Abstract
Maxillofacial and craniofacial surgery is on the increase, which exposes more patients at risk of acquiring microbial infections. The use of antibiotic-loaded calcium phosphate bone cements has been shown to reduce the incidence of infection. A marked increase in antibiotic-resistant pathogens, including multidrug-resistant pathogens, has been reported. This has led to the investigation of various compounds as alternatives to conventional treatments. In this paper, we report on the incorporation and release of a broad-spectrum class II antimicrobial peptide, bacteriocin ST4SA produced by Enterococcus mundtii, into a calcium orthophosphate-based bone cement. Our results suggest class II bacteriocins may be incorporated into self-setting bone cements to produce implants with antimicrobial activity over extended periods of time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the increase in life expectancy, orthopaedic implant surgery is on the rise, which in turn puts more patients at risk of acquiring microbial infections [17, 26]. In maxillofacial and craniofacial surgery, antibiotics are usually administered prophylactically to prevent post-operative infections. However, the increase in the number of antibiotic-resistant bacteria, especially methicillin-resistant Staphylococcus aureus (MRSA), has narrowed the choice of antibiotics down to only a few [13, 14, 26, 34]. Furthermore, antibiotics administered intravenously or orally are not always effective [13, 14]. The main reason for this is the restriction in blood flow in areas surrounding the implant and hence less contact between the antibiotic and site of infection [14, 37]. In severe cases of infection, surgical intervention is often the only alternative to a cure [37, 38].
A number of papers have been published on the incorporation of antibiotics and therapeutic agents in bone cement [2, 6, 15, 18, 20, 32, 33, 36]. Thornes et al. [34] observed a decrease in Streptococcus epidermis infection with gentamicin incorporated into bone cement (41% infection rate compared with 73% in the absence of gentamicin). However, the number of S. epidermis cells that developed resistance to gentamicin increased from 19 to 78% [34]. In other studies, antibiotic-loaded bone cements used as a prophylactic significantly reduced the incidence of prosthetic joint–associated infection [13, 19].
Bacteriocins with a broad or narrow spectrum of activity, and mechanisms of action different from conventional antibiotics, may serve as an alternative in the treatment of antibiotic-resistant pathogens. Some of these peptides have shown activity against MRSA, vancomycin-resistant enterococci as well as other clinical isolates [7, 11, 22, 23, 28]. Most of the bacteriocins are small, ribosomally synthesized, cationic and hydrophobic/amphiphilic peptides with 20–60 amino acid residues. They are grouped into two main classes [9, 10, 21]. Class I bacteriocins are defined as lantibiotics and are divided into subgroups A and B based on structure and mode of action [10]. Class II bacteriocins are heat-stable, non-lanthionine-containing peptides and separated into four subgroups [4]. Pediocin-like bacteriocins [1, 35] are grouped into class IIa, bacteriocins that require two peptides for activity are grouped in class IIb, cyclic peptides in class IIc and single non-pediocin-like peptides in class IId [10]. Class III contains the bacteriolysins [10].
Bacteriocin ST4SA, produced by Enterococcus mundtii ST4SA, belongs to the class IIa bacteriocins and is active against various Gram-positive bacteria, including S. pneumonia and S. aureus, as well as the Gram-negative bacterium Pseudomonas aeruginosa [22]. This paper describes the incorporation of bacteriocin ST4SA in calcium orthophosphate-based cement (CPC) and reports on the release rate and antimicrobial activity of the peptide in vitro. We have chosen CPC due to its excellent osteoconductive properties and stability during in situ moulding [29]. Furthermore, CPCs are usually resorbed by osteoclasts and do not need to be removed, thus reducing the risk of infection caused by secondary operations [8, 27].
Materials and Methods
The β-tricalcium phosphate (βTCP, 95%) and monocalcium phosphate monohydrate (MCPM, 85%) were acquired from Sigma–Aldrich (Sigma–Aldrich, Germany). Tri-sodium citrate dihydrate was obtained from Saarchem (Saarchem, Gauteng, South Africa) and ammonium sulphate (99.5%) and Listeria Enrichment Broth (LEB) from Merck (Merck, Darmstadt, Germany). Dialysis membranes (1 kD, Spectra/Por® 6) were from Spectrumlabs (Spectrum Inc., CA, USA). The BCA protein assay kit was from Thermo Scientific (Pierce Biotechnology, Rockford, IL, USA). All other growth media used were from Biolab (Biolab Diagnostics, Midrand, South Africa).
