Abstract
Selective microbes used as probiotics can enhance epithelial cell protection. We have previously shown that a Lactobacillus plantarum strain 299v (Lp299v) has the ability to induce mucin genes. In the current study, we utilized a cytokine model of inflammation in cell culture to study the modulation of apoptosis by this probiotic. HT-29 cells were pre-incubated with the Lp299v or L. plantarum strain adh- (Lpadh-), a non-adherent derivative of Lp299v. Cells were challenged with a mixture of cytokines (TNF-α, IFN-γ, and IL-1a) to imitate conditions of inflammation. To assess for cell death, we evaluated TUNEL, multi-caspase, and caspase-3 and caspase-7 activity assays. There was a marked decrease in apoptosis as measured by TUNEL+ cells in samples pre-treated with Lp299v (18.7 ± 4.1%, p < 0.01) and Lpadh- (16.6 ± 3.2%, p < 0.05) prior to cytokine exposure when compared to cells (43.6 ± 6.2%) exposed to the cytokine mixture. Lp299v pre-incubation with HT-29 cells reduced caspase+ cells in the multi-caspase activity assay (3.6 ± 0.6%, p < 0.05) compared to cells exposed to cytokines (68.9 ± 5.1%) whereas Lpadh- did not (46.8 ± 17.5%, p > 0.05). Similarly, caspase-3, caspase-7 activity was also reduced by Lp299v. Selected probiotics may confer an exogenous protective effect at the mucosal–luminal interface for intestinal epithelial cells via alteration of caspase-dependent apoptotic pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Probiotics are non-pathogenic microbes that can confer a number of health benefits to their host. They can exert some of their effects at the mucosal–luminal interface through their actions on intestinal epithelial cells (IECs). Select probiotics can upregulate IEC mucin expression to impede enteropathogen access to IECs [1]; maintain barrier function following enteropathogen insult and reduce enteropathogen colonization and adherence [2, 3]; modulate Toll-like receptor 2, critical in maintaining barrier function when cells are faced with inflammatory stress [4]; and prevent apoptosis to enhance survival of mucosal cells to subsequently provide enhanced mucosal barrier function [5].
IECs constitute the mucosal layer of the small intestine and act as the cellular barrier between luminal contents and the host. IEC homeostasis is maintained in the gastrointestinal tract mainly via proliferative activity and apoptosis [6]; cells shed naturally are rapidly replenished by stem cells in villi crypts, every 3–5 days on average [7]. Due to this large degree of cell turnover and the physiological requirement for apoptotic cell death, it follows that dysregulated apoptosis is indicated in a number of gastrointestinal diseases [6, 8–11]. Since a reduction in disproportionate apoptosis improves intestinal health [12], it is worthwhile to further delineate the nature of this physiological process. Previous research has shown that a secreted product of Lactobacillus rhamnosus strain GG (LrGG) can inhibit cytokine-induced apoptosis [5]. However, both the physical nature of the microbial–IEC relationship as it relates to apoptosis as well as the extent of caspase involvement is not well described.
The goal of this study was to further understand the relationship that exists between probiotics and IECs. It was hypothesized that intimate probiotic interaction requiring adherence with IECs enhances cell survival through alteration of programmed cell death (PCD) mechanisms. Specific aims included evaluating the extent of L. plantarum strain 299v (Lp299v)-mediated anti-apoptosis in a model of cytokine-induced stress and assessing the role of adherence with any anti-apoptotic effect noted in vitro.
