Abstract
Twenty-five strains of lactic acid bacteria (LAB) isolated from South Indian traditional fermented foods Kallappam batter, Koozh and Mor Kuzhambu. Further 6 strains were selected based on their antimicrobial activity. They were identified according to morphological, biochemical and physiological criteria. Identification by 16S rDNA sequence homology of the isolates revealed the presence of Weissella paramesenteroides, Lactobacillus plantarum and Lactobacillus fermentum. Lactobacillus plantarum AS1 showed maximum antimicrobial activity among 6 strains and this strain was chosen for biopreservation. When male Albino Wistar rats were fed with L. plantarum AS1 (approx. 109 cells/mL for a month), there was no sign of any illness and they were on par with control rats in terms of weight gain/week. In the L. plantarum AS1–treated group, there was reduction in the populations of indigenous microflora of coliforms, yeast and molds; however, the lactobacilli population increased comparatively. L. plantarum AS1 was able to retain its normal growth in the presence of increasing concentration of bile salt in the MRS and it also tolerated the artificial gastric juice simulating the condition inside the stomach where it was viable for 24 h with bacterial count of 6.079 logCFU/mL. L. plantarum AS1 reduced the cholesterol in the MRS broth by 57.3%. Hence, all these properties established it as an effective probiont. L. plantarum AS1 found to be an effective biopreservative in cheese, where it decreased the population of Salmonella typhi by 2.95 log cycles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fermented foods in many countries are consumed, in part, because of a belief in their health promotion and disease prevention capacities. Most of these traditional fermented foods contain beneficial bacteria named probiotics. India is traditionally rich in fermented foods. But the nature of the products and the base material vary from region to region [21]. Appam or hoppers are a common type of food in South Indian cuisine especially in Tamil Nadu and Kerala. Another form of appam is “Kallappam”, which looks like a pancake. The name originated from “Kallu,” which means toddy and is used for fermentation [http://en.wikipedia.org/wiki/Appam]. Koozh is the Tamil name for a porridge made from millet. Finger millet, a traditional South Indian weaning food, is also consumed in the fermented form as koozh in rural and urban households [4]. Koozh is made from Kezhvaragu or Cumbu flour and broken rice (called noyee in Tamil) in a mud pot. Mor Kuzhambu is a dish from Tamil Nadu, made from curd or buttermilk [http://premascookbook.blogspot.com/2007/04/mor-kuzhambu.html]. These probiotic foods are mainly consisting of groups of bacteria known as lactic acid bacteria (LAB). LAB have a long history of safe use, and members of the genera Lactococcus and Lactobacillus have been given generally regarded as safe (GRAS) status [19]. The antibacterial effect of LAB is a result of fermentation and is attributed to organic acids (particularly lactate and acetate), lowered pH, hydrogen peroxide, diacetyl, competition and nutrient depletion, altered redox potentials, bacteriocins, deconjugation of bile acids and stimulation of the immune system, which act in concert [2, 5].
The aim of this work was to isolate and characterize lactic acid bacteria that produce antibacterial compounds from the South Indian fermented foods Kallappam, Koozh and Mor kuzhambu and thereafter to determine the efficacy of best strain as probiont and biopreservative.
Materials and Methods
Strains and Chemicals
The indicator organisms, viz. Vibrio parahaemolyticus 451, Vibrio vulnificus1145, Vibrio fischeri1738, Vibrio anguillarum, Escherichia coli DH5α, Lactobacillus acidophilus 447, Lactobacillus rhamnosus 1408, Salmonella typhi 734, Listeria monocytogenes 1143 and Proteus vulgaris 426, were procured from the Microbial Type Culture Collection (MTCC) at the Institute of Microbial Technology, Chandigarh, India. LAB were isolated from the Kallappam batter, Koozh and Mor kuzhambu.
The bacteriological media and analytical grade chemicals were obtained from Hi-Media, Mumbai, while the proteolytic enzymes, molecular weight markers, 16S rDNA PCR kit and purification kit were purchased from Bangalore Genei, India.
Preparation of South Indian Fermented Food
Kallappam Batter
Two hundred grams of boiled or raw rice was soaked in 300 ml of water for 3 h and then water was drained off through a strainer. The soaked rice was grinded in a mixer along with 3-day-old 100 ml of fermented coconut kallu (toddy). The grinded rice along with toddy was kept for 1 day to allow the mixture to ferment at room temperature.
Koozh
About 300 gm of raggi (finger millet) flour was mixed with 250 mL of water and allowed to ferment at room temperature for 2 days.