Preparation of Bacteriocin ST4SA
Enterococcus mundtii ST4SA was cultured in 1 L MRS broth for 24 h at 30 °C. Cells were harvested at 8,000g for 10 min at 4 °C. The pH of the resulting supernatant was adjusted to 6.5–7.0 and then incubated at 80 °C for 10 min to inactivate proteolytic enzymes. Proteins were precipitated from the cell-free supernatant with 80% saturated ammonium sulphate [25]. The precipitate was collected by centrifugation (10,000g for 1 h at 4 °C), and the pellet was re-suspended in 10 mL sterile distilled water. The concentrated bacteriocin was dialysed against 4 L sterile distilled water using a 1-kDa cut-off dialysis membrane (Spectrumlabs). The dialysed product was concentrated by freeze-drying and stored at −20 °C. Antimicrobial activity was determined using the agar-spot method [22]. Activity was expressed in arbitrary units per mL (AU/mL). One AU is defined as the reciprocal of the highest serial twofold dilution showing a clear zone of growth inhibition. Listeria monocytogenes EGD-e, grown in LEB supplemented with 7.5 μg/mL chloramphenicol and incubated at 37 °C on a shaker, was used as sensitive strain model.
Preparation of Bone Cement
Cement samples were prepared according to the method described by Barralet et al. [3]. β-TCP and MCPM were ground to a fine powder with a pestle and mortar and mixed in equimolar amounts. The powder (250 mg) was mixed with 75.76 μL 500 mM tri-sodium citrate (P/L ratio of 3.3 g/mL), which served as retardant. Samples containing bacteriocin ST4SA were prepared by first adding the peptide to the dry cement powder (5%, w/w) before mixing with 500 mM tri-sodium citrate. Mixing was performed on a glass slab for 30 s to form a homogeneous paste. The paste was moulded into insulin syringes, from which the tips have been cut off, and dried at 37 °C for 2 h. The set cement cylinders were approx. 8 mm long and 4 mm in diameter.
Characterization of Bone Cement
The crystal structure of the bone cement was studied using a Zeiss EVO MA15VP scanning electron microscope (SEM). The cement cylinders were fractured, placed on an adhesive stub and coated with gold before SEM analysis. Powder X-ray diffraction (XRD) patterns of the set cements were recorded on a PANalytical X’Pert PRO MPD (Multi-Purpose Diffractomator). Data were collected from 2θ = 10°–60° with a step size of 0.02° and a normalized count time of 1 s/step using Cu Kaα radiation. Phase composition was checked by means of ICDD (international centre for diffraction data) reference patterns for β-TCP (00-009-0348), MCPM (01-070-0359), brushite (00-009-0077 & 01-072-0713) and hydroxyapatite (00-001-1008).
Release of Bacteriocin ST4SA from Bone Cement
Bone cement cylinders, made from 250 mg β-TCP/MCPM powder mixed with 12.5 mg bacteriocin ST4SA preparation, were incubated in 5 mL PBS buffer (pH 7.4) at 37 °C and slowly stirred. At selected time intervals, the entire volume of buffer was collected and centrifuged at 8,000g for 1 min to remove non-dissolved particles. Protein concentration (mg/mL) in the particle-free buffer was determined using the colorimetric BCA protein assay method at 562 nm to determine the release of bacteriocin ST4SA. Readings were recorded from standard curves prepared with bovine serum albumin. Buffer extracted was replaced with fresh buffer, and the bone cement cylinders were incubated until the next reading. Readings were recorded over 120 h. Release of protein from the bone cement was expressed as percentage of the original protein content present in the sample.
In a separate experiment, the bone cement cylinders were removed from the PBS buffer (pH 7.4), inserted into 10 mL soft agar (1.0%, w/v) and seeded with 1 mL L. monocytogenes EGD-e (1 × 107 cfu/mL). The plates were incubated at 37 °C for 24 h, and bacteriocin ST4SA activity was recorded by the formation of a clear zone of growth inhibition around the cement cylinders.
Antimicrobial Activity of Peptide ST4SA Released from Bone Cement
To determine the rate at which bacteriocin ST4SA is released from the bone cement, a delayed agar diffusion method was used [5]. Bone cement (250 mg) containing 12.5 mg peptide ST4SA, equivalent to 102,400 AU/mL, was incubated in 20 mL 1% LEB soft agar seeded with 1 mL L. monocytogenes EGD-e (1 × 107 cfu/mL). After 24 h of incubation at 37 °C, the diameter (cm) of growth inhibition zones was recorded. The cement cylinders were then aseptically removed and placed into fresh soft agar seeded with L. monocytogenes EGD-e. The experiment was repeated until no zones of growth inhibition zones could be detected.