Methods
Bacteria and Growth Conditions
Bacteria used in the study included L. plantarum strain 299v (Lp299v; supplied by Dr. Siv Ahrne, University of Lund, Lund, Sweden) that is a member of a genetically well-defined sub-group of L. plantarum isolated from intestinal mucosa [13]. An adherent-negative derivative strain of Lp299v named L. plantarum strain adh- (Lpadh-; supplied by Dr. Siv Ahrne) is a spontaneous mutant of Lp299v that lacks adherin (personal communication, Dr. S Ahrne), has reduced intestinal epithelial cell binding, and lacks the ability to modulate mucin gene expression by intestinal epithelial cells [14]. RFLP analysis did not detect differences between Lp299v and its derivative Lpadh- [13]. Stock cultures were stored at −80 °C or maintained at 4 °C on Lactobacilli MRS agar (Becton, Dickinson and Company, Sparks, MD) until grown overnight at 37 °C in Lactobacilli MRS broth (Becton–Dickinson Canada Inc., Oakville, ON). Bacteria were harvested by centrifugation and quantified as previously described [1].
Cell Culture
As Lp299v was initially isolated from small intestinal biopsies, the HT-29 cells (American Type Culture Collection, Manassas, VA) were progressively transferred from a glucose-containing McCoy’s 5A media to a glucose-free, 5 mM galactose-containing McCoy’s 5A culture medium as previously described [15] to transform the cells to display features of small intestinal cells [16]. Culture medium was supplemented with 10% heat-inactivated fetal bovine serum at 0.25% (Invitrogen Canada Inc.) and an antibiotic/antimycotic mixture containing 100 U/mL penicillin G, 100 mg/mL streptomycin sulfate, and 0.25 mg/mL amphotericin B (Invitrogen Canada Inc.). Cells were washed twice with PBS and incubated in antibiotic-free media prior to exposure to bacterial strains. Cells were cultured at 37 °C in 75-cm2 culture flasks (Corning Inc., NY) in a ThermoForma Series II Water Jacketed CO2 (ThermoForma, Mariette, OH) with 5% CO2 and were routinely passaged after washing with PBS using Trypsin–EDTA (Invitrogen Canada Inc.). For the in vitro assessment of cytokine-induced apoptosis, HT-29 cells were grown to approximately 80% confluence on sterilized microscope slide cover slips.
Cytokine-Mediated Induction of Apoptosis
Cytokine profiles in the bowel with active inflammation include IL-1, IFNγ, and TNFα [17]. This mixture has also been previously found to induce IEC apoptosis in vitro [5]. We therefore chose a mixture of these three cytokines for our in vitro analysis of apoptosis. Cells on cover slips were incubated with fresh antibiotic-free media and 1 × 109 CFU/mL Lp299v, or 1 × 109 CFU/mL Lpadh- for 1 h. Following microbial exposure for 1 h, 100 ng/mL of IFNγ, 100 ng/mL of TNFα, and 10 ng/mL of IL-1α were administered to HT-29 cells for a period of 15 min, after which time cells were washed twice in PBS before further analysis of the extent of apoptosis using two distinct methods.
TUNEL Assay
Cells were assessed for the relative extent of total apoptosis using the ApopTag Peroxidase In Situ Apoptosis Detection Kit (Chemicon International, Temecula, CA). This kit is a version of the TUNEL assay based on the detection of 180-bp DNA fragments that are the hallmark of apoptotic cell death. Cleaved DNA fragments were detected by dNTP TdT enzyme labeling to 3′ OH ends. An anti-digoxigenin antibody was then used which in the presence of peroxidase substrate such as diaminobenzidine (DAB) produced a brown color indicative of apoptosis. Cells were counterstained with hematoxylin for 10 s and viewed with a light microscope to distinguish stained cells. An ApopTag Fluorescein In Situ Apoptosis Detection Kit (Chemicon International, Temecula, CA) as a fluorescent TUNEL assay was also employed as a means of verification of TUNEL+ cells due to the increased sensitivity of this method. This assay uses a fluorescent-conjugated primary antibody for detection of TdT enzyme activity instead of the peroxidase system.