Mor Kuzhambu
Rice and dals were soaked together for 15–20 min. Green chillies, ginger, garlic and coconut were grinded with the soaked rice and dal to a fine paste. In a pan, a half cup water and veggies were added and halfway cooked, after which the ground paste and salt were added. Buttermilk curd was whisked/beated with turmeric powder and added to above-mentioned curry. This Mor Kuzhambu was allowed to ferment at room temperature for a day.
Isolation and Screening of LAB from Fermented Foods
LAB were isolated from the above-mentioned fermented foods, namely Kallappam batter, Koozh and Mor Kuzhambu, by appropriate dilutions with saline, plated onto MRS (de Mann Rogosa Sharpe) agar and incubated at 37 °C for 2–3 days. Twenty-five well-isolated colonies were picked up and transferred to MRS broth. Colonies were propagated twice and streaked on MRS agar to check the purity of the isolates and then stored in MRS soft agar (0.5%) overlaid with glycerol at −20 °C for further study.
All the 25 isolates were picked and inoculated into MRS broth and incubated at 37 °C for 24 h. Cell-free supernatants, adjusted to pH 6.0 with 2 N NaOH, were concentrated to one-tenth of the original volume by flash evaporator, sterilized by passing through a 0.22-μm membrane filter and evaluated for antimicrobial activity by the agar well diffusion method against the indicator organisms [23].
Characterization of LAB Strains Showing Antimicrobial Activity
LAB strains showing antimicrobial activity were Gram stained and examined microscopically for cellular morphology and Gram stain phenotype. Catalase activity test was performed by spotting colonies with 3% hydrogen peroxide. Strains were also observed visually by different sizes and color appearance of their colonies.
Growth was determined in MRS broth at 10, 15, 37 and 45 °C as well as at pH of 4.5, 5.0, 8.6 and 9.0 and incubated at 37 °C for 48 h. A citrate utilization test was carried out by Simmon’s citrate agar [20]. Assay for nitrate reduction was performed as described previously [8]. IMViC tests were also performed on the isolated strains.
Identification of LAB Strains by 16S rDNA Sequencing
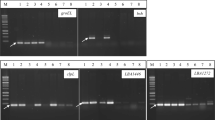
Genomic DNA from each isolate was isolated by the method described earlier [16]. The 16S rDNA was amplified from the isolated genomic DNA with the forward and reverse primers 16S1 (5′-AGAGTTTGATCCTGGCTCAG-3′) and 16S2 (5′-ACGGCTACCTTGTTACGACTT-3′), Taq DNA polymerase and buffers in the thermocycler for 30 cycles comprising 95 °C denaturation for 30 s, 55 °C annealing for 30 s and 72 °C for extension. The PCR-amplified rDNAs were purified using the quick PCR purification kit (Bangalore Genie, India). The sequencing was performed at MACROGEN, SOUTH KOREA. The analysis of alignment, homology and the construction of a phylogenetic tree for the partial nucleotide sequence of LAB was carried out by the BLAST and MEGA4 softwares.
In Vitro Test for Probiotic Characteristics of Potential LAB Strain
L. plantarum AS1 was selected to determine its probiotic-like characteristic as the strain displayed better antimicrobial activity against pathogens. The putative strain was assayed for bile tolerance, acid resistance and cholesterol reduction:
Bile Tolerance
Bile tolerance of L. plantarum AS1 was determined as described [12]. L. plantarum AS1 grew in MRS broth at 37 °C for 16 h. Cells were harvested by centrifugation at 5,000×g for 10 min and washed twice with 0.1 M phosphate buffer, pH 7.0. Cells were resuspended in the original volume with the buffer by vortexing, 0.5% from this suspension was used to inoculate sterilized MRS and MRSO broth (MRS broth supplemented with 0.05, 0.1, 0.15 and 0.3% of bile oxgall) and incubated at 37 °C. Absorbance was read at 560 nm at every 2 h for the 24 h of incubation.
Acid Resistance
Artificial juice (NaCl 0.2%, pepsin 3.2 g/l, pH 2.0) was prepared and sterilized by filtration (filter membrane 0.22 μm). As a control, artificial gastric juice adjusted at a final pH 6.0 with 1 N NaOH was taken. It was inoculated with 2% L. plantarum AS1 cell suspension containing 8.929 logCFU/mL viable cells and both media were incubated at 37 °C in an orbital shaker at 100 rpm. Samples were taken at 0, 1, 2, 3 and 4 h and after 24 h for cell viability by plating in MRS agar from 10-fold serial dilution prepared in 0.1% peptone water as described earlier [12]. Plates were incubated at 37 °C for 24 h. Results were expressed as colony-forming unit per milliliter (CFU/mL).
