant variants of Listeria innocua grown in the presence of high nisin concentration. FEMS Microbiol Lett 140:29–35
Mazzotta AS, Montville TJ (1997) Nisin induces changes in membrane fatty acid composition of Listeria monocytogenes nisin-resistant strains at 10 °C and 30 °C. J Appl Microbiol 82:32–38
Mehla J, Sood SK (2011) Substantiation in Enterococcus faecalis of dose-dependent resistance and cross-resistance to pore-forming antimicrobial peptides by use of a polydiacetylene-based colorimetric assay. Appl Environ Microbiol 77(3):786–793
Ming X, Daeschel MA (1993) Nisin resistance of food bore bacteria and the specific responses of Listeria monocytogenes Scott A. J Food Prot 56:944–948
Ming X, Daeschel MA (1995) Correlation of cellular phospholipids content with nisin resistance of Listeria monocytoges Scott A. J Food Prot 58:416–420
Núñez M, Rodríguez JL, García E, Gaya P, Medina M (1997) Inhibition of Listeria monocytogenes by enterocin 4 during the manufacture and ripening of Manchego cheese. J Appl Microbiol 83:671–677
Naghmouchi K, Drider D, Kheadr E, Lacroix C, Prévost H, Fliss I (2006) Multiple characterizations of Listeria monocytogenes sensitive and insensitive variants to divergicin M35, a new pediocin like bacteriocin. J Appl Microbiol 100:29–39
Rekhif N, Atrih A, Lefebvre G (1994) Selection and properties of spontaneous mutants of Listeria monocytogenes ATCC 15313 resistant to different bacteriocins produced by lactic acid bacteria strains. Curr Microbiol 28:237–241
Revol-Junelles AM, Mathis R, Krier F, Fleury Y, Delfour A, Lefebvre G (1996) Leuconostoc mesenteroides subsp. mesenteroides FR52 synthesizes two distinct bacteriocins. Lett Appl Microbiol 23:120–124
Rocourt J, Cossart P (1997) Listeria monocytogenes. In: Doyle MP, Buechat LR, Montville TJ (eds) Food microbiology: fundamentals and frontiers. American Society for Microbiology (ASM) press, Washington, DC, pp 337–352
Sarantinopoulos P, Leroy F, Leontopoulou E, Georgalaki MD, Kalantzopoulos G, Tsakalidou E, Vuyst LD (2002) Bacteriocin production by Enterococcus faecium FAIR-E 198 in view of its application as adjunct starter in Greek Feta cheese making. Int J Food Microbiol 72(1–2):125–136
Schved F, Lalazar A, Linder P, Juven BJ (1994) Interaction of the bacteriocin produced by Pediococcus acidilactici SJ-1 with the cell envelope of Lactobacillus spp. Lett Appl Microbiol 19:281–283
Torri Tarelli G, Carminati D, Giraffa G (1994) Production of bacteriocins active against Listeria monocytogenes and Listeria innocua from dairy enterococci. Food Microbiol 11:243–252
Ulhman U, Schillinger U, Rupnow JR, Holzapfel WH (1992) Identification and characterization of two bacteriocin-producing strains of Lactococcus lactis isolated from vegetables. Int J Food Microbiol 16:141–151
Vadyvaloo V, Hastings JW, van der Merwe MJ, Rautenbach M (2002) Membranes of class IIa bacteriocin-resistant Listeria monocytogenes cells contain increased levels of desaturated and short-acyl-chain phosphatidylglycerols. Appl Environ Microbiol 68(11):5223–5230
Vadyvaloo V, Snoep JL, Hastings JW, Rautenbach M (2004) Physiological implications of class IIa bacteriocins resistance in Listeria monocytogenes. Microbiology 150:335–340
Zajdel JA, Ceglowski P, Dobrzanski WI (1985) Mechanism of action of lactostrepsin 5, a bacteriocin produced by Streptococcus cremoris 202. Appl Errviron Microbiol 49:969–974
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaur, G., Singh, T.P., Malik, R.K. et al. Mechanism of Nisin, Pediocin 34, and Enterocin FH99 Resistance in Listeria monocytogenes . Probiotics & Antimicro. Prot. 4, 11–20 (2012). https://doi.org/10.1007/s12602-011-9085-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-011-9085-4