Caspase Activity Assay
Caspase activity assays can detect cells in the early stages of apoptosis that would otherwise be missed in the TUNEL assay and also account for the degree of TUNEL+ cells that were in fact necrotic and not truly apoptotic, as TUNEL assesses non-specific breaks in DNA. Experiments were conducted using a multi-caspase activity assay measuring the activity of caspases 1–9. Additional experiments were conducted using a caspase-3 and -7 activity assays to determine more specifically the relative involvement of these two main effector caspases [18]. Both the CaspaTag Multi-Caspase In Situ Assay Kit and the CaspaTag Caspase-3/7 In Situ Assay Kit (Chemicon International, Temecula, CA) use fluorochrome inhibitors of caspases (FLICA) reagent to detect and bind active caspases. The caspase-3/7 kit uses a carboxyfluorescein-labeled fluoromethyl ketone peptide inhibitor of caspase-3 (FAM-DEVD-FMK) to produce a green fluorescence. For both assays, Hoechst stain was used to label the nuclei of dividing cells and as a means of enumerating a total cell count. Cover slips were inverted onto a clean glass microscope slide on top of a drop of 1× wash buffer before being analyzed via fluorescent microscopy. Green fluorescence indicative of apoptotic cell death was viewed using a bandpass filter (excitation 490 nm, emission 520 nm) and cell nuclei were viewed using a filter set to excitation 365 nm and emission 480 nm.
TUNEL and Caspase Activity Analysis
Upon completion of the TUNEL assays, samples were inverted onto microscope slides and analyzed by counting the number of TUNEL+ cells per 200 total cells detected via light microscopy (for peroxidase TUNEL) and fluorescent microscopy (fluorescent TUNEL) and displayed as a percentage of TUNEL+ cell death. Similarly, caspase+ cells were counted per 200 cells and graphically represented as a percentage of caspase-dependent cell death (PCDI). Caspase+ cells were noted as green fluorescent cells with Hoescht blue nuclear stain to delineate total cell nuclei.
Statistical Analysis
Data from all groups were analyzed using 1-way ANOVA and the Tukey post-test for inter-condition comparison. Statistics were generated using Prism Version 4.0 (GraphPad Software, San Diego, CA) and are presented as mean ± SE with a CI of 95%; p < 0.05 was considered statistically significant.
Results
Lp299v and Lpadh- Similarly Protect Intestinal Epithelial Cells from Cytokine-Induced Cell Death
Control cells not exposed to cytokines were assayed for TUNEL positivity and fell within an anticipated range (23.5 ± 3.2%). Following cytokine exposure, there was a significant increase in TUNEL+ cells (43.6 ± 6.2%, p < 0.05 versus control). Pre-treatment of HT-29 cells with the adherent-negative Lpadh- leads to a substantial decrease in this value (16.6 ± 3.2%, p < 0.05) (Fig. 1a, compare panels 1 and 2; and 1b, second and third bars) compared to cells treated with cytokines. Similarly, Lp299v incubation prior to cytokine exposure mimicked this effect (18.7 ± 4.1%, p < 0.05) (Fig. 1a, compare panels 1 and 3; and 1b, second and fourth bars). These results suggest that both Lp299v and Lpadh- pre-incubation on IECs similarly confer protection against cytokine-induced cell death involving DNA cleavage, as detected by TUNEL.