Cholesterol Reduction Assay
Freshly prepared MRS broth was supplemented with 0.30% oxgall as a bile salt. Water-soluble cholesterol (Sigma, USA) was filter-sterilized and added to the broth at a final concentration of 100 μg/mL, inoculated with each strain (at 1%), and incubated at 37 °C for 20 h. After the incubation period, cells were centrifuged and the remaining cholesterol concentration in the broth was determined using a modified colorimetric method as described [18]. One milliliter of the aliquot was added with 1 mL of KOH (33% wt/vol) and 2 mL of absolute ethanol, vortexed for 1 min and heated at 37 °C for 15 min. After cooling, 2 mL of distilled water and 3 mL of hexane were added and vortexed for 1 min. One milliliter of the hexane layer was transferred into a glass tube and evaporated under nitrogen. The residue was immediately dissolved in 2 mL of o-phthalaldehyde reagent. After complete mixing, 0.5 mL of concentrated sulfuric acid was added, and the mixture was vortexed for 1 min. Absorbance was read at 550 nm (Hitachi, Japan) after 10 min. All experiments were replicated twice.
In Vivo Characterization of LAB Strain for Probiotics Characteristics
Approval to work with Wistar rats was obtained from the Ethics Committee of the Pondicherry University (ethics reference number: PU/SLS/IAEC/15/08-09). Six-week-old male Albino Wistar rats were purchased from King’s Institute, Guindy, Chennai. They were acclimatized for 2 weeks. These rats were divided into two groups (6 rats each). One group was fed orally with 1 mL of 0.8% saline containing approx. 109 cells of L. plantarum AS1 daily for a month. Another group fed with 1 mL of saline alone and served as a control. Weights of animals were checked weekly. Fecal samples of treated and untreated rats were enumerated for microbiological population. One gram of feces sample was diluted in 0.8% saline. 0.1 mL of diluents were plated on MRS agar, yeast and mold agar, and violet red bile agar. Plates were incubated for 24 h at 37 °C, and resultant colonies were counted.
Efficacy of Isolated LAB Strain in Preservation of Cheese
L. plantarum AS1 was chosen for the preservation of cheese as the strain displayed better antimicrobial activity against pathogens and passed both in vivo and in vitro criteria to be a probiont. Cottage cheese was sliced into small cubes weighing 5 g each. These cubes were distributed to sterile petriplates. Four groups were formed for biopreservation study: Control (uninoculated cheese), L. plantarum AS1 treated cheese, Salmonella typhi-treated cheese, L. plantarum AS1 + S.typhi treated cheese. L. plantarum AS1 and S.typhi were grown overnight in MRS broth and tryptic soy broth (TSB), respectively, at 37 °C. Cultures were centrifuged at 5,000×g for 10 min, and pellet was dissolved in the same volume of 0.1 M phosphate buffer; 8.079 logCFU/mL of L. plantarum AS1 and 7.968 logCFU/mL of S.typhi were inoculated to cheese. Both L. plantarum AS1 and S.typhi were applied by spraying over the surface of cheese bacterial sample containing above-mentioned number of viable cells to 5-g cheese cube. Cheeses were incubated for 15 days at 4 °C. Bacterial counts were enumerated at day 0, 5, 10 and 15. At every interval, 5 g cheese cube was dispensed into 50 mL of 0.8% saline, mixed thoroughly and plated onto selective media: MRS agar for L. plantarum AS1 and bismuth sulfite agar (Hi-Media, Mumbai) for S.typhi. Uninoculated cheese was plated on tryptic soy agar (TSA). Samples were diluted appropriately to make the colonies countable. Plates were incubated for 24 h at 37 °C. All tests were performed in triplicate.
Statistical Analysis
One-way analysis of variance (ANOVA) was done using the SPSS 7.5 statistical program.