Statistical Analysis
Data were collected in Microsoft Excel 2007, and data points, indicated with standard deviations, represent an average of three repeats.
Results
Cement Characteristics
Addition of bacteriocin ST4SA to the β-TCP/MCPM powder produced a slightly more viscous cement paste compared with samples without the peptide. SEM of cement cylinders with and without bacteriocin ST4SA revealed similar textures, i.e. a dense surface with characteristic plates and blocks (Fig. 1a, b). XRD patterns corresponded well with that recorded for ICDD patterns of brushite (00-009-0077 & 01-072-0713), although some peaks for β-TCP (00-009-0348) and MCPM (01-070-0359) could be seen in samples with and without bacteriocin ST4SA (Fig. 2). No peaks characteristic of hydroxyapatite were observed in any of the samples.
Release of Bacteriocin ST4SA from the Bone Cement
Most of bacteriocin ST4SA (approx. 80%) was released from the bone cement during the first 12 h (Fig. 3). This was followed by a 4% increase in release over the next 108 h. After 120 h of submersion in PBS buffer, bacteriocin ST4SA-loaded bone cement was still active, i.e. repressed the growth of L. monocytogenes (not shown).
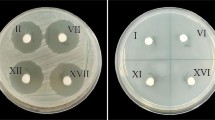
Bacteriocin ST4SA incorporated into the bone cement inhibited the growth of L. monocytogenes EGD-e for up to 13 consecutive days, as shown by clear zones of growth inhibition (Fig. 4a). Zone sizes ranged from 3.5 cm in diameter, reported at day 2, to approx. 1.7 cm in diameter on day 13 (Fig. 4b).
a Antimicrobial activity of bacteriocin ST4SA incorporated into brushite cement. L. monocytogenes EGD-e served as sensitive strain. Images of antimicrobial activity are as recorded on days 1, 8 and 13. b Release of peptide ST4SA (5%, w/w) from brushite cement over 13 days. L. monocytogenes EGD-e served as sensitive strain
Discussion
Incorporation of bacteriocin ST4SA into β-TCP/MCPM did not lead to drastic structural changes (Fig. 1), suggesting that crystal formation and setting of the cement were not altered. XRD results indicated that the main phase of the set cement is brushite, and addition of bacteriocin ST4SA did not cause any significant changes (Fig. 2). Peaks for β-TCP and MCPM could be seen in control samples and bacteriocin ST4SA-loaded samples, possibly due to the presence of non-reacting starting material [6]. Similar results have been reported with the incorporation of other therapeutic agents in bone cement [24, 33].
Release of the active agent from bone cement depends on several factors, such as cement–drug interaction, porosity of the cement and solubility of the drug [16, 20, 33]. Release of most therapeutic agents from bone cement is characterized by a sudden burst, followed by slow release over several hours [6, 15, 20, 33, 36]. Rapid release of bacteriocin ST4SA (Fig. 3) suggests a high initial diffusion from the bone cement, which is supported by the small size (3.5 kDa) of the peptide. Bacteriocin ST4SA, on the other hand, was not completely released from the bone cement, as evident by the prolonged antimicrobial activity recorded (Fig. 4b) as well as the release study (Fig. 3). The release profile of bacteriocin ST4SA out of the cement was slightly higher than that reported for other therapeutic agents in unmodified cement [6, 15, 20, 33, 36]. Solubility also plays a major role in the release rate. Hofmann et al. [20] reported a higher burst release rate for vancomycin (80% in 24 h) as opposed to the less soluble ciprofloxacin (60% in 24 h). Concluded from results obtained in our study, bacteriocin ST4SA was released from bone cement for at least 120 h, suggesting that a certain percentage of the peptide takes longer to dissolve. It may also be that the cationic peptide adheres to the bone cement by electrostatic forces. However, XRD and SEM results did not indicate chemical or structural changes (Figs. 2, 3). Another possibility may be that the peptide is only released upon cement dissolution/resorption [20, 33]. L. monocytogenes EGD-e was used as a model pathogen in this study. In future in vitro and in vivo experiments, Streptococcus epidermidis and S. aureus will be included.
Release of bacteriocin ST4SA from the bone cement may be enhanced by incorporating the peptide into micro- or nanoparticles. The concept has been shown in several other studies [12, 29–32]. The porosity of bone cement is easily changed by increasing the powder:liquid ratio, which in turn will enhance the release of a therapeutic agent [20].