Lp299v and Lpadh- similarly protect cells from cytokine-induced apoptosis. Panel a Peroxidase in situ TUNEL staining of HT-29 Gal cells in vitro following cytokine exposure (1), incubation with Lpadh- for 1 h prior to cytokine exposure (2), and incubation with Lp299v for 1 h prior to cytokine exposure (3). Images were obtained using an Axioskop microscope and Northern Eclipse software for analysis. Panel b Average number (mean ± SE) of TUNEL+ intestinal epithelial cells reported as a percentage of total cells counted in 9 randomly selected microscopic fields in 4 independent experiments; * p<0.05, ** p<0.01 versus cytokine-treated cells
Lp299v Significantly Decreases Caspase-Dependent Apoptosis In Vitro
To differentiate caspase-dependent programmed cell death (PCDI) from necrosis and caspase-independent programmed cell death (PCDII), HT-29 cells were analyzed for caspase involvement using the caspase activity assay as a means of caspase+ cell detection. When HT-29 Gal cells were incubated for 15 min with the pro-inflammatory cytokines in the absence of either the adherent or non-adherent probiotic strain, there were a large number of caspase+ cells noted (68.9 ± 5.1%) when compared to control cells (25.0 ± 2.0%) (Fig. 2a, panel 1; and 2b, first and second bars). There was a significant decrease in caspase+ cells observed in samples pre-treated with Lp299v prior to 15 min cytokine exposure (3.6 ± 0.6%, p < 0.05) compared to cytokine-treated cells (Fig. 2a, compare panels 1 and 3; and 2b, second and fourth bars). Caspase reactivity (46.8 ± 17.5%, p > 0.05) with Lpadh- treated cells was not statistically different than cytokine-treated cells.
Incubation of cells with Lp299v decreases caspase activation following cytokine exposure. Panel a Fluorescent caspase activity assay showing caspase+ cells following cytokine exposure (1), incubation with Lpadh- for 1 h prior to cytokine exposure (2), and incubation with Lp299v for 1 h prior to cytokine exposure (3). Caspase+ cells are indicated by the presence of green fluorescence on a blue Hoescht counterstain which indicate cell nuclei. Images were obtained using an Axioskop microscope and Northern Eclipse software for analysis. Panel b Caspase activity assay results following enumeration of apoptotic cells per total cells counted in randomly selected microscopic fields. Values represent mean ± SE and are the result of three independent experiments. * p<0.01 versus cytokine treatment
Lp299v and Lpadh- Demonstrate Opposing Effects on Caspase-3 and -7 Activity
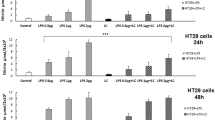
To distinguish between the number of caspase+ cells and caspase-3, 7+ cells, HT-29 cells were subjected to experimental conditions similar to the caspase activity assay except using a fluorescent dye specific to activated caspase-3 and -7. This was to determine whether Lp299v and Lpadh- exclusively inhibited the activity of executioner caspases-3 and -7 or instead exerted a global effect on caspase family members. Control cells not subjected to experimental treatment were assessed using the assay and found to have levels of caspase reactivity that fell within the normal range indicated by the manufacturer (control data not shown). When cells were exposed to cytokines for 15 min, there was a large degree of caspase-3, 7+ reactivity observed (73%) (Fig. 3, first bar). In contrast, cells incubated with Lp299v for 1 h prior to cytokine exposure demonstrated a significant attenuation of caspase-3, 7+ reactivity (4%), once again implicating their role in IEC protection from cytokine-mediated apoptosis (Fig. 3, third bar). Cells exposed to Lpadh- prior to cytokine exposure showed minimal decrease in percentage of caspase-3, 7+ cells compared to cytokine exposed cells alone (Fig. 3, second bar).
Discussion
In this study, both Lp299v and Lpadh- pre-incubation with HT-29 cells ameliorated the effects of HT-29 cell by cytokines exposure as measured by an in vitro TUNEL assay, an assay that measures the extent of cell death involving the DNA cleavage characteristic of apoptosis.