Results
Isolation and Antimicrobial Activity of LAB Strains
Six of 25 randomly picked colonies exhibited antimicrobial activity against the indicator organisms: Vibrio parahaemolyticus 451, Vibrio vulnificus1145, Vibrio fischeri1738, Vibrio anguillarum, Escherichia coli DH5α, Lactobacillus acidophilus 447, Lactobacillus rhamnosus 1408, Salmonella typhi 734, Listeria monocytogenes 1143 and Proteus vulgaris 426. Two isolates, CS1 and CS2, were from Koozh; AS1, AS2 and AS5 were isolated from Kallappam; and one strain MS1 was from Mor Kuzhambhu. The cell-free supernatant of these isolates showed antimicrobial activity against other bacterial strains (Table 1). AS1 showed best antimicrobial activities among 6 isolated strains.
Morphological and Biochemical Characterization
All the 6 strains were found to be Gram positive, and morphologically, most of the strains were observed to be rod shaped. CS1 was catalase positive among the isolates. All isolates were oxidase negative and did not utilize citrate from the medium. CS1, AS1 and AS5 were able to reduce nitrate. Only AS1 was able to utilize tryptophan in the medium to liberate indole as by-product. CS2 and AS5 produced acidic end products and were methyl red positive. None of the strains fermented carbohydrate with the production of non-acidic end product and were negative for Voges-Proskauer test. Biochemical parameters showed all strains except CS1 to be Lactobacilli but confirmation could only be established after 16S rDNA sequence homology. All the strains grew well at 37 °C, but only AS1, AS2 and AS5 grew at 45 °C; strains CS1, CS2, AS1 and MS1 showed weak growth at 10 °C. Only strain AS1 showed growth at all temperatures. At pH 8.6, all the strains showed luxurious growth, while very weak growth was observed at pH 9.0 (Table 2). Here too, AS1 survived better at a wide pH range compared to other strains.
Identification by 16S rDNA Sequencing and Phylogenetic Relationships
Using BLAST software, 16S rDNA sequences of 6 isolates CS1, CS2, AS1, AS2, AS5 and MS1 were compared with sequence available in the Gene bank database, and sequences were deposited in the NCBI Gene bank. CS1 matched with Weissella paramesenteroides (FJ821316) and also MS1 matched with Weissella paramesentroides (GQ468311), sequence of AS1 matched with Lactobacillus plantarum (GQ468312); similarly, AS2, AS5 (FJ821317) and CS2 (GQ478018) matched with Lactobacillus fermentum.
In Vitro Test for Probiotic Characteristics of Potential LAB Strain
Bile Tolerance
L. plantarum AS1 was found to be tolerant to increased bile salt concentration, i.e. 0.05, 0.1, 0.15 and 0.3% as shown by the growth curve of bacteria in Fig. 1. The initial growth of bacteria in MRSO was delayed up to the 6th hour when compared with MRS broth, but increased to a growth rate similar to that found in MRS broth. Therefore, L. plantarum AS1 could grow normally in the presence of bile salt concentration.
Acid Tolerance
L. plantarum AS1 was viable at pH 2.0 even after 24 h of incubation; however, the bacterial count of L. plantarum AS1 was lower compared to control (pH 6.0). Initially, when L. plantarum AS1 was inoculated, its count was 6.973 logCFU/mL at pH 2.0 and 8.146 logCFU/mL at pH 6.0, which was reduced to 6.079 logCFU/mL and 7.732 logCFU/mL, respectively, after 24 h (Table 3). In other sense, there was 12.82% reduction in bacterial count at pH 2.0 compared to 5.08% at pH 6.0. Hence, majority of cells were viable at pH 2.0 even after 24 h of incubation.
Cholesterol Reduction
Absorbance at 550 nm was 0.248 ± 0.005 for standard 100 μg/mL cholesterol (uninoculated MRSCHO broth). Absorbance of test sample (inoculated MRSCHO broth) was 0.106 ± 0.004. Residual cholesterol in the inoculated MRSCHO broth (MRS broth with 0.3% bile salt and 100 μg/mL cholesterol) was determined to be 42.7 μg/mL, i.e., total cholesterol reduced or assimilated was 57.3 μg/mL. Thus, L. plantarum AS1 reduced cholesterol by 57.3%.
In Vivo Characterization of LAB Strain for Probiotics Characters
When male Albino Wistar rats were fed with L. plantarum AS1 (approx. 109 cells/mL for a month), there was no sign of any illness and they were on par with control rats in terms of weight gain/week (Fig. 2). There was a marked decrease in the count of yeast & mold and Coliform bacteria in L. plantarum AS1 treated samples compared to untreated rats (Table 4). These results indicate the antimicrobial nature of L. plantarum AS1 inside the gut of the rat.