Conclusions
Bacteriocin ST4SA proved stable and active for at least 13 days in β-TCP/MCPM cement. The incorporation of bacteriocins into bone cement and developing implants with inherent antimicrobial activity seems possible. The challenge would be to select antimicrobial peptides with broad-spectrum activity that would not elicit a host immune response and with a mode of action that would prevent the emergence of microbial resistance. The possibility of combining antibiotics with a suitable bacteriocin may also reduce the chances of microbial resistance. Encapsulation of antimicrobial peptides with micro- and nanoparticles in vitro as well as in vivo trials with mice is currently being investigated.
References
Albano H, Todorov SD, Van Reenen CA, Hogg T, Dicks LMT, Teixeira P (2007) Characterization of two bacteriocins produced by Pediococcus acidilactici isolated from “Alheira”, a fermented sausage traditionally produced in Portugal. Int J Food Microbiol 116:239–247
Alkhraisat MH, Rueda C, Marino FT, Torres J, Jerez LB, Gbureck U, Cabarcos EL (2009) The effect of hyaluronic acid on brushite cement cohesion. Acta Biomater 5:3150–3156
Barralet J, Grover L, Gbureck U (2004) Ionic modification of calcium phosphate cement viscosity. Part II: hypodermic injection and strength improvement of brushite cement. Biomaterials 25:2197–2203
Bauer R, Dicks LMT (2005) Mode of action of lipid II-targeting lantibiotics. Int J Food Microbiol 101:201–216
Bayston R, Milner R (1982) The sustained release of antimicrobial drugs from bone cement. An appraisal of laboratory investigations and their significance. J Bone Joint Surg (Br) 64:460–465
Bohner M, Lemaître J, Van Landuyt P, Zambelli PY, Merkle HP, Gander B (1997) Gentamicin-loaded hydraulic calcium phosphate bone cement as antibiotic delivery system. J Pharm Sci 86:565–572
Brand AM, De Kwaadsteniet M, Dicks LMT (2010) The ability of nisin F to control Staphylococcus aureus infection in the peritoneal cavity, as studied in mice. Lett Appl Microbiol 51:645–649
Chambers T, Thomson B, Fuller K (1984) Effect of substrate composition on bone resorption by rabbit osteoclasts. J Cell Sci 70:61–71
Chen H, Hoover D (2003) Bacteriocins and their food applications. Compr Rev Food Sci Food Safety 2:82–100
Cotter PD, Hill C, Ross RP (2005) Bacteriocins: developing innate immunity for food. Nat Rev Microbiol 3:777–788
De Kwaadsteniet M, Doeschate K, Dicks LMT (2009) Nisin F in the treatment of respiratory tract infections caused by Staphylococcus aureus. Lett Appl Microbiol 48:65–70
De la Riva B, Sánchez E, Hernández A, Reyes R, Tamimi F, López-Cabarcos E, Delgado A, Évora C (2009) Local controlled release of VEGF and PDGF from a combined brushite-chitosan system enhances bone regeneration. J Controlled Rel 143:45–52
Espehaug B, Engesaeter L, Vollset S, Havelin L, Langeland N (1997) Antibiotic prophylaxis in total hip arthroplasty: review of 10 905 primary cemented total hip replacements reported to the Norwegian arthroplasty register, 1987 to 1995. J Bone Joint Surg (Br) 79:590–595
Frommelt L, Kühn KD (2005) Properties of bone cements: antibiotic-loaded bone cements. In: Breusch SJ, Malchau H (eds) The well-cemented total hip arthoplasty: theory and practice. Springer Medizin, Germany, pp 86–92
Fullana G, Ternet H, Freche M, Lacout JL, Rodriguez F (2010) Controlled release properties and final macroporosity of a pectin microspheres–calcium phosphate composite bone cement. Acta Biomater 6:2294–2300
Ginebra M, Traykova T, Planell J (2006) Calcium phosphate cements as bone drug delivery systems: a review. J Controlled Rel 113:102–110
Górecki A, Babiak I (2008) Infection of joint prosthesis and local drug delivery. In: Kienapfel H, Kühn KD (eds) The infected implant. Springer Medizin, Germany, pp 19–26
Hamanishi C, Kitamoto K, Tanaka S, Otsuka M, Doi Y, Kitahashi T (1996) A self-setting TTCP-DCPD apatite cement for release of vancomycin. J Biomed Mater Res-B 33:139–143