To assess the relative degree of whole caspase involvement measuring caspase-1 involvement and caspase-8 activity consistent with inflammation, a multi-caspase activity assay to measure the activity of caspases 1–9 was performed. Consistent with TUNEL assay results, the multi-caspase activity assay revealed an increase in apoptotic HT-29 cell death following cytokine exposure in the absence of probiotics (68.9 ± 5.1%). However, only Lp299v pre-incubation reduced caspase+ cells (3.6 ± 0.6%, p < 0.01). The failure of Lpadh- to protect against PCDI underlines the importance of the microbial adherence factor in eliciting IEC protection from caspase-dependent apoptosis. This same adherence has been previously found to be important in in vivo mucin expression [19] and in vitro attenuation of enteropathogen adherence to IECs [15]. Taken together, these results suggest that direct interaction between probiotic organisms and IECs enhance cellular-derived protection. Polk et al. [5] showed the LrGG secreted a soluble factor that was responsible for prevention of apoptosis following cytokine exposure of IECs and this strain is also capable of attenuation of enteropathogen adherence to IECs [1]. An epithelial cell adhesin could be expected to enhance delivery of such a soluble compound to cells to which they are bound. Alternatively, Lpadh- may act on caspase-independent PCDII or necrosis.
Our further experiments were conducted using a caspase-3 and -7 activity assay to determine more specifically whether key executioner caspases were being inhibited in the prevention of apoptosis. As with the multi-caspase activity assay, when cells were incubated with Lp299v for 1 h prior to cytokine exposure, the percentage of caspase-3, -7+ cells markedly decreased, suggesting that the strain’s reduction of caspase-dependent programmed cell death may be mediated by caspase-3 and -7 inhibition. The lack of protection conferred by the non-adherent Lpadh- points both to the suspected role of adherence in preventing cytokine-induced caspase activation.
Successful disease interference would be aided by knowledge of the form of cell death indicated for the specific disease in question [20]. If probiotics impinge selectively on caspase-3 and -7, mainly affecting the end stage of apoptosis, this may permit the activity of other caspases involved in differentiation and cell development which would also be of benefit to the host [8]. Probiotics have been found to be of benefit in the prevention of development of necrotizing enterocolitis [7] that is associated with a large degree of necrosis and IEC death [21, 22]. Since the comparatively circumscribed level of apoptosis is thought to trigger opportunistic necrotic cell death, PCDI inhibition could potentially block subsequent necrosis and chronic inflammation, attributed in part by necrosis. Preventing apoptosis as such could preclude subsequent necrosis and inflammation characteristic of necrotizing enterocolitis and provide some additional explanation for the mechanism of action behind probiotic success in preventing the development of this condition [23, 24]. Probiotics that closely interact with IECs and demonstrate an anti-apoptotic phenotype associated with caspase-3 and -7 inhibition and therapeutic interference of PCDI would provide another explanation of probiotic effect and could potentially be used to maximize benefit at the mucosal–luminal interface.
References
Mack DR, Michail S, Wei S, McDougall L, Hollingsworth MA (1999) Probiotics inhibit enteropathogenic E coli adherence in vitro by inducing intestinal mucin gene expression. Am J Physiol 276(4 Pt 1):G941–G950
Sherman PM, Johnson-Henry KC, Yeung HP, Ngo PS, Goulet J, Tompkins TA (2005) Probiotics reduce enterohemorrhagic Escherichia coli O157:H7- and enteropathogenic E. coli O127:H6-induced changes in polarized T84 epithelial cell monolayers by reducing bacterial adhesion and cytoskeletal rearrangements. Infec Immun 73(8):5183–5188