Efficacy of Strain AS1 as Biopreservatives
L. plantarum AS1 effectively controlled S.typhi population in cheese during 15 days of storage at 4 °C. The appearance and texture of the cheese remained stable and solid compared to the cheese infected with pathogen. Initial count of L. plantarum AS1 in the cheese was 9.991 logCFU/mL. This probably also includes the bacteria naturally present in cheese, which were enumerated to be 2.342 logCFU/mL. In the cheese sample groups inoculated with both L. plantarum AS1 and S.typhi, L. plantarum AS1 count was 9.963 logCFU/mL, whereas S.typhi count reduced drastically from 9.819 logCFU/mL to 4.869 logCFU/mL (Fig. 3). Hence, L. plantarum AS1 decreased the S.typhi population by 2.95 log cycles.
Discussion
Traditional fermented foods are one of the rich sources for isolation of LAB. In recent years, many papers have been published on isolation and characterization of LAB from traditional fermented foods. Fifty bacteriocin-producing Lactobacillus species were isolated from traditional Nigerian fermented foods such as Fufu, Garri, Nono and Ogi [3]. Twelve bacteriocin-producing LAB strains were isolated from Senegal fermented foods [6]. Similarly, two LAB Lactobacillus acidophilus and Lb. casei were isolated from appam batter and pickles and characterized [10]. Lactobacillus plantarum and Lactobacillus paracasei were isolated from Sudanese fermented camel’s milk product Garris [22]. LAB belonging to Lactobacilli and Pediococcus were also isolated from borde an Ethopian cereal beverage [1] and from Nigerian fermented dairy products wara, nunu and unpasteurized yogurts [17]. This is the first study wherein 25 LAB strains were isolated from traditional South Indian fermented foods Kallappam batter, Koozh and Mor Kuzhambhu. Among 25 isolates, six showed good antimicrobial activities. The strains were identified by 16S rDNA sequencing as Weisella paramesenteroides (CS1, MS1), L. plantarum (AS1) and L. fermentum (AS2, AS5 and CS2).
These isolates showed antibacterial activity toward Salmonella typhi, Vibrio parahaemolyticus and Listeria monocytogenes, which are food pathogens. Among these six strains, L. plantarum AS1 displayed better activity and it was selected to study its probiotic characteristics to determine its efficacy as biopreservative in cheese.
L. plantarum AS1 was screened for probiotic characteristics and found to be an effective probiont. L. plantarum AS1 was found to be tolerant to bile but there was initial delay in growth rate. This was probably due to the unfavorable condition conferred by bile in the medium. The bacterium was subsequently able to synthesize bile hydrolase enzyme to breakdown bile in the medium and could survive normally. Kaushik et al. [13] observed similar results with L. plantarum LP9. Bacterial number decreased by 1 log cycle after 2-h incubation at 37 °C in MRS broth containing 1.5–2.0% bile. Similarly, there was delay in growth rate in 0.3% oxgall containing MRS broth for L. acidophilus strains C28, FR1 and FR2 [7]. L. plantarum AS1 was tolerant to artificial gastric juice at pH 2.0. Hence, it could be administered orally as food supplement. In other studies, LP9 isolate found to be tolerant at pH 2.0 but its initial log (CFU/mL) of 8.9 decreased to 8.4 [13]. Cecal lactobacilli strain could survive at pH 2.0 for up to 2 h of incubation [11]. L. plantarum AS1 was found to be very effective in cholesterol reduction (up to 57.3%). Cholesterol reduction is a special property of probiotic bacteria, and many bacteria have been characterized to possess this property but the range of cholesterol reduction or assimilation varied among the Lactobacilli strains. In one study, cholesterol assimilation ranged from 0% for strains ATCC4356 and 14F1 to 50% for strain ATCC43121 [9].
L. plantarum AS1 was able to control the pathogen Salmonella typhi population in cottage cheese. Though cheese itself may contain LAB, the addition of L. plantarum AS1 increased the shelf life and improved the texture and quality of the cheese. Similarly, reduction in L. monocytogenes population was observed by 2.7 log cycles in Saint-Paulin cheese inoculated with bacteriocin preparation of E. faecium 4231 [14]. Reduction in pathogens was observed using a bacteriocin-producing E. faecium as a co-culture in cheddar cheese manufacture [15]. L. plantarum AS1 exhibited good antimicrobial activity, probiotic characteristics and biopreservation effect in cheese. Hence, this strain could be used as a probiont in food or dairy industries.