Hanssen AD, Rand JA, Osmon DR (1994) Treatment of the infected total knee arthroplasty with insertion of another prosthesis: the effect of antibiotic-impregnated bone cement. Clin Orthop 309:44–55
Hofmann M, Mohammed A, Perrie Y, Gbureck U, Barralet J (2009) High-strength resorbable brushite bone cement with controlled drug-releasing capabilities. Acta Biomater 5:43–49
Klaenhammer TR (1993) Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev 12:39–85
Knoetze H, Todorov SD, Dicks LMT (2008) A class IIa peptide from Enterococcus mundtii inhibits bacteria associated with otitis media. Int J Antimicrob Agents 31:228–234
Kruszewska D, Sahl HG, Bierbaum G, Pag U, Hynes SO, Ljungh Å (2004) Mersacidin eradicates methicillin-resistant Staphylococcus aureus (MRSA) in a mouse rhinitis model. J Antimicrob Chemother 54:648–653
Le Nihouannen D, Komarova SV, Gbureck U, Barralet JE (2008) Bioactivity of bone resorptive factor loaded on osteoconductive matrices: stability post-dehydration. E J Pharma Biopharm 70:813–818
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
National Institutes of Health (1994) Total Hip Replacement. NIH Consensus Statement 12:1–31
Pierce AM, Lindskog S, Hammarström L (1991) Osteoclasts: structure and function. Electron Microsc Rev 4:1–45
Piper C, Hill C, Cotter PD, Ross RP (2010) Bioengineering of a Nisin A-producing Lactococcus lactis to create isogenic strains producing the natural variants Nisin F, Q and Z. Microb Biotech. doi:10.1111/j.1751-7915.2010.00207
Ruhe P, Boerman O, Russel F, Spauwen P, Mikos AG, Jansen JA (2005) Controlled release of rhBMP-2 loaded poly (dl-lactic-co-glycolic acid)/calcium phosphate cement composites in vivo. J Controlled Rel 106:162–171
Ruhe PQ, Hedberg EL, Padron NT, Spauwen PHM, Jansen JA, Mikos AG (2003) rhBMP-2 release from injectable poly (DL-lactic-co-glycolic acid)/calcium-phosphate cement composites. J Bone Joint Surg 85:75–81
Salmaso S, Elvassore N, Bertucco A, Lante A, Caliceti P (2004) Nisin-loaded poly-L-lactide nano-particles produced by CO2 anti-solvent precipitation for sustained antimicrobial activity. Int J Pharm 287:163–173
Schnieders J, Gbureck U, Thull R, Kissel T (2006) Controlled release of gentamicin from calcium phosphate–poly (lactic acid-co-glycolic acid) composite bone cement. Biomaterials 27:4239–4249
Tamimi F, Torres J, Bettini R, Ruggera F, Rueda C, López-Ponce M, Lopez-Cabarcos E (2008) Doxycycline sustained release from brushite cements for the treatment of periodontal diseases. J Biomed Mater Res-A 85:707–714
Thornes B, Murray P, Bouchier-Hayes D (2004) Development of resistant strains of Staphylococcus epidermidis on gentamicin-loaded bone cement in vivo. J Bone Joint Surg (Br) 84:758–760
Van Reenen CA, Chikindas ML, Van Zyl WH, Dicks LMT (2003) Characterization and heterologous expression of a class IIa bacteriocin, plantaricin 423 from Lactobacillus plantarum 423, in Saccharomyces cerevisiae. Int J Food Microbiol 81:29–40
Young AM, Ng PYJ, Gbureck U, Nazhat SN, Barralet JE, Hofmann MP (2008) Characterization of chlorhexidine-releasing, fast-setting, brushite bone cements. Acta Biomater 4:1081–1088
Zimmerli W (2006) Prosthetic-joint-associated infections. Best Prac Res Cl Rh 20:1045–1063
Zimmerli W, Ochsner P (2003) Management of infection associated with prosthetic joints. Infection 31:99–108
Acknowledgments
This work was supported by a grant from Cipla Medpro, South Africa, and the National Research Foundation, South Africa.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Staden, A.D., Heunis, T.D.J. & Dicks, L.M.T. Release of Enterococcus mundtii Bacteriocin ST4SA from Self-Setting Brushite Bone Cement. Probiotics & Antimicro. Prot. 3, 119–124 (2011). https://doi.org/10.1007/s12602-011-9074-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-011-9074-7