Michail S, Abernathy F (2003) Lactobacillus plantarum inhibits the intestinal epithelial migration of neutrophils induced by enteropathogenic Escherichia coli. J Pediatr Gastroenterol Nutr 36(3):385–391
Cario E, Gerken G, Podolsky DK (2007) Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 132(4):1359–1374
Yan F, Polk DB (2002) Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem 277(52):50959–50965
Husain KD, Coopersmith CM (2003) Role of intestinal epithelial apoptosis in survival. Curr Opin Crit Care 9(2):159–163
Deshpande G, Rao S, Patole S, Bulsara M (2010) Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm infants. Pediatrics 125(5):921–930
Hotchkiss RS, Coopersmith CM, Karl IE (2005) Prevention of lymphocyte apoptosis-a potential treatment of sepsis? Clin Infect Dis 41(Suppl 7):S465–S469
Palejwala AA, Watson AJM (2000) Apoptosis and gastrointestinal disease. J Pediatr Gastroenterol Nutr 31(4):356–361
Lichtenberger GS, Flavell RA, Alexopoulou L (2004) Innate immunity and apoptosis in IBD. Inflamm Bowel Dis 10(Suppl 1):S58–S62
Grishin A, Ford H, Wang J, Li H, Salvador-Recatala V, Levitan ES, Zaks-Makhina E (2005) Attenuation of apoptosis in enterocytes by blockade of potassium channels. Am J Physiol Gastrointest Liver Physiol 289(5):G815–G821
Mahida YR (2004) Microbial-gut interactions in health and disease: epithelial cell responses. Best Pract Res Clin Gastroenterol 18(2):241–253
Johansson ML, Quednau M, Ahrne S, Molin G (1995) Classification of Lactobacillus plantarum by restriction endonuclease analysis of total chromosomal DNA using conventional agarose gel electrophoresis. Int J Syst Bacteriol 45(4):670–675
Mack DR, Ahrne S, Hyde L, Wei S, Hollingsworth MA (2003) Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut 52(6):827–833
Mack DR, Hollingsworth MA (1994) Alteration in expression of MUC2 and MUC3 mRNA levels in HT29 colonic carcinoma cells. Biochem Biophys Res Commun 199(2):1012–1018
Mack DR, Cheng PW, Perini F, Wei S, Hollingsworth MA (1998) Altered expression of sialylated carbohydrate antigens in HT29 colonic carcinoma cells. Glycoconjugate J 15(12):1155–1163
Ferrero-Miliani L, Seidelin JB, Nielsen OH (2006) Regulation of cytokine production in inflammatory bowel disease. Ugeskr Laeger 168(19):1847–1850
Liston P, Fong WG, Korneluk RG (2003) The inhibitors of apoptosis: there is more to life than Bcl2. Oncogene 22(53):8568–8580
Dykstra NS, Hyde L, Adawi D, Kulik D, Ahrne S, Molin G, Jeppsson B, Mackenzie A, Mack DR (2011) Pulse probiotic administration induces repeated small intestinal Muc3 expression in rats. Pediatr Res 69(3):206–211
Tsujimoto Y, Shimizu S (2005) Another way to die: autophagic programmed cell death. Cell Death Differ 12(Suppl 2):1528–1534
Clark JA, Lane RH, Maclennan NK, Holubec H, Dvorakova K, Halpern MD, Williams CS, Payne CM, Dvorak B (2005) Epidermal growth factor reduces intestinal apoptosis in an experimental model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 288(4):G755–G762
Neu J, Caicedo R (2005) Probiotics: protecting the intestinal ecosystem? J Pediatr 147(2):143–146
Hunter CJ, Williams M, Petrosyan M, Guner Y, Mittal R, Mock D, Upperman JS, Ford HR, Prasadarao NV (2009) Lactobacillus bulgaricus prevents intestinal epithelial cell injury caused by Enterobacter sakazakii-induced nitric oxide both in vitro and in the newborn rat model of necrotizing enterocolitis. Infect Immun 77(3):1031–1043
Lin PW, Nasr TR, Berardinelli AJ, Berardinelli AJ, Kumar A, Neish AS (2008) The probiotic Lactobacillus GG may augment intestinal host defense by regulating apoptosis and promoting cytoprotective responses in the developing murine gut. Pediatr Res 64(5):511–516
Competing interests
Work was supported in part through research grants from Probi AB (Lund, Sweden).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dykstra, N.S., Hyde, L., MacKenzie, A. et al. Lactobacillus plantarum 299v Prevents Caspase-Dependent Apoptosis In Vitro. Probiotics & Antimicro. Prot. 3, 21–26 (2011). https://doi.org/10.1007/s12602-011-9066-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-011-9066-7