References
Abegaz K (2007) Isolation, characterization and identification of lactic acid bacteria involved in traditional fermentation of borde, an Ethiopian cereal beverage. Afr J Biotechnol 6:1469–1478
Adams MR (1990) Topical aspects of fermented foods. Trends in Food Science and Technology 1:140–144
Ogunshel AAO, Omotoso MA, Adeleye JA (2007) In vitro antimicrobial characteristics of bacteriocin producing Lactobacillus strains from Nigerian indigenous fermented foods. Afr J Biotechnol 6(4):445–453
Antony U, George ML, Chandra TS (1998) Inhibition of Salmonella typhimurium and Escherichia coli by fermented Finger millet (Eleusine coracana). W J Microbiol & Biotechnol 14:883–886
Daeschel MA (1989) Antimicrobial substances from lactic acid bacteria for use as food preservatives. Food Technol 43:164–166
Diop MK, Dubios-Dauphin R, Tine E, Nigom A, Destain J, Thonaut P (2007) Bacteriocin producers from traditional food products. Biotechnol Agron Soc Environ 11(4):275–281
Gilliland SE, Stanley TE, Bush LJ (1984) Importance of bile tolerance of Lactobacillus acidophilus used as a dietary adjunct. J Dairy Sci 67:3045–3051
Harrigan WF (1998) Laboratory methods in food microbiology. Academic press, California
James WA, Stanley EG (1999) Effect of fermented milk (yoghurt) containing Lactobacillus acidophilus L1 on serum cholesterol in hypercholesterolemic humans. J American College Nutr 18(1):43–50
Jamuna M, Jeevaratnam K (2004) Isolation and characterization of Lactobacilli from some traditional fermented foods and evaluation of the bacteriocins. J Gen Appl Microbiol 50:79–90
Jin LZ, Ho YW, Abdullah N, Jalaludin S (1998) Acid and bile tolerance of Lactobacillus isolated from chicken intestine. Lett Appl Microbiol 27:183–185
John Spencer FT, Alicia Ragout LS (eds) (2001) Food microbiology protocols, 14th edn. Humana press, Totowa. New Jersey
Kaushik JK, Kumar A, Duary RK, Mohanty AK, Grover S et al (2009) Functional and probiotic attributes of an indigenous isolate of Lactobacillus plantarum. PLoS ONE 4(12):e8099
Laukova A, Vlaemynek G (2003) Use of bacteriocin preparation with anti-microbial activity in saint-paulin cheese. Bull Vet Res Inst Pulawy 47(2):497–505
Leroy F, Foulquie Moreno MR, De vuyst L (2003) Enterococcus faecium RZSC5, an interesting bacteriocin producer to be used as a co-culture in food fermentation. Int J Food Microbiol 88:235–240
Marmur LJ (1961) A procedure for the isolation of deoxyribonucleic acid from microorganism. J Mol Biol 3:208–218
Osuntoki AA, Ejide OR, Omonigbehin EA (2008) Antagonistic effects on Enteropathogens and plasmid analysis of Lactobacilli isolated from fermented dairy products. Biotechnology 7(2):311–316
Rudel LL, Morris MD (1973) Determination of cholesterol using o-phthaldehyde. J Lipid Res 14:354–366
Salminen S, Von Wright A, Morelli L, Marteau P, Brassart D (1998) Demonstration of safety of probiotics—a review. Internat J Microbiol 44:93–106
Schillinger U, Geisen R, Holzapfel WH (1996) Potential of antagonistic microorganisms and bacteriocins for the biological preservation of foods. Trends Food Sci Tech 7:158–164
Sekar S, Mariappan S (2007) Usage of traditional fermented products by Indian rural folks and IPR. Ind J Traditional Knowledge 6(1):111–120
Sulieman AME, Osawa R, Tsenkova R (2007) Isolation and identification of lactobacilli from Garris, a Sudanese fermented Camel’s milk product. Res J Microbiol 2(2):125–132
Tagg JR, McGiven AR (1971) Assay systems for bacteriocins. Appl Microbiol 21:125
Acknowledgments
This work was supported by a grant from Department of Biotechnology (DBT), India. R.S.K. was supported by an ICMR fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Satish Kumar, R., Ragu Varman, D., Kanmani, P. et al. Isolation, Characterization and Identification of a Potential Probiont from South Indian Fermented Foods (Kallappam, Koozh and Mor Kuzhambu) and Its Use as Biopreservative. Probiotics & Antimicro. Prot. 2, 145–151 (2010). https://doi.org/10.1007/s12602-010-9052-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-010-9